The Global Prevalence of Antibiotic Resistance and Shiga Toxin-Producing Escherichia coli in Chickens: A Systematic Review and Meta-Analysis (2011–2024)
Abstract
1. Introduction
2. Results
2.1. Study Selection and Identification
2.2. Study Characteristics
2.3. Results of Meta-Analysis of Overall Prevalence
2.4. Subgroup Analyses
2.4.1. Subgroup Analysis of Shiga Toxin-Producing E. coli
2.4.2. Antibiotic-Resistant STEC Subgroup Analysis
3. Discussion
4. Materials and Methods
4.1. The Design of the Study
4.2. Ethics
4.3. Review Question
4.4. Search Strategy
4.5. Inclusion and Exclusion Criteria
4.6. Data Extraction
4.7. Quality Assessment
4.8. Outcome
4.9. Data Processing and Analysis
4.10. Test for Publication Bias Due to Small-Study Effects
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momtaz, H.; Jamshidi, A. Shiga toxin-producing Escherichia coli isolated from chicken meat in Iran: Serogroups, virulence factors, and antimicrobial resistance properties. Poult. Sci. 2013, 92, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.S.; Carvalho, R.C.; Conte-Junior, C.A.; Figuiredo, E.E. Shiga-toxin producing Escherichia coli: Pathogenicity, supershedding, diagnostic methods, occurrence, and foodborne outbreaks. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1269–1280. [Google Scholar] [CrossRef]
- Pakbin, B.; Brück, W.M.; Rossen, J.W. Virulence factors of enteric pathogenic Escherichia coli: A review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Lee, W.; Ha, J.; Choi, J.; Jung, Y.; Kim, E.; An, E.S.; Kim, S.; Shin, H.; Ryu, S.; Kim, S.H.; et al. Genetic and virulence characteristics of hybrid Shiga toxin-producing and atypical enteropathogenic Escherichia coli strains isolated in South Korea. Front. Microbiol. 2024, 15, 1398262. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Sant’Ana, A.S. Pathogen subtyping tools for risk assessment and management of produce-borne outbreaks. Curr. Opin. Food Sci. 2020, 32, 83–89. [Google Scholar] [CrossRef]
- Nada, H.G.; El-Tahan, A.S.; El-Didamony, G.; Askora, A. Detection of multidrug-resistant Shiga toxin-producing Escherichia coli in some food products and cattle faeces in Al-Sharkia, Egypt: One health menace. BMC Microbiol. 2023, 23, 127. [Google Scholar] [CrossRef]
- Ramatla, T.; Mokgokong, P.; Lekota, K.; Thekisoe, O. Antimicrobial resistance profiles of Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae strains isolated from broiler chickens. Food Microbiol. 2024, 120, 104476. [Google Scholar] [CrossRef]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The diversity of Escherichia coli pathotypes and vaccination strategies against this versatile bacterial pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Ullah, F.; Irshad, H.; Ahmed, S.; Shakeela, Q.; Mian, A.H. Molecular detection of Shiga toxin-producing Escherichia coli (STEC) O157 in sheep, goats, cows and buffaloes. Mol. Biol. Rep. 2021, 48, 6113–6121. [Google Scholar] [CrossRef]
- Mukwevho, F.N.; Mbanga, J.; Bester, L.A.; Ismail, A.; Essack, S.Y.; Abia, A.L. Potential environmental transmission of antibiotic-resistant Escherichia coli and Enterococcus faecium harbouring multiple antibiotic resistance genes and mobile genetic elements in surface waters close to informal settlements: A tale of two cities. Sci. Total Environ. 2025, 976, 179321. [Google Scholar] [CrossRef]
- Mir, R.A.; Kudva, I.T. Antibiotic-resistant Shiga toxin-producing Escherichia coli: An overview of prevalence and intervention strategies. Zoonoses Public Health 2019, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ramatla, T.; Tutubala, M.; Motlhaping, T.; de Wet, L.; Mokgokong, P.; Thekisoe, O.; Lekota, K. Molecular detection of Shiga toxin and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolates from sheep and goats. Mol. Biol. Rep. 2024, 51, 57. [Google Scholar] [CrossRef] [PubMed]
- de Assis, D.C.; da Silva, T.M.; Brito, R.F.; da Silva, L.C.; Lima, W.G.; Brito, J.C. Shiga toxin-producing Escherichia coli (STEC) in bovine meat and meat products over the last 15 years in Brazil: A systematic review and meta-analysis. Meat Sci. 2021, 173, 108394. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Cheng, Y.; Ma, L.; Cai, Y.; Li, Y.; Liu, Y. A Systematic Review and Meta-Analysis of the Detection of Shiga Toxin–Producing Escherichia coli in Cattle in China in the Past 10 Years. Foodborne Pathog. Dis. 2024. [Google Scholar] [CrossRef]
- Kintz, E.; Brainard, J.; Hooper, L.; Hunter, P. Transmission pathways for sporadic Shiga-toxin producing E. coli infections: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2017, 220, 57–67. [Google Scholar] [CrossRef]
- Dewsbury, D.M.; Cernicchiaro, N.; Sanderson, M.W.; Dixon, A.L.; Ekong, P.S. A systematic review and meta-analysis of published literature on prevalence of non-O157 Shiga toxin-producing Escherichia coli serogroups (O26, O45, O103, O111, O121, and O145) and virulence genes in feces, hides, and carcasses of pre-and peri-harvest cattle worldwide. Anim. Health Res. Rev. 2022, 23, 1–24. [Google Scholar]
