A Retrospective Analysis of Intravenous Push versus Extended Infusion Meropenem in Critically Ill Patients
Abstract
1. Introduction
2. Results
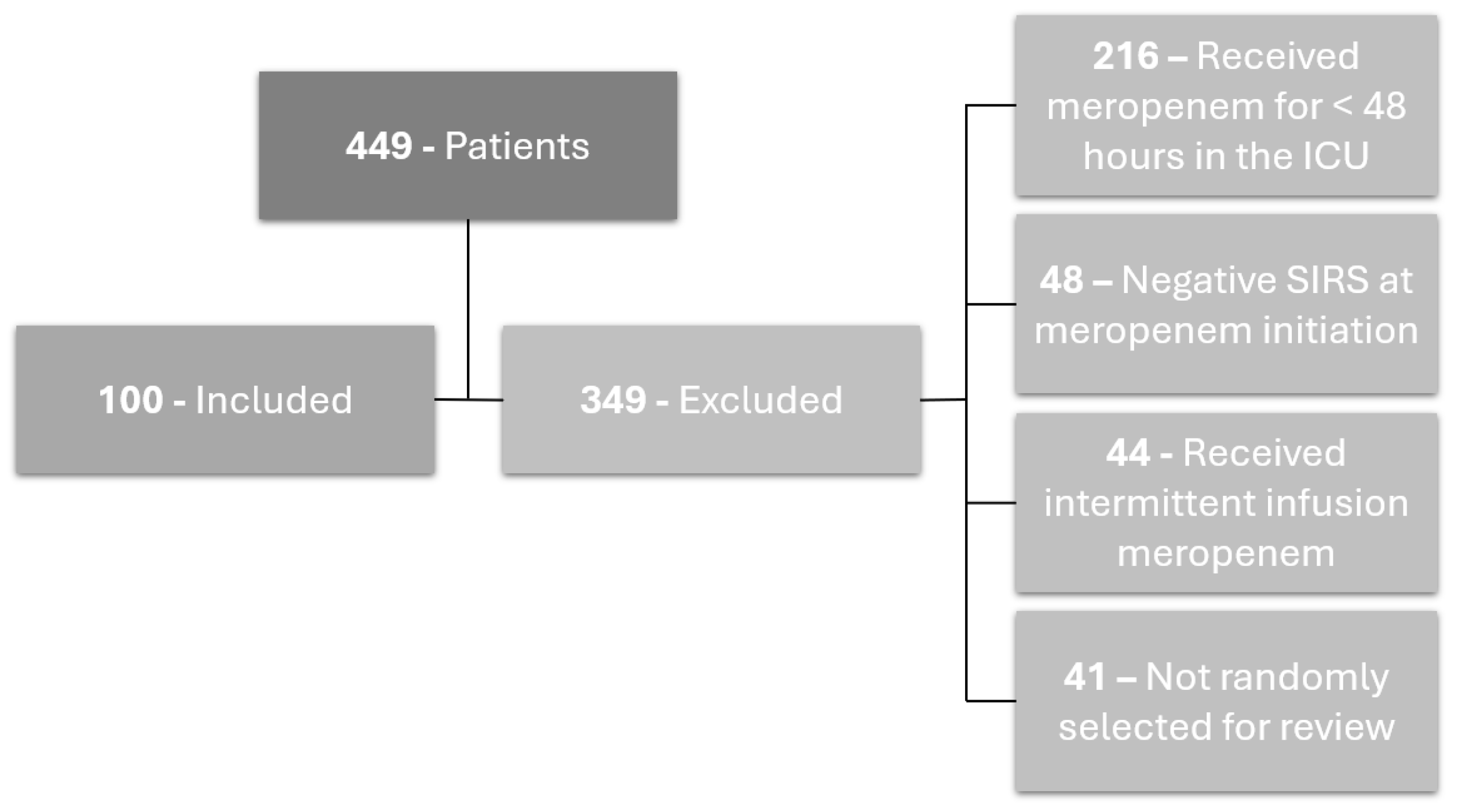
2.1. Patients
2.2. Primary Outcome
2.3. Secondary Outcomes
3. Discussion
4. Limitations
5. Materials and Methods
5.1. Study Design
5.2. Patient Selection
5.3. Primary and Secondary Outcomes
5.4. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monti, G.; Bradić, N.; Marzaroli, M.; Konkayev, A.; Fominskiy, E.; Kotani, Y.; Likhvantsev, V.V.; Momesso, E.; Nogtev, P.; Lobreglio, R.; et al. Continuous vs Intermittent Meropenem Administration in Critically Ill Patients with Sepsis: The MERCY Randomized Clinical Trial. JAMA 2023, 330, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-Y.; Gu, J.; Lyu, J.; Liu, D.; Wang, Y.-T.; Liu, F.; Zhu, F.-X.; An, Y.-Z. Pharmacokinetic and Pharmacodynamic Efficacies of Continuous versus Intermittent Administration of Meropenem in Patients with Severe Sepsis and Septic Shock: A Prospective Randomized Pilot Study. Chin. Med. J. Engl. 2017, 130, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Tilanus, A.; Drusano, G. Optimizing the Use of Beta-Lactam Antibiotics in Clinical Practice: A Test of Time. Open Forum Infect. Dis. 2023, 10, ofad305. [Google Scholar] [CrossRef] [PubMed]
- Charmillon, A.; Novy, E.; Agrinier, N.; Leone, M.; Kimmoun, A.; Levy, B.; Demoré, B.; Dellamonica, J.; Pulcini, C. The ANTIBIOPERF study: A nationwide cross-sectional survey about practices for β-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin. Microbiol. Infect. 2016, 22, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Dulhunty, J.M.; Paterson, D.; Webb, S.A.R.; Lipman, J. Antimicrobial utilisation in 37 Australian and New Zealand intensive care units. Anaesth. Intensive Care 2011, 39, 231–237. [Google Scholar] [CrossRef]
- Tabah, A.; De Waele, J.; Lipman, J.; Zahar, J.R.; Cotta, M.O.; Barton, G.; Timsit, J.-F.; Roberts, J.A. The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef]
- Buyle, F.M.; Decruyenaere, J.; Waele, J.; Tulkens, P.M.; Audenrode, T.; Depuydt, P.; Claeys, G.; Robays, H.; Vogelaers, D. A survey of beta-lactam antibiotics and vancomycin dosing strategies in intensive care units and general wards in Belgian hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 763–768. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Sulaiman, H.; Mat-Nor, M.