- Somda, N.S.; Adesoji, T.O.; Tetteh-Quarcoo, P.B.; Donkor, E.S. A Systematic Review and Meta-Analysis on the Presence of Escherichia coli O157: H7 in Africa from a One Health Perspective. Microorganisms 2025, 13, 902. [Google Scholar] [CrossRef]
- Rasheed, M.U.; Jamil, K.; Thajuddin, N.; Pasupuleti, M.; Ahamed, P.; Muthukumaresan, K.P. Distribution of the stx1, stx2 and hlyA genes: Antibiotic profiling in Shiga-toxigenic E. coli strains isolated from food sources. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 348–361. [Google Scholar]
- Rashid, M.; Kotwal, S.K.; Malik, M.A.; Singh, M. Prevalence, genetic profile of virulence determinants and multidrug resistance of Escherichia coli isolates from foods of animal origin. World 2013, 6, 139–142. [Google Scholar] [CrossRef]
- Runa, J.A.; Lijon, M.B.; Rahman, M.A. Detection of multidrug resistant and shiga toxin producing Escherichia coli (STEC) from apparently healthy broilers in Jessore, Bangladesh. Front. Environ. Microbiol. 2018, 4, 16–21. [Google Scholar] [CrossRef]
- Saikia, P.; Joshi, S.R. A study on the occurrence of non-O157 Shiga toxin producing Escherichia coli isolates in retail chicken meats marketed in North-East India. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 337–342. [Google Scholar] [CrossRef]
- Salehi, M. Determination of intimin and Shiga toxin genes in Escherichia coli isolates from gastrointestinal contents of healthy broiler chickens in Kerman City, Iran. Comp. Clin. Path. 2014, 23, 175–179. [Google Scholar] [CrossRef]
- Sarwar, A.; Aslam, B.; Rasool, M.H.; Bekhit, M.M.; Sasanya, J. A Health Threat from Farm to Fork: Shiga Toxin-Producing Escherichia coli Co-Harboring blaNDM-1 and mcr-1 in Various Sources of the Food Supply Chain. Pathogens 2024, 13, 659. [Google Scholar] [CrossRef]
- Selim, S.A.; Ahmed, S.F.; Aziz, M.H.; Zakaria, A.M.; Klena, J.D.; Pangallo, D. Prevalence and characterization of Shiga-toxin O157: H7 and non-O157: H7 enterohemorrhagic Escherichia coli isolated from different sources. Biotechnol. Biotechnol. Equip. 2013, 27, 3834–3842. [Google Scholar] [CrossRef]
- Shawish, R. Serotypes and virulence profiles of non-o157 shiga toxin producing E. coli isolated from beef, chicken meat and its products. Assiut Vet. Med. J. 2015, 61, 171–178. [Google Scholar]
- Shokoohizadeh, L.; Hossainpour, H.; Alikhani, M.Y. Prevalence of shiga toxin-producing Escherichia coli isolated from chicken meat in west of Iran. Res. Sq. 2019. preprint. [Google Scholar] [CrossRef]
- Sirikaew, S.; Sukkua, K.; Rattanachuay, P.; Khianngam, S.; Sukhumungoon, P. High level of shiga toxin-producing Escherichia coli and occurrence of STX-Negative E. Coli O157 from raw meats: Characterization of virulence profile and genetic relatedness. Southeast Asian. J. Trop. Med. Public Health 2016, 47, 1008–1009. [Google Scholar]
- Theyazan, A.Q.; Aboueisha, A.; Fadel, H.; Youssef, A. Zoonotic potential of Escherichia Coli in poultry intestinal contents in Ismailia city, Egypt with special reference to Shiga toxin-producing (STEC) strains. Suez Canal Vet. Med. J. 2021, 26, 219–241. [Google Scholar] [CrossRef]
- Treier, A.; Stephan, R.; Stevens, M.J.; Cernela, N.; Nüesch-Inderbinen, M. High occurrence of Shiga toxin-producing Escherichia coli in raw meat-based diets for companion animals—A public health issue. Microorganisms 2021, 9, 1556. [Google Scholar] [CrossRef]
- Trung, N.V.; Nhung, H.N.; Carrique-Mas, J.J.; Mai, H.H.; Tuyen, H.T.; Campbell, J.; Nhung, N.T.; Van Minh, P.; Wagenaar, J.A.; Mai, N.T.; et al. Colonization of Enteroaggregative Escherichia coli and Shiga toxin-producing Escherichia coli in chickens and humans in southern Vietnam. BMC Microb. 2016, 16, 208. [Google Scholar] [CrossRef]
- Vinayananda, C.O.; Fairoze, N.; Madhavaprasad, C.B.; Byregowda, S.M.; Nagaraj, C.S.; Bagalkot, P.; Karabasanavar, N. Studies on occurrence, characterisation and decontamination of emerging pathogenic Escherichia coli (STEC, ETEC and EIEC) in table eggs. Br. Poult. Sci. 2017, 58, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, G.; Gao, Y.; Xu, H.; Mohamed, L.; Zhao, J.; Gai, W.; Zou, M.; Cui, Z.; Yan, S.; et al. Virulence and Antimicrobial Characteristics of Escherichia Coli Isolated from Diseased Chickens in China and Algeria. J. Adv. Agric. Technol. 2019, 10, 1821–1833. [Google Scholar] [CrossRef]
- Zarei, O.; Shokoohizadeh, L.; Hossainpour, H.; Alikhani, M.Y. The Prevalence of Shiga Toxin-Producing Escherichia coli and Enteropathogenic Escherichia coli Isolated from Raw Chicken Meat Samples. Int. J. Microbiol. 2021, 2021, 3333240. [Google Scholar] [CrossRef] [PubMed]
- Abdelmonem, M.A.; Kelany, M.A.; Fawzy, M.; sheta, R.; Ageez, A.; Ismail, A.A.; El-Moez, S.I.A. Detection of Shiga-toxin producing E. coli in some retail markets in Egypt using Qpcr assay with special reference to serotyping. Adv. Environ. Biol. 2022, 16, 1–12. [Google Scholar] [CrossRef]