-B.; Rai, V.; Wong, K.K.; Hasan, M.S.; Rahman, A.N.A.; Jamal, J.A.; Wallis, S.C.; Lipman, J.; et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): A prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med. 2016, 42, 1535–1545. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; Paterson, D.L.; et al. Continuous infusion of beta-lactam antibiotics in severe sepsis: A multicenter double-blind, randomized controlled trial. Clin. Infect. Dis. 2013, 56, 236–244. [Google Scholar] [CrossRef]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Clinical outcomes of continuous vs intermittent meropenem infusion for the treatment of sepsis: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2020, 29, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Hammond, N.E.; Brett, S.J.; Cotta, M.O.; De Waele, J.J.; Devaux, A.; Di Tanna, G.L.; Dulhunty, J.M.; Elkady, H.; Eriksson, L.; et al. Prolonged vs Intermittent Infusions of β-Lactam Antibiotics in Adults with Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. JAMA 2024, 332, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pang, X.; Wu, X.; Shan, C.; Jiang, S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PLoS ONE 2018, 13, e0201667. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.N.; Mynatt, R.P.; Kaye, K.S.; Zhao, J.J.; Pogue, J.M. Clinical Outcomes with Extended Versus Intermittent Infusion of Anti-Pseudomonal Beta-Lactams in Patients with Gram-Negative Bacteremia. Open Forum Infect. Dis. 2023, 10, ofad170. [Google Scholar] [CrossRef]
- Dulhunty, J.M.; Brett, S.J.; De Waele, J.J.; Rajbhandari, D.; Billot, L.; Cotta, M.O.; Davis, J.S.; Finfer, S.; Hammond, N.E.; Knowles, S.; et al. Continuous vs Intermittent β-Lactam Antibiotic Infusions in Critically Ill Patients with Sepsis: The BLING III Randomized Clinical Trial. JAMA 2024, 332, 629–637. [Google Scholar] [CrossRef]
- Hong, L.T.; Downes, K.J.; FakhriRavari, A.; Abdul-Mutakabbir, J.C.; Kuti, J.L.; Jorgensen, S.; Young, D.C.; Alshaer, M.H.; Bassetti, M.; Bonomo, R.A.; et al. International consensus recommendations for the use of prolonged-infusion beta-lactam antibiotics: Endorsed by the American College of Clinical Pharmacy, British Society for Antimicrobial Chemotherapy, Cystic Fibrosis Foundation, European Society of Clinical Microbiology and Infectious Diseases, Infectious Diseases Society of America, Society of Critical Care Medicine, and Society of Infectious Diseases Pharmacists. Pharmacotherapy 2023, 43, 740–777, Erratum in Pharmacotherapy 2024, 44, 8. [Google Scholar] [CrossRef]
- Lokhandwala, A.; Patel, P.; Isaak, A.K.; Yousaf, R.F.; Maslamani, A.N.J.; Khalil, S.K.; Riaz, E.; Hirani, S. Comparison of the Effectiveness of Prolonged Infusion and Intermittent Infusion of Meropenem in Patients with Sepsis: A Meta-Analysis. Cureus 2023, 15, e46990. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Rhodes, N.J.; Liu, J.; O’donnell, J.N.; Dulhunty, J.M.; Abdul-Aziz, M.H.; Berko, P.Y.; Nadler, B.; Lipman, J.; Roberts, J.A. Prolonged infusion piperacillin-tazobactam decreases mortality and improves outcomes in severely ill patients: Results of a systematic review and meta-analysis. Crit. Care Med. 2018, 46, 236–243. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Davis, J.S.; Dulhunty, J.M.; Cotta, M.O.; Myburgh, J.; Bellomo, R.; Lipman, J. Continuous versus Intermittent β-Lactam Infusion in Severe Sepsis. A Meta-analysis of Individual Patient Data from Randomized Trials. Am. J. Respir. Crit. Care Med. 2016, 194, 681–691. [Google Scholar] [CrossRef]
- Karaba, S.M.; Cosgrove, S.E.; Lee, J.H.; Fiawoo, S.; Heil, E.L.; Quartuccio, K.S.; Shihadeh, K.C.; Tamma, P.D. Extended-Infusion β-Lactam Therapy, Mortality, and Subsequent Antibiotic Resistance Among Hospitalized Adults with Gram-Negative Bloodstream Infections. JAMA Netw. Open. 2024, 7, e2418234. [Google Scholar] [CrossRef]
- Marsh, K.; Dubrovskaya, Y.; Jen, S.P.; Ahmed, N.; Decano, A.; Siegfried, J.; Papadopoulos, J.; Merchan, C. Intravenous push versus intravenous piggyback beta-lactams for the empiric management of gram-negative bacteremia. J. Clin. Pharm. Ther. 2021, 46, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.C.; Smith, S.E.; Bland, C.M.; Newsome, A.S.; Branan, T.N.; Hawkins, W.A. Hidden Fluids in Plain Sight: Identifying Intravenous Medication Classes as Contributors to Intensive Care Unit Fluid Intake. Hosp. Pharm. 2022, 57, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; O’sullivan, D.; Krawczynski, M. Cefepime Intravenous Push Versus Intravenous Piggyback on Time to Administration of First-Dose Vancomycin in the Emergency Department. J. Pharm. Pract. 2018, 31, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Gregorowicz, A.J.; Costello, P.G.; Gajdosik, D.A.; Purakal, J.; Pettit, N.N.; Bastow, S.; Ward, M.A. Effect of IV Push Antibiotic Administration on Antibiotic Therapy Delays in Sepsis. Crit. Care Med. 2020, 48, 1175–1179. [Google Scholar] [CrossRef]
- Chakraborty, R.K.; Burns, B. Systemic Inflammatory Response Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547669/ (accessed on 1 July 2024).