- Agusi, E.R.; Kabantiyok, D.; Mkpuma, N.; Atai, R.B.; Okongwu-Ejike, C.; Bakare, E.L.; Budaye, J.; Sule, K.G.; Rindaps, R.J.; James, G.K.; et al. Prevalence of multidrug-resistant Escherichia coli isolates and virulence gene expression in poultry farms in Jos, Nigeria. Front. Microbiol. 2024, 15, 1298582. [Google Scholar] [CrossRef] [PubMed]
- Amir, M.; Riaz, M.; Chang, Y.F.; Ismail, A.; Hameed, A.; Ahsin, M. Antibiotic resistance in diarrheagenic Escherichia coli isolated from broiler chickens in Pakistan. J. Food Qual. Hazards Control 2021, 8, 78–86. [Google Scholar] [CrossRef]
- Bagheri, M.; Ghanbarpour, R.; Alizade, H. Shiga toxin and beta-lactamases genes in Escherichia coli phylotypes isolated from carcasses of broiler chickens slaughtered in Iran. Int. J. Food Microbiol. 2014, 177, 16–20. [Google Scholar] [CrossRef]
- Bai, X.; Wang, H.; Xin, Y.; Wei, R.; Tang, X.; Zhao, A.; Sun, H.; Zhang, W.; Wang, Y.; Xu, Y.; et al. Prevalence and characteristics of Shiga toxin-producing Escherichia coli isolated from retail raw meats in China. Int. J. Food Microbiol. 2015, 200, 31–38. [Google Scholar] [CrossRef]
- Benameur, Q.; Gervasi, T.; Giarratana, F.; Vitale, M.; Anzà, D.; La Camera, E.; Nostro, A.; Cicero, N.; Marino, A. Virulence, antimicrobial resistance and biofilm production of Escherichia coli isolates from healthy broiler chickens in western algeria. Antibiotics 2021, 10, 1157. [Google Scholar] [CrossRef]
- Bonyadian, M.; Haidari, F.I.; Sami, M. Virulence genes and pulsed-field gel electrophoresis profiles of Shiga toxin-producing Escherichia coli isolated from different food samples and patients with acute diarrhea. Iran. J. Microbiol. 2024, 16, 329. [Google Scholar] [CrossRef]
- Chen, F.C.; Godwin, S.; Green, A.; Chowdhury, S.; Stone, R. Prevalence of Salmonella, Campylobacter, and Shiga Toxin–Producing Escherichia coli on the surfaces of raw poultry packages. J. Food Prot. 2018, 81, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Koo, M.S.; Jang, H.J. Characterization of diarrheagenic Escherichia coli isolated from fresh beef, pork, and chicken meat in korean markets. Microbiol. Biotechnol. Lett. 2020, 48, 121–128. [Google Scholar] [CrossRef]
- Daoud, J.R.; Mohamed, K.; Nasef, S.A.; Ahmed, R.Y. Detection of Shiga toxin produced by Escherichia coli in poultry and meat in Luxor city using multiplex PCR. Benha Vet. Med. J. 2016, 31, 40–44. [Google Scholar] [CrossRef]
- Darwish, W.S.; Abd El-Moaty, A.M.; Reda, L.M.; Mohamed, T. Evaluation of the sanitary status and prevalence of shiga-toxin producing E. coli in chicken meat products with a reduction trial using organic acids. Benha Vet. Med. J. 2017, 32, 239–247. [Google Scholar]
- Dishan, A.D.; Hizlisoy, H.A.; Barel, M.U.; Disli, H.B.; Gungor, C.; Ertas Onmaz, N.; Gonulalan, Z.; Al, S.E.; Yildirim, Y.E. Biofilm formation, antibiotic resistance and genotyping of Shiga toxin-producing Escherichia coli isolated from retail chicken meats. Br. Poult. Sci. 2023, 64, 63–73. [Google Scholar] [CrossRef]
- Doregiraee, F.; Alebouyeh, M.; Fasaei, B.N.; Charkhkar, S.; Tajedin, E.; Zali, M.R. Isolation of atypical enteropathogenic and shiga toxin encoding Escherichia coli strains from poultry in Tehran, Iran. Gastroenterol. Hepatol. Bed Bench 2016, 9, 53. [Google Scholar] [PubMed]
- Duc, H.M.; Ha, C.T.; Hoa, T.T.; Hung, L.V.; Thang, N.V.; Son, H.M. Prevalence, molecular characterization, and antimicrobial resistance profiles of Shiga toxin-producing Escherichia coli isolated from raw beef, pork, and chicken meat in Vietnam. Foods 2024, 13, 2059. [Google Scholar] [CrossRef]
- Dutta, T.K.; Roychoudhury, P.; Bandyopadhyay, S.; Wani, S.A.; Hussain, I. Detection & characterization of Shiga toxin producing Escherichia coli (STEC) & enteropathogenic Escherichia coli (EPEC) in poultry birds with diarrhoea. Indian J. Med. Res. 2011, 133, 541–545. [Google Scholar]
- Eid, S.A.; Nasef, S.O.M.; Erfan, A.H. Multidrug resistant bacterial pathogens in eggs collected from backyard chickens. Assiut Vet. Med. J. 2015, 61, 87–103. [Google Scholar]
- El-Ashmony, A.L.; Mostafa, A.E.; Tarabees, R. Molecular Characterization of Virulence and Antimicrobial Resistance Genes of E. coli Isolated from Different Animal Sources. Alex. J. Vet. Sci. 2022, 74, 6. [Google Scholar]
- Elsayed, M.S.; Eldsouky, S.M.; Roshdy, T.; Bayoume, A.M.; Nasr, G.M.; Salama, A.S.; Akl, B.A.; Hasan, A.S.; Shahat, A.K.; Khashaba, R.A.; et al. Genetic and antimicrobial resistance profiles of non-O157 Shiga toxin-producing Escherichia coli from different sources in Egypt. BMC Microb. 2021, 21, 257. [Google Scholar] [CrossRef] [PubMed]
- Elsyaed, M.S.; Mounir, M. Virulence factors and antimicrobial resistance patterns of non-o157 Shiga toxin-producing Escherichia coli isolated from different sources at Sadat city. Microbiol. Res. J. Int. 2020, 30, 64–73. [Google Scholar] [CrossRef]
- Gharieb, N.M.; Twad, A.E.; Ashraf, A.; El Oksh, A.S. Prevalence of multidrug resistant shiga toxin-producing Escherichia coli in broiler. Benha Med. J. 2023, 44, 64–69. [Google Scholar] [CrossRef]
- Gökmen, M.; İlhan, Z.; Tavşanlı, H.; Önen, A.; Ektik, N.; Göçmez, E.B. Prevalence and molecular characterization of shiga toxin-producing Escherichia coli in animal source foods and green leafy vegetables. Food Sci. Technol. Int. 2024, 30, 30–36. [Google Scholar] [CrossRef]
- Hasona, I.F.; Helmy, S.M.; El Gamal, A.M. Prevalence, virulence factors, and antimicrobial resistance profiles of Shiga toxin-producing Escherichia coli isolated from broiler chickens in Egypt. Vet. Res. Forum 2023, 14, 131. [Google Scholar] [PubMed]
- Himi, H.A.; Parvej, M.S.; Rahman, M.B.; Nasiruddin, K.M.; Ansari, W.K.; Ahamed, M.M. PCR based detection of shiga toxin producing E. coli in commercial poultry and related environments. Turk. J. Agric. Food Sci. Technol. 2015, 3, 361–364. [Google Scholar] [CrossRef][Green Version]
- Kalwaniya, M.K.; Gaurav, A.; Kumar, H.; Choudhary, D.; Kumari, A. Molecular characterization of Escherichia coli isolated from meat and meat products. Pharma Innov. J. 2020, 9, 452–456. [Google Scholar]
- Kaushik, P.; Anjay, A.; Kumari, S.; Dayal, S.; Kumar, S. Antimicrobial resistance and molecular characterisation of E. coli from poultry in Eastern India. Vet. Ital. 2018, 54, 197–204. [Google Scholar]
- Khan, J.A.; Rathore, R.S.; Abulreesh, H.H.; Al-thubiani, A.S.; Khan, S.; Ahmad, I. Diversity of antibiotic-resistant Shiga toxin-producing Escherichia coli serogroups in foodstuffs of animal origin in northern India. J. Food Saf. 2018, 38, e12566. [Google Scholar] [CrossRef]
- Kholdi, S.; Motamedifar, M.; Fani, F.; Mohebi, S.; Bazargani, A. Virulence factors, serogroups, and antibiotic resistance of Shiga-toxin producing Escherichia coli from raw beef, chicken meat, and vegetables in Southwest Iran. Iran. J. Vet. Res. 2021, 22, 180. [Google Scholar]
- Li, M.C.; Wang, F.; Li, F. Identification and molecular characterization of antimicrobial-resistant shiga toxin–producing Escherichia coli isolated from retail meat products. Foodborne Pathog. Dis. 2011, 8, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tan, X.; Xiao, J.; Wang, H.; Liu, Z.; Zhou, M.; Bi, W.; Miyamoto, T. Molecular screening and characterization of Shiga toxin-producing Escherichia coli in retail foods. Food Control 2016, 60, 180–188. [Google Scholar] [CrossRef]
- Lopes, H.P.; Alves, L.C.; Costa, G.A.; Dias, T.S.; Machado, L.S.; Cunha, N.C.; Pereira, V.L.; Abreu, D.L. Detection and Antimicrobial Resistance Profile of Enteropathogenic (EPEC) and Shigatoxigenic Escherichia coli (STEC) in Conventional and Organic Broiler Chickens. Braz. J. Poult. Sci. 2023, 25, eRBCA-2022. [Google Scholar] [CrossRef]
- Madoroba, E.; Malokotsa, K.P.; Ngwane, C.; Lebelo, S.; Magwedere, K. Presence and Virulence Characteristics of Shiga Toxin Escherichia coli and Non-Shiga Toxin–Producing Escherichia coli O157 in Products from Animal Protein Supply Chain Enterprises in South Africa. Foodborne Pathog. Dis. 2022, 19, 386–393. [Google Scholar] [CrossRef]
- Mokhtar, A.; Karmi, M. Surveillance of food poisoning Escherichia coli (STEC) in ready-to-eat meat products in Aswan, Egypt. In Proceedings of the 9th International Conference of Veterinary Research Division National Research Centre, Giza, Egypt, 27–29 September 2021; Volume 52, pp. 41–50. [Google Scholar]
- Morshdy, A.E.; Hussein, M.A.; Tharwat, A.E.; Moustafa, N.A.; Hussein, O.K. Prevalence of shiga toxigenic and multi drug resistant Escherichia coli in ready to eat chicken products’sandwiches. Slov. Vet. Res. 2018, 55, 349. [Google Scholar]
- Mousavi, R.; Rahimi, E.; Shakerian, A. Incidence and profiles of antibiotic resistance and virulence markers of the Escherichia coli O157 bacteria recovered from poultry meat. Egypt. J. Vet. Sci. 2020, 51, 215–223. [Google Scholar] [CrossRef]
- Naidu, S.T.; Bodempudi, B.; Kiranmayi, B.C.; Pedada, V.C.; Nelapati, S.; Tumati, S.R.; Gottapu, C.; Talluri, H.L.; Puvvada, S.; Chekuri, N.; et al. Prevalence of β-lactamase Producing Shiga Toxigenic E. coli (STEC) in Retail Meats and Chicken Cloacal Swabs. J. Anim. Res. 2021, 11, 263–271. [Google Scholar] [CrossRef]
- Nasef, S.E.L.; Oksh, A.M.; Ibrahim, G. Studies on Enterohaemorrhagic Escherichia coli (EHCE) strains Non O157: H7 in chicken with regard to antibiotic resistance gene on plasmid. Assiut Vet. Med. J. 2017, 63, 157–165. [Google Scholar]
- Oluyege, A.O.; Famurewa, O. Shiga toxin and non-shiga toxin-producing Escherichia coli O157 from cattle, goats and chicken in Ado-Ekiti, South West, Nigeria. Int. J. Trop. Dis. Health 2015, 6, 108–118. [Google Scholar] [CrossRef]
- Ornellas, R.P.; Lopes, H.P.; de Queiroz Baptista, D.; Dias, T.S.; de Almeida Figueira, A.; Costa, G.A.; dos Santos Machado, L.; da Cunha, N.C.; de Almeida Pereira, V.L.; da Costa Abreu, D.L. Multidrug resistance in Shiga toxin-producing Escherichia coli (STEC) isolated from broiler chickens at slaughter. Semin. Ciências Agrárias 2021, 42, 3813–3824. [Google Scholar] [CrossRef]
- Panahee, M.; Pourtaghi, H. Virulence gene profiles of Shiga-toxin producing Escherichia coli isolates from retail raw meat in Iran. Bulg. J. Vet. Med. 2016, 1311–1477. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, J.W.; Heo, E.J.; Ko, E.K.; Kim, K.Y.; Kim, Y.J.; Yoon, H.J.; Wee, S.H.; Park, Y.H.; San Moon, J. Antibiotic resistance and virulence potentials of Shiga toxin-producing Escherichia coli isolates from raw meats of slaughterhouses and retail markets in Korea. J. Microbiol. Biotechnol. 2015, 25, 1460–1466. [Google Scholar] [CrossRef]
- Pewleang, T.; Rattanachuay, P.; Themphachana, M.; Sukhumungoon, P. Quantification of enterohemorrhagic and Shiga toxinproducing Escherichia coli from retailed meats. Glob. Vet. 2014, 12, 244–249. [Google Scholar]
- Swetha, C.S.; Kannan, P.; Elango, A.; Ronald, B.S.; Senthil, K.T.; Amal, R.T. Characterization of E. coli isolates from meat samples for shiga toxin producing virulence markers. Biomed Res. Int. 2014, 2014, 795104. [Google Scholar] [CrossRef]
- Tayh, G.; Nsibi, F.; Chemli, K.; Daâloul-Jedidi, M.; Abbes, O.; Messadi, L. Occurrence, antibiotic resistance and molecular characterisation of Shiga toxin-producing Escherichia coli isolated from broiler chickens in Tunisia. Br. Poult. Sci. 2024, 65, 751–761. [Google Scholar] [CrossRef]
- Alizade, H.; Teshnizi, S.H.; Azad, M.; Shojae, S.; Gouklani, H.; Davoodian, P.; Ghanbarpour, R. An overview of diarrheagenic Escherichia coli in Iran: A systematic review and meta-analysis. J. Res. Med. Sci. 2019, 24, 23. [Google Scholar] [PubMed]
- O’Loughlin, E.V.; Robins-Browne, R.M. Effect of Shiga toxin and Shiga-like toxins on eukaryotic cells. Microbes Infect. 2001, 3, 493–507. [Google Scholar] [CrossRef]
- Swelum, A.A.; Elbestawy, A.R.; El-Saadony, M.T.; Hussein, E.O.; Alhotan, R.; Suliman, G.M.; Taha, A.E.; Ba-Awadh, H.; El-Tarabily, K.A.; Abd El-Hack, M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: An updated overview. Poult. Sci. 2021, 100, 101039. [Google Scholar] [CrossRef]
- Yousef, H.M.; Hashad, M.E.; Osman, K.M.; Alatfeehy, N.M.; Hassan, W.M.; Elebeedy, L.A.; Salem, H.M.; Shami, A.; Al-Saeed, F.A.; El-Saadony, M.T.; et al. Surveillance of Escherichia coli in different types of chicken and duck hatcheries: One health outlook. Poult. Sci. 2023, 102, 103108. [Google Scholar] [CrossRef]
- Assefa, A.; Bihon, A. A systematic review and meta-analysis of prevalence of Escherichia coli in foods of animal origin in Ethiopia. Heliyon 2018, 4, e00716. [Google Scholar] [CrossRef]
- Ramatla, T.; Ramaili, T.; Lekota, K.E.; Ndou, R.; Mphuti, N.; Bezuidenhout, C.; Thekisoe, O. A systematic review and meta-analysis on prevalence and antimicrobial resistance profile of Escherichia coli isolated from water in africa (2000–2021). Heliyon 2023, 9, e16123. [Google Scholar] [CrossRef]
- Monyama, M.C.; Onyiche, E.T.; Taioe, M.O.; Nkhebenyane, J.S.; Thekisoe, O.M. Bacterial pathogens identified from houseflies in different human and animal settings: A systematic review and meta-analysis. Vet. Med. Sci. 2022, 8, 827–844. [Google Scholar] [CrossRef]
- Kalule, J.B.; Keddy, K.H.; Smith, A.; Nicol, M.P.; Robberts, L. Development of a real-time PCR assay and comparison to CHROMagarTM STEC to screen for Shiga toxin-producing Escherichia coli in stool, Cape Town, South Africa. Afr. J. Lab. Med. 2017, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jafari, Z.; Motamedi, M.; Jalalizand, N.; Shokoohi, G.R.; Charsizadeh, A.; Mirhendi, H. Comparison of CHROMagar, polymerase chain reaction-restriction fragment length polymorphism, and polymerase chain reaction-fragment size for the identification of Candida species. Curr. Med. Mycol. 2017, 3, 10. [Google Scholar]
- Bording-Jorgensen, M.; Parsons, B.; Szelewicki, J.; Lloyd, C.; Chui, L. Molecular detection of non-O157 shiga toxin-producing Escherichia coli (STEC) directly from stool using multiplex qPCR assays. Microorganisms 2022, 10, 329. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.T.; Sowers, E.G.; Wells, J.G.; Greene, K.D.; Griffin, P.M.; Hoekstra, R.M.; Strockbine, N.A. Non-O157 Shiga toxin–producing Escherichia coli infections in the United States, 1983–2002. J. Infect. Dis. 2005, 192, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Tarr, G.A.; Lin, C.Y.; Lorenzetti, D.; Chui, L.; Tarr, P.I.; Hartling, L.; Vandermeer, B.; Freedman, S.B. Performance of commercial tests for molecular detection of Shiga toxin-producing Escherichia coli (STEC): A systematic review and meta-analysis protocol. BMJ Open 2019, 9, e025950. [Google Scholar] [CrossRef]
- Ibrahim, R.A.; Cryer, T.L.; Lafi, S.Q.; Basha, E.A.; Good, L.; Tarazi, Y.H. Identification of Escherichia coli from broiler chickens in Jordan, their antimicrobial resistance, gene characterization and the associated risk factors. BMC Vet. Res. 2019, 15, 159. [Google Scholar] [CrossRef]
- Paintsil, E.K.; Ofori, L.A.; Adobea, S.; Akenten, C.W.; Phillips, R.O.; Maiga-Ascofare, O.; Lamshoeft, M.; May, J.; Obiri Danso, K.; Krumkamp, R.; et al. Prevalence and antibiotic resistance in Campylobacter spp., isolated from humans and food-producing animals in West Africa: A systematic review and meta-analysis. Pathogens 2022, 11, 140. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Teng, Y.; Ou, L.; Xi, Y.; Chen, S.; Duan, G. The resistance mechanism of Escherichia coli induced by ampicillin in laboratory. Infect. Drug Resist. 2019, 12, 2853–2863. [Google Scholar] [CrossRef]
- García-Béjar, B.; García de Blas Martín, I.; Arévalo-Villena, M.; Briones Pérez, A. High prevalence of antibiotic-resistant Escherichia coli isolates from retail poultry products in Spain. Animals 2021, 11, 3197. [Google Scholar] [CrossRef] [PubMed]
- Fatoba, D.O.; Amoako, D.G.; Abia, A.L.; Essack, S.Y. Transmission of antibiotic-resistant Escherichia coli from chicken litter to agricultural soil. Front. Environ. Sci. 2022, 9, 751732. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Grande, H.; Weaver, B.; Papp, K.; Horwinski, J.; Koch, B.; Hungate, B.A.; Liu, C.M.; et al. Antibiotic-resistant Escherichia coli from retail poultry meat with different antibiotic use claims. BMC Microb. 2018, 18, 174. [Google Scholar] [CrossRef] [PubMed]
- Buvens, G.; Bogaerts, P.; Glupczynski, Y.; Lauwers, S.; Piérard, D. Antimicrobial resistance testing of verocytotoxin-producing Escherichia coli and first description of TEM-52 extended-spectrum β-lactamase in serogroup O26. Antimicrob. Agents Chemother. 2010, 54, 4907–4909. [Google Scholar] [CrossRef]
- Rahman, A.; Rahman Chowdhury, M.S.; Hossain, H.; Elsaid, F.G.; Almutairi, L.A.; Begum, R.; Sabrin, M.S.; Akanda, M.R.; Hossain, M.M.; Islam, M.R.; et al. Identification of Virulence Genes and Multidrug Resistance in Shiga-Toxin Producing Escherichia coli (STEC) from Migratory and Captive Wild Birds. Pak. Vet. J. 2024, 44, 1120–1130. [Google Scholar]
- Melton-Celsa, A.R.; O’Brien, A.D. New therapeutic developments against Shiga toxin-producing Escherichia coli. In Enterohemorrhagic Escherichia coli and Other Shiga Toxin-Producing, E. coli; American Society for Microbiology: Washington, DC, USA, 2015; pp. 341–358. [Google Scholar]
- Hunt, J.M. Shiga toxin–producing Escherichia coli (STEC). Clin. Lab. Med. 2010, 30, 21–45. [Google Scholar] [CrossRef]
- Ramatla, T.A.; Mphuthi, N.; Ramaili, T.; Taioe, M.O.; Thekisoe, O.M.; Syakalima, M. Molecular detection of virulence genes in Salmonella spp. isolated from chicken faeces in Mafikeng, South Africa. J. S. Afr. Vet. Assoc. 2020, 91, 1–7. [Google Scholar] [CrossRef]
- Abreu, R.; Semedo-Lemsaddek, T.; Cunha, E.; Tavares, L.; Oliveira, M. Antimicrobial drug resistance in poultry production: Current status and innovative strategies for bacterial control. Microorganisms 2023, 11, 953. [Google Scholar] [CrossRef]
- Al Sattar, A.; Chisty, N.N.; Irin, N.; Uddin, M.H.; Hasib, F.Y.; Hoque, M.A. Knowledge and practice of antimicrobial usage and resistance among poultry farmers: A systematic review, meta-analysis, and meta-regression. Vet. Res. Commun. 2023, 47, 1047–1066. [Google Scholar] [CrossRef]
- Nkhebenyane, S.J.; Khasapane, N.G.; Lekota, K.E.; Thekisoe, O.; Ramatla, T. Insight into the Prevalence of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Vegetables: A Systematic Review and Meta-Analysis. Foods 2024, 13, 3961. [Google Scholar] [CrossRef]
- Khasapane, N.G.; Nkhebenyane, S.J.; Lekota, K.; Thekisoe, O.; Ramatla, T. “One Health” Perspective on Prevalence of ESKAPE Pathogens in Africa: A systematic review and meta-analysis. Pathogens 2024, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.; Trikalinos, T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: A large survey. CMAJ 2007, 176, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Leone, C.; Xu, X.; Mishra, A.; Thippareddi, H.; Singh, M. Interventions to reduce Salmonella and Campylobacter during chilling and post-chilling stages of poultry processing: A systematic review and meta-analysis. Poult. Sci. 2024, 103, 103492. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vaddu, S.; Bhumanapalli, S.; Mishra, A.; Applegate, T.; Singh, M.; Thippareddi, H. A systematic review and meta-analysis of the sources of Salmonella in poultry production (pre-harvest) and their relative contributions to the microbial risk of poultry meat. Poult. Sci. 2023, 102, 102566. [Google Scholar] [CrossRef]
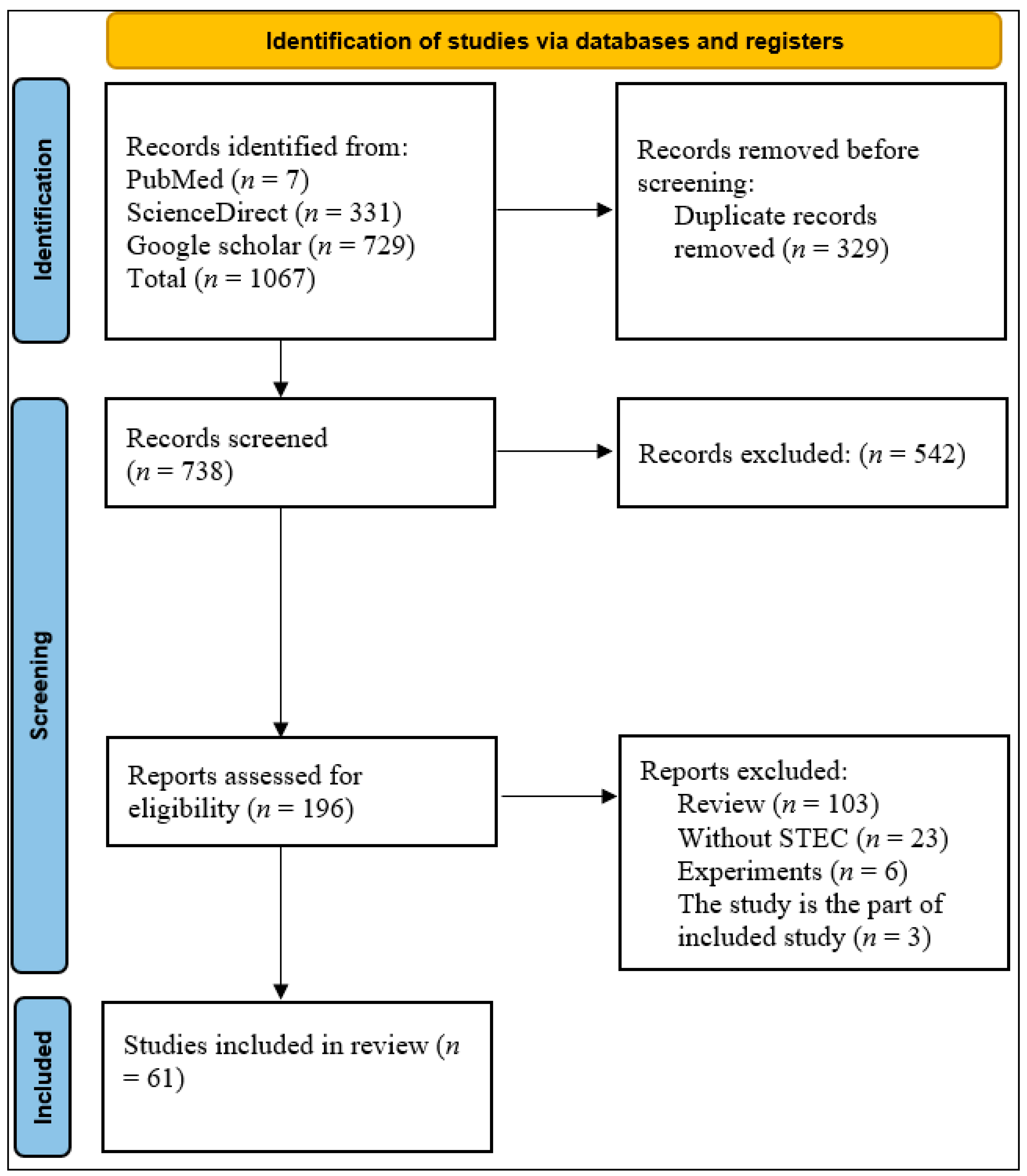
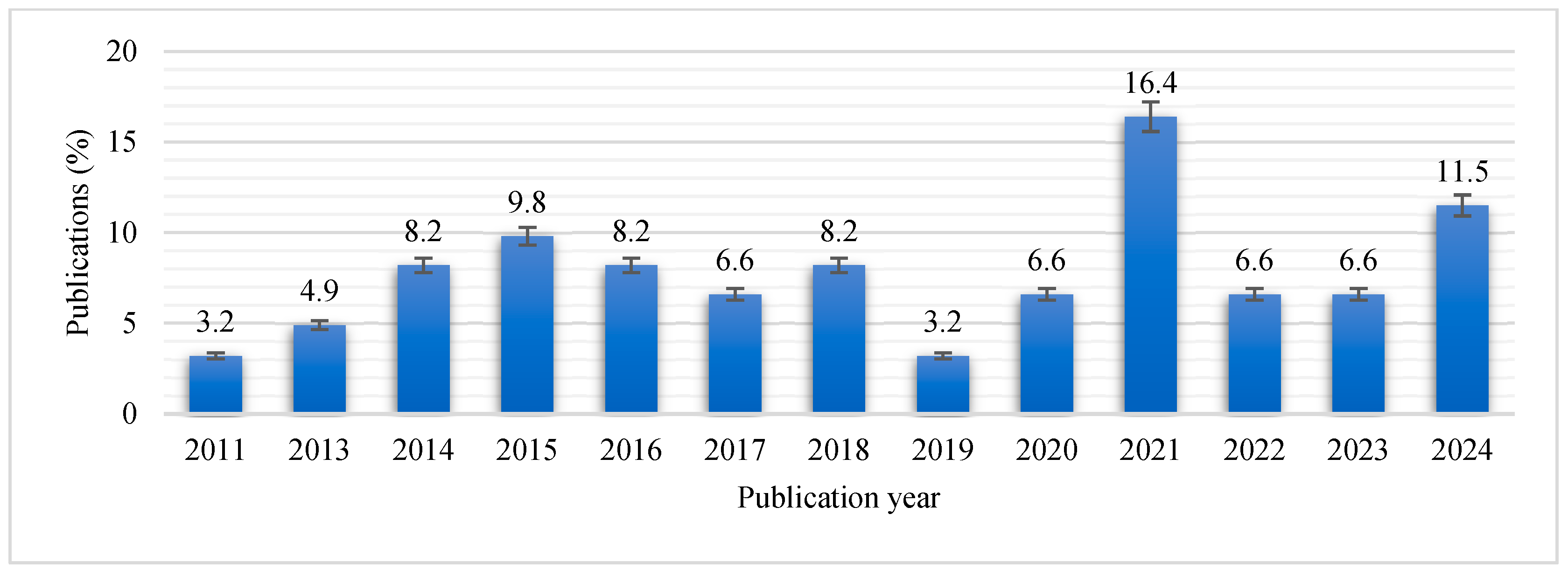
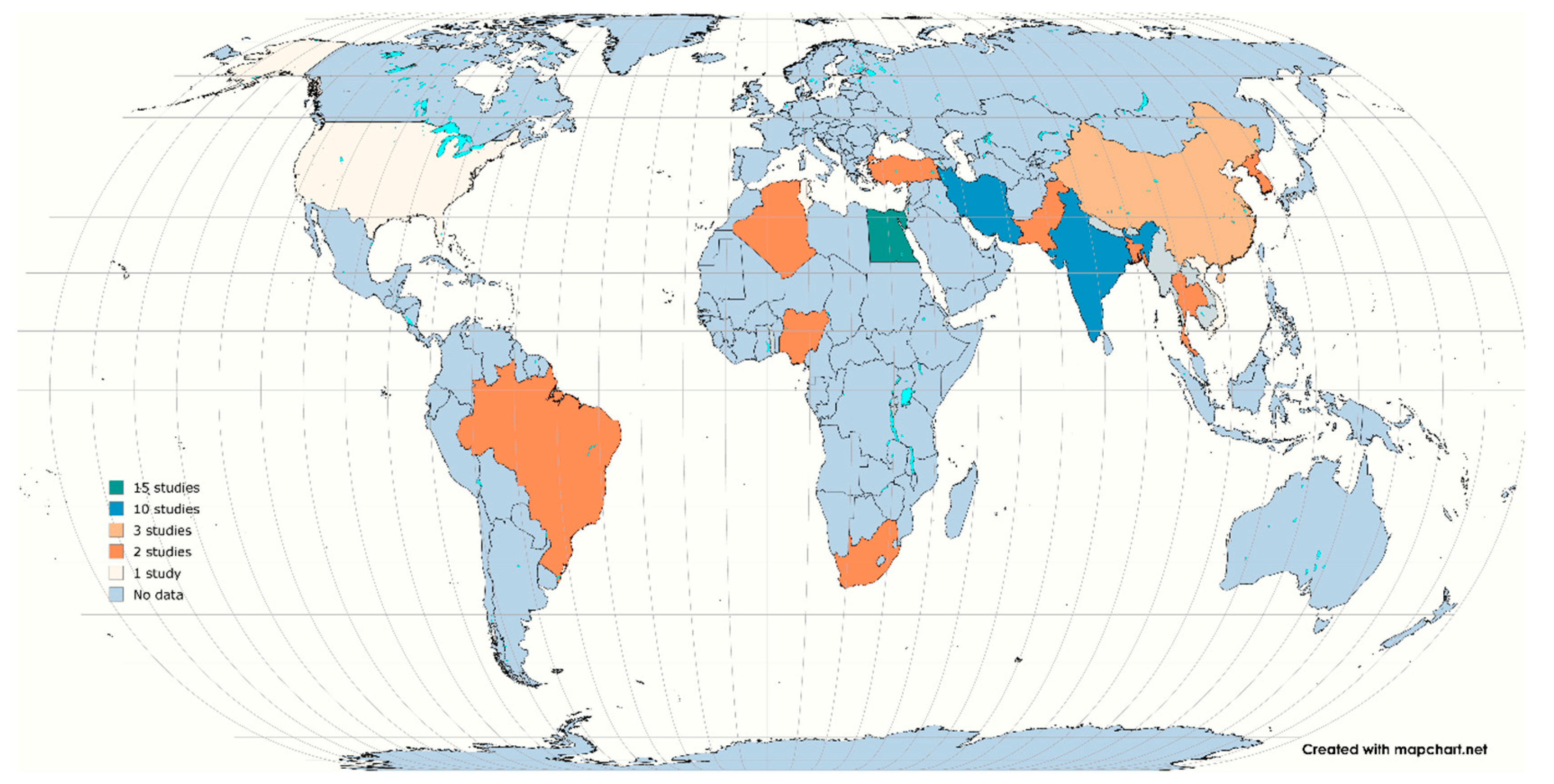

| No. | Citation | Country | Samples | Diagnostic Methods | Antibiotic Methods | Total No. | No. of Isolates | stx1 | stx2 | STEC |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Rasheed et al. [18] | India | Meat and eggs | Culture media and PCR | − | 90 | 45 | 13 | 11 | 13 |
| 2 | Rashid et al. [19] | India | Meat | Culture media, serology, and PCR | − | 50 | 20 | 9 | 5 | 15 |
| 3 | Runa et al. [20] | Bangladesh | Cloacal swabs | Culture media, serology, and PCR | − | 8 | 5 | − | − | 5 |
| 4 | Saiki and Joshi, [21] | India | Meat | Culture media and PCR | − | 336 | 22 | 22 | 0 | 22 |
| 5 | Salehi [22] | Iran | GIT content | Culture media and PCR | − | 145 | 290 | 0 | 3 | − |
| 6 | Sarwar et al. [23] | Pakistan | Multiple | MALDI-TOF, VAGs, and PCR | DD | 75 | 29 | 6 | 7 | 9 |
| 7 | Selim et al. [24] | Egypt | Meat | Culture media and PCR | − | 14 | 3 | 1 | − | − |
| 8 | Shawish [25] | Egypt | Meat | Culture media, serology, and PCR | − | 150 | 57 | 14 | 14 | 56 |
| 9 | Shokoohizadeh et al. [26] | Iran | Meat | Culture media, serology, and PCR | DD | 257 | 93 | − | − | 31 |
| 10 | Sirikaew et al. [27] | Thailand | Meat | Culture media, serology, and PCR | − | 12 | 2 | − | − | 2 |
| 11 | Theyazan et al. [28] | Egypt | Intestines | CHROMagar STEC | − | 200 | 158 | 8 | 25 | 144 |
| 12 | Treier et al. [29] | Switzerland and Germany | Meat | Brolacin STEC agar or CHROMagar | − | 7 | 3 | 1 | 1 | 1 |
| 13 | Trung et al. [30] | Vietnam | Faeces | Culture media and PCR | − | 188 | 1 | 1 | − | − |
| 14 | Vinayananda et al. [31] | India | Eggs | Culture media, serology, and PCR | − | 840 | 239 | − | − | 9 |
| 15 | Wang et al. [32] | Algeria | Meat | Culture media, serology, and PCR | − | 248 | 141 | − | 3 | − |
| 16 | Zarei et al. [33] | Iran | Meat | Culture media, serology, and PCR | DD | 257 | 93 | 15 | 31 | 36 |
| 17 | Abdelmonem et al. [34] | Egypt | Meat | PCR | − | 20 | 1 | − | 1 | 1 |
| 18 | Agusi et al. [35] | Nigeria | Cloacal swabs | PCR | − | 179 | 178 | 5 | ||
| 19 | Amir et al. [36] | Pakistan | Faeces and meat | PCR | DD | 400 | 19 | 18 | 21 | |
| 20 | Bagheri et al. [37] | Iran | Carcasses | PCR | − | 102 | 204 | 1 | ||
| 21 | Bai et al. [38] | China | Meat | CHROMagar STEC | − | 205 | − | − | − | 6 |
| 22 | Benameur et al. [39] | Algeria | Faeces | MALDI-TOF-MS | DD | 32 | − | − | 1 | − |
| 23 | Bonyadian et al. [40] | Iran | Meat | Not specified | − | 100 | 84 | − | 1 | 4 |
| 24 | Chen et al. [41] | USA | Meat | PCR | − | 105 | 25 | − | − | 6 |
| 25 | Cho et al. [42] | Korea | Meat | CHROMagar STEC | − | 133 | 79 | 1 | 1 | 2 |
| 26 | Daoud et al. [43] | Luxor city | Meat | PCR | − | 50 | 6 | 2 | 5 | |
| 27 | Darwish et al. [44] | Egypt | Meat | PCR | − | 40 | 2 | 2 | ||
| 28 | Dishan et al. [45] | Türkiye | Meat | PCR | DD | 100 | 77 | 24 | 23 | 35 |
| 29 | Doregiraee et al. [46] | Iran | Cloacal swabs | PCR | − | 500 | 444 | − | 2 | − |
| 30 | Duc et al. [47] | Vietnam | Meat | PCR | − | 72 | 7 | − | − | 2 |
| 31 | Dutta et al. [48] | India | Faeces | m-PCR | − | 19 | 42 | 8 | 6 | 10 |
| 32 | Eid et al. [49] | Egypt. | Eggs | PCR | − | 200 | 36 | − | 4 | |
| 33 | El-Ashmony et al. [50] | Egypt | Meat | VITEK2/PCR | − | − | 30 | 2 | 3 | 4 |
| 34 | Elsayed et al. [51] | Egypt | Faecal swabs | PCR | − | 15 | 7 | 6 | 6 | 7 |
| 35 | Elsyaed et al. [52] | Egypt | Faeces | PCR | − | 20 | 17 | 17 | 15 | − |
| 36 | Gharieb et al. [53] | Egypt | Visceral organs | PCR | − | 200 | 110 | 1 | − | − |
| 37 | Gökmen et al. [54] | Turkey | Meat | m-PCR | − | 31 | − | 3 | − | − |
| 38 | Hasona et al. [55] | Egypt | Cloacal swabs and internal organs | PCR | − | 410 | 29 | 5 | 5 | 18 |
| 39 | Himi et al. [56] | Bangladesh | Cloacal swabs and eggs | PCR | − | 120 | 66 | − | 66 | 66 |
| 40 | Kalwaniya et al. [57] | India | Meat | PCR | − | 30 | − | 1 | − | − |
| 41 | Kaushik et al. [58] | India | Meat and eggs | m-PCR | − | 252 | 62 | − | 13 | − |
| 42 | Khan et al. [59] | India | Meat | PCR | DD | 200 | 34 | − | − | 8 |
| 43 | Kholdi et al. [60] | Iran | Meat | PCR | − | 100 | − | − | − | 8 |
| 44 | Li et al. [61] | China | Meat | PCR | − | 50 | − | − | − | 2 |
| 45 | Li et al. [62] | China | Meat | PCR | − | 52 | 1 | − | 2 | |
| 46 | Lopes et al. [63] | Brazil | Cloacae and carcases | PCR | DD | 213 | − | − | − | 35 |
| 47 | Madoroba et al. [64] | South Africa | Meat | PCR | − | 1758 | − | − | − | 4 |
| 48 | Mokhtar and Karmi [65] | Egypt | Meat | PCR | − | 30 | − | − | − | 1 |
| 49 | Momtaz and Jamshidi [1] | Iran | Meat | PCR | DD | 422 | 146 | 80 | 5 | 82 |
| 50 | Morshdy et al. [66] | Egypt | Chicken sandwiches | m-PCR | − | 250 | 73 | 5 | 2 | − |
| 51 | Mousavi et al. [67] | Iran | Meat | PCR | − | 100 | − | 12 | 4 | − |
| 52 | Naidu et al. [68] | India | Multiple | PCR | − | 65 | 13 | 3 | − | |
| 53 | Nasef et al. [69] | Egypt | Multiple | PCR | − | 50 | 20 | 9 | 13 | − |
| 54 | Oluyege and Famurewa [70] | Nigeria | Faeces | PCR | − | 293 | 87 | 1 | − | − |
| 55 | Ornellas et al. [71] | Brazil | Carcasses and cloacae | PCR | DD | − | 171 | − | − | 36 |
| 56 | Panahee and Pourtaghi [72] | Iran | Meat | PCR | − | 84 | 9 | − | 5 | − |
| 57 | Park et al. [73] | Korea | Meat | PCR | − | 233 | 176 | − | − | 4 |
| 58 | Pewleang et al. [74] | Thailand | Meat | PCR | − | 62 | − | 3 | 1 | − |
| 59 | Ramatla et al. [7] | South Africa | Faeces | PCR | − | 480 | 62 | 29 | 38 | − |
| 60 | Swetha et al. [75] | India | Meat | PCR | − | 150 | 16 | 8 | 8 | |
| 61 | Tayh et al. [76] | Tunisia | Caecum | PCR | − | 222 | 61 | 10 | 72 |
| Risk Factors | Number of Studies | Pooled Estimates | Measure of Heterogeneity | Publication Bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sample Size | STEC-Positive | I2 (95%CI) | Q Value | I2 | Q | Tau2 | p-Value (p < 0.05) | ||
| Overall | |||||||||
| STEC | 61 | 9973 | 940 | 8.9% (6.2–12.6) | 1299.759 | 95.4 | <0.001 | 2.158 | 0.353 |