| Characteristics | Intravenous Push (n = 50) | Extended Infusion (n = 50) | p-Value |
|---|---|---|---|
| Age (years), median (IQR) | 61 (49–73) | 66 (53–72) | 0.57 |
| Male sex, n (%) | 24 (48) | 32 (64) | 0.11 |
| Race, n (%) White Black Asian More than one Declined to answer/unknown | 36 (72) 11 (22) 0 (0) 1 (2) 2 (4) | 42 (84) 6 (12) 1 (2) 0 (0) 1 (2) | 0.39 |
| Hispanic or Latino, n (%) | 4 (8) | 8 (16) | 0.36 |
| Weight (kg), median (IQR) | 82 (67–96) | 82 (66–95) | 0.89 |
| Height (inches), median (IQR) | 67 (64–70) | 67 (65–71) | 0.49 |
| Body mass index (kg/m2), median (IQR) | 27 (22–34) | 26 (24–30) | 0.63 |
| Creatinine clearance (mL/min), median (IQR) | 62 (28–86) | 68 (32–95) | 0.46 |
|
Antibiotic therapy 3 months
prior to admission, n (%) | 14 (28) | 17 (34) | 0.52 |
|
Hospitalization 3 months
prior to admission, n (%) | 16 (32) | 18 (36) | 0.67 |
| Charlson Comorbidity Index, median (IQR) | 4 (1–5) | 4 (2–5) | 0.96 |
| Oxygen status, n (%) Room air Non-invasive oxygen Invasive oxygen | 5 (10) 24 (48) 21 (42) | 1 (2) 17 (34) 32 (64) | 0.06 |
| Vasopressor use, n (%) | 34 (68) | 30 (60) | 0.41 |
| Time from admission to first dose of meropenem (hours), median (IQR) | 23 (5–194) | 119 (20–372) | 0.003 |
| Febrile at meropenem start, n (%) | 22 (44) | 23 (46) | 0.84 |
| Abnormal WBC at meropenem start, n (%) | 45 (90) | 42 (84) | 0.37 |
| Elevated respiratory rate at meropenem start, n (%) | 40 (80) | 47 (94) | 0.04 |
| Elevated heart rate at meropenem start, n (%) | 42 (84) | 45 (90) | 0.37 |
| Meropenem dose, n (%) * 500 mg 1000 mg 2000 mg | 8 (16) 41 (82) 1 (2) | 2 (4) 45 (90) 3 (6) | 0.09 0.39 0.62 |
| Frequency, n (%) * Q6 h Q8 h Q12 h Q24 h | 2 (4) 28 (56) 19 (38) 1 (2) | 0 (0) 38 (76) 9 (18) 3 (6) | 0.49 0.03 0.03 0.62 |
| Appropriate renal adjustment, n (%) | 49 (98) | 48 (96) | 1.00 |
| Primary source of infection, n (%) Respiratory tract Gastrointestinal tract/intra-abdominal Genitourinary tract Bloodstream Skin and soft tissue Central nervous system Other | 21 (42) 12 (24) 5 (10) 4 (8) 5 (10) 1 (2) 2 (4) | 24 (48) 7 (14) 4 (8) 6 (12) 3 (6) 6 (12) 0 (0) | 0.26 |
| Outcome | IVP (n = 50) | EI (n = 50) | p-Value |
|---|---|---|---|
|
Achieved Clinical
Stabilization, n (%) | 24 (48) | 22 (44) | 0.84 |
|
Median Time to Clinical
Stabilization (hours), median (IQR) | 66.2 (14.4–177.3) | 20.4 (73.8–89.7) | 0.01 |
| Outcomes | IVP Group (n = 50) | EI Group (n = 50) | p-Value |
|---|---|---|---|
| Time to complete defervescence (hours), median (IQR) * | 26.14 (13.98–61.21) | 14.78 (9.41–35.17) | 0.29 |
| Time to WBC normalization (hours), median (IQR) ** | 127.25 (77.45–166.98) | 70.63 (33.94–136.98) | 0.08 |
| Hospital length of stay (days), median (IQR) *** | 16.92 (12.16–29.92) | 13.03 (6.22–20.13) | 0.05 |
| ICU length of stay (days), median (IQR) *** | 9.19 (4.67–15.71) | 6.07 (2.97–8.39) | 0.02 |
| Mortality, n (%) | 13 (26) | 19 (38) | 0.20 |
| Treatment failure, n (%) | 19 (38) | 25 (50) | 0.23 |
| Microbiological cure, n (%) | 12 (24) | 6 (12) | 0.14 |
| Recurrence of resistant isolates, n (%) | 3 (6) | 2 (4) | 1.00 |
| Isolates resistant to meropenem, n (%) | 1 (2) | 1 (2) | 1.00 |
| Characteristics | Intravenous Push (n = 50) | Extended Infusion (n = 50) | p-Value |
|---|---|---|---|
| No pathogen cultured, n (%) | 24 (48) | 15 (30) | 0.07 |
| Organism(s) cultured, n (%) Acinetobacter spp. Klebsiella spp. Pseudomonas spp. Escherichia coli Enterococcus spp. Staphylococcus spp. Streptococcus spp. Proteus spp. Candida Other | 0 (0) 1 (2) 3 (6) 1 (2) 3 (6) 8 (16) 2 (4) 1 (2) 4 (8) 6 (12) | 2 (4) 5 (10) 8 (16) 8 (16) 4 (8) 9 (18) 3 (6) 2 (4) 1 (2) 10 (20) | 0.50 0.20 0.20 0.03 1.00 1.00 1.00 1.00 0.37 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, E.G.; Maki Ortiz, K.; Adams, D.T.; Kaur, S.; Faust, A.C.; Yang, H.; Alvarez, C.A.; Hall, R.G., 2nd. A Retrospective Analysis of Intravenous Push versus Extended Infusion Meropenem in Critically Ill Patients. Antibiotics 2024, 13, 835. https://doi.org/10.3390/antibiotics13090835
Johnson EG, Maki Ortiz K, Adams DT, Kaur S, Faust AC, Yang H, Alvarez CA, Hall RG 2nd. A Retrospective Analysis of Intravenous Push versus Extended Infusion Meropenem in Critically Ill Patients. Antibiotics. 2024; 13(9):835. https://doi.org/10.3390/antibiotics13090835
Chicago/Turabian StyleJohnson, Emory G., Kayla Maki Ortiz, David T. Adams, Satwinder Kaur, Andrew C. Faust, Hui Yang, Carlos A. Alvarez, and Ronald G. Hall, 2nd. 2024. "A Retrospective Analysis of Intravenous Push versus Extended Infusion Meropenem in Critically Ill Patients" Antibiotics 13, no. 9: 835. https://doi.org/10.3390/antibiotics13090835
APA StyleJohnson, E. G., Maki Ortiz, K., Adams, D. T., Kaur, S., Faust, A. C., Yang, H., Alvarez, C. A., & Hall, R. G., 2nd. (2024). A Retrospective Analysis of Intravenous Push versus Extended Infusion Meropenem in Critically Ill Patients. Antibiotics, 13(9), 835. https://doi.org/10.3390/antibiotics13090835