| stx1 | 34 | 2874 | 385 | 12.9% (8.1–19.9) | 440.987 | 92.5 | <0.001 | 1.969 | 0.116 |
| stx2 | 37 | 3281 | 364 | 11.8% (7.7–17.6) | 286.650 | 90.7 | <0.001 | 1.765 | 0.107 |
| Samples | |||||||||
| Meat | 35 | 4415 | 379 | 9.7% (5.8–15.8) | 515.654 | 93.4 | <0.001 | 2.456 | 0.754 |
| Cloacal swabs | 4 | 659 | 30 | 12.2% (0.8–69.4) | 85.235 | 96.5 | <0.001 | 7.790 | 0.496 |
| Faeces | 6 | 359 | 74 | 25.6% (7.9–57.8) | 58.324 | 91.4 | <0.001 | 2.555 | 0.188 |
| Visceral organs | 4 | 630 | 220 | 36.7% (0.6–98.3) | 153.763 | 98.0 | <0.001 | 21.009 | 0.500 |
| Mix samples | 10 | 798 | 203 | 26.6% (16.8–39.3) | 84.168 | 90.5 | <0.001 | 0.688 | 0.835 |
| Eggs | 2 | 275 | 13 | − | − | − | − | − | |
| Virulence genes | |||||||||
| eaeA | 18 | 1257 | 227 | 14.8% (8.7–24.1) | 193.306 | 91.2 | <0.001 | 1.409 | 0.471 |
| HlyA | 5 | 185 | 45 | 22.6% (12.6–37.0) | 11.671 | 65.7 | <0.001 | 0.383 | 0.141 |
| exhA | 2 | 246 | 43 | − | − | − | − | − | |
| Serotypes | |||||||||
| O157 | 4 | 580 | 18 | 80.5% (52.0–94.0) | 2.656 | 0.00 | <0.001 | 2.656 | 0.497 |
| O103 | 3 | 77 | 6 | 12.3% (2.1–47.3) | 7.880 | 74.6 | <0.001 | 2.003 | 0.117 |
| O26 | 7 | 143 | 22 | 5.1% (2.8–9.1) | 11.413 | 47.4 | <0.001 | 0.322 | 0.880 |
| O111 | 5 | 186 | 14 | 3.8% (1.8–7.9) | 6.258 | 35.9 | <0.001 | 0.258 | 0.624 |
| O145 | 3 | 106 | 5 | 4.9% (2.1–11.3) | 0.480 | 0.00 | <0.001 | 0.000 | 0.601 |
| Methods | |||||||||
| PCR | 52 | 6437 | 882 | 17.6% (11.9–25.2) | 921.522 | 94.5 | <0.001 | 1.551 | 0.956 |
| m-PCR | 5 | 237 | 49 | 21.0% (8.8–42.0) | 31.846 | 87.4 | <0.001 | 1.131 | 0.624 |
| CHROMagar STEC | 2 | 284 | 8 | − | − | − | − | − | − |
| MALDI-TOF-MS | 2 | 107 | 10 | − | − | − | − | − | − |
| Years | |||||||||
| 2011–2016 | 21 | 3293 | 301 | 6.6% (3.6–11.7) | 346.308 | 94.2 | <0.001 | 1.823 | 0.277 |
| 2017–2019 | 11 | 2334 | 100 | 6.0% (3.0–11.7) | 106.741 | 90.6 | 1.309 | 0.937 | |
| 2020–2022 | 18 | 3518 | 317 | 10.7% (5.0–21.6) | 447.748 | 96.2 | 2.831 | 0.704 | |
| 2023–2024 | 11 | 2082 | 222 | 8.3% (4.4–15.1) | 170.819 | 94.1 | <0.001 | 1.113 | 0.311 |
| Country | |||||||||
| India | 10 | 889 | 112 | 20.2% (10.8–34.6) | 95.044 | 90.5 | <0.001 | 1.224 | 0.089 |
| Egypt | 15 | 702 | 279 | 26.4% (10.1–53.3) | 238.745 | 94.1 | <0.001 | 4.760 | 0.805 |
| Iran | 10 | 1638 | 184 | 7.7% (3.0–18.4) | 214.427 | 95.8 | <0.001 | 2.302 | 0.001 |
| China | 3 | 307 | 10 | 3.3% (1.8–6.0) | 0.214 | 0.00 | <0.001 | 0.000 | 0.117 |
| Continent | |||||||||
| Asia | 36 | 3779 | 605 | 15.6% (9.3–24.8) | 696.504 | 94.9 | <0.001 | 2.813 | 0.683 |
| Africa | 21 | 2977 | 259 | 15.5% (7.1–30.5) | 330.906 | 93.9 | <0.001 | 3.566 | 0.856 |
| North America | 2 | 384 | 71 | − | − | − | − | − | |
| South America | 1 | 25 | 6 | − | − | − | − | − | |
| Europe | 1 | 7 | 3 | − | − | − | − | − | |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Covariates | R2 | p-Value | R2 (%) | p-Value |
| Overall | 0.144 | - | ||
| Samples | 0.000 | 0.000 | ||
| Countries | 0.000 | 0.000 | ||
| Methods | 0.000 | 0.000 | ||
| Years | 0.144 | - | ||
| Antimicrobial Agents | Number of Studies | Number of Isolates | % Prevalence (95%CI) | I2 (95%CI) | Tau2 | Publication Bias p-Value |
|---|---|---|---|---|---|---|
| TET | 10 | 359 | 25.2% (11.9–45.7) | 97 | 1.977 | 0.788 |
| CIP | 10 | 59 | 4.9% (2.1–11.1) | 86 | 1.211 | 0.620 |
| N | 3 | 65 | 23.3% (5.9–59.6) | 80 | 1.504 | 0.601 |
| CAF | 3 | 66 | 5.3% (0.3–50.9) | 97 | 6.414 | 0.117 |
| AMP | 11 | 322 | 28.8% (14.5–49.0) | 96 | 1.954 | 0.815 |
| GEN | 7 | 109 | 8.7% (4.8–15.2) | 88 | 0.617 | 0.024 |
| AML | 3 | 9 | 3.0% (0.06–13.4) | 79 | 1.527 | 0.601 |
| AMC | 5 | 58 | 8.7% (3.1–22.1) | 89 | 1.183 | 0.815 |
| MDR | 4 | 7 | 0.7% (0.3–1.5) | 00 | 0.0 | 0.734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramatla, T.; Jane, N.; Dineo, M.; Mpho, T.; Tshegofatso, M.; Khasapane, N.G. The Global Prevalence of Antibiotic Resistance and Shiga Toxin-Producing Escherichia coli in Chickens: A Systematic Review and Meta-Analysis (2011–2024). Antibiotics 2025, 14, 568. https://doi.org/10.3390/antibiotics14060568
Ramatla T, Jane N, Dineo M, Mpho T, Tshegofatso M, Khasapane NG. The Global Prevalence of Antibiotic Resistance and Shiga Toxin-Producing Escherichia coli in Chickens: A Systematic Review and Meta-Analysis (2011–2024). Antibiotics. 2025; 14(6):568. https://doi.org/10.3390/antibiotics14060568
Chicago/Turabian StyleRamatla, Tsepo, Nkhebenyane Jane, Mohapi Dineo, Tawana Mpho, Motlhaoloa Tshegofatso, and Ntelekwane George Khasapane. 2025. "The Global Prevalence of Antibiotic Resistance and Shiga Toxin-Producing Escherichia coli in Chickens: A Systematic Review and Meta-Analysis (2011–2024)" Antibiotics 14, no. 6: 568. https://doi.org/10.3390/antibiotics14060568
APA StyleRamatla, T., Jane, N., Dineo, M., Mpho, T., Tshegofatso, M., & Khasapane, N. G. (2025). The Global Prevalence of Antibiotic Resistance and Shiga Toxin-Producing Escherichia coli in Chickens: A Systematic Review and Meta-Analysis (2011–2024). Antibiotics, 14(6), 568. https://doi.org/10.3390/antibiotics14060568







