Vancomycin-Resistant E. faecium: Addressing Global and Clinical Challenges
Abstract
1. Introduction
2. Determinants of VREfm Infections and Resistance
2.1. Biological Factors
2.2. Behavioural Factors
2.3. Social, Economic, and Environmental Factors
3. Social and Global Disparities in VREfm Infections
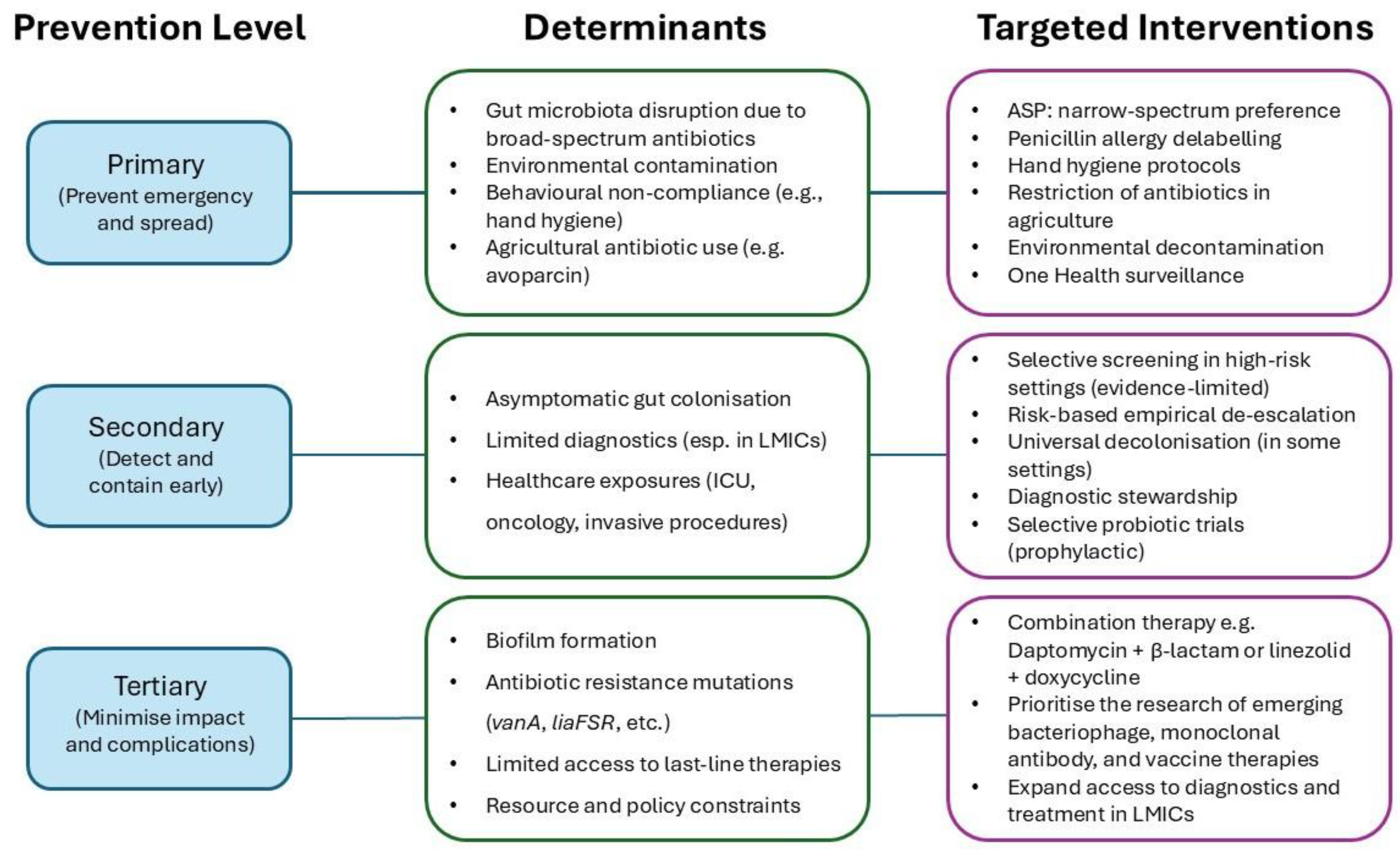
4. Mapping Prevention Activities Across the Disease Pathway
4.1. Primary Prevention (Preventing Emergence and Spread)
4.2. Secondary Prevention (Early Detection and Containment)
4.3. Tertiary Prevention (Minimising Complications and Impact)
5. The Evolving Role of Antimicrobial Stewardship Programmes (ASP) in Preventing VREfm
6. Limitations and Future Directions
6.1. Biological Challenges
6.2. Clinical Management Challenges
6.3. Stewardship Gaps, Surveillance Disparities, and Implementation Challenges
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AMR | Antimicrobial resistance |
| ASP | Antimicrobial Stewardship Programme |
| BSI | Bloodstream infection |
| CAUTI | Catheter-associated urinary tract infection |
| CLABSI | Central line-associated bloodstream infection |
| GI | Gastrointestinal |
| HAI | Hospital-acquired infection |
| HICs | High-income countries |
| ICU | Intensive care unit |
| LMICs | Low- and middle-income countries |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| VBNC | Viable but non-culturable |
| VRE | Vancomycin-resistant enterococci |
| VREfm | Vancomycin-resistant Enterococcus faecium |
References
- Fleming, A. Penicillin. Presented at the Nobel Lecture. 1945. Available online: https://www.nobelprize.org/prizes/medicine/1945/fleming/lecture/ (accessed on 10 April 2025).
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial Resistance: A Concise Update. Lancet Microbe 2025, 6, 100947. [Google Scholar] [CrossRef] [PubMed]
- UKHSA. Laboratory Surveillance of Enterococcus spp. Bacteraemia (England): 2022; UK Health Security Agency: London, UK, 2024.
- CDC. Antibiotic Resistance Threats in the United States, 2019; CDC: Atlanta, GA, USA, 2019.
- Horner, C.; Mushtaq, S.; Allen, M.; Hope, R.; Gerver, S.; Longshaw, C.; Reynolds, R.; Woodford, N.; Livermore, D.M. Replacement of Enterococcus faecalis by Enterococcus faecium as the Predominant Enterococcus in UK Bacteraemias. JAC Antimicrob. Resist. 2021, 3, dlab185. [Google Scholar] [CrossRef]
- UKHSA. Surveillance of Bloodstream Infections in Critical Care Units, England: May 2016 to March 2024 Report; UK Health Security Agency: London, UK, 2024.
- Brinkwirth, S.; Ayobami, O.; Eckmanns, T.; Markwart, R. Hospital-Acquired Infections Caused by Enterococci: A Systematic Review and Meta-Analysis, WHO European Region, 1 January 2010 to 4 February 2020. Eurosurveillance 2021, 26, 2001628. [Google Scholar] [CrossRef]
- del, M.; Lleò, M.; Bonato, B.; Benedetti, D.; Canepari, P. Survival of Enterococcal Species in Aquatic Environments. FEMS Microbiol. Ecol. 2005, 54, 189–196. [Google Scholar] [CrossRef]
- Wagenvoort, J.H.T.; Brauwer, E.I.G.B.D.; Penders, R.J.R.; Willems, R.J.; Top, J.; Bonten, M.J. Environmental Survival of Vancomycin-Resistant Enterococcus faecium. J. Hosp. Infect. 2011, 77, 282–283. [Google Scholar] [CrossRef]
- Michiels, J.E.; Van den Bergh, B.; Verstraeten, N.; Fauvart, M.; Michiels, J. In Vitro Emergence of High Persistence upon Periodic Aminoglycoside Challenge in the ESKAPE Pathogens. Antimicrob. Agents Chemother. 2016, 60, 4630–4637. [Google Scholar] [CrossRef]
- Ch’ng, J.-H.; Chong, K.K.L.; Lam, L.N.; Wong, J.J.; Kline, K.A. Biofilm-Associated Infection by Enterococci. Nat. Rev. Microbiol. 2019, 17, 82–94. [Google Scholar] [CrossRef]
- Montealegre, M.C.; Singh, K.V.; Somarajan, S.R.; Yadav, P.; Chang, C.; Spencer, R.; Sillanpää, J.; Ton-That, H.; Murray, B.E. Role of the Emp Pilus Subunits of Enterococcus faecium in Biofilm Formation, Adherence to Host Extracellular Matrix Components, and Experimental Infection. Infect. Immun. 2016, 84, 1491–1500. [Google Scholar] [CrossRef]
- Almohamad, S.; Somarajan, S.R.; Singh, K.V.; Nallapareddy, S.R.; Murray, B.E. Influence of Isolate Origin and Presence of Various Genes on Biofilm Formation by Enterococcus faecium. FEMS Microbiol. Lett. 2014, 353, 151–156. [Google Scholar] [CrossRef]
- Paganelli, F.L.; Willems, R.J.L.; Jansen, P.; Hendrickx, A.; Zhang, X.; Bonten, M.J.M.; Leavis, H.L. Enterococcus faecium Biofilm Formation: Identification of Major Autolysin AtlAEfm, Associated Acm Surface Localization, and AtlAEfm-Independent Extracellular DNA Release. mBio 2013, 4, e00154. [Google Scholar] [CrossRef] [PubMed]
- Heikens, E.; Singh, K.V.; Jacques-Palaz, K.D.; van Luit-Asbroek, M.; Oostdijk, E.A.N.; Bonten, M.J.M.; Murray, B.E.; Willems, R.J.L. Contribution of the Enterococcal Surface Protein Esp to Pathogenesis of Enterococcus faecium Endocarditis. Microbes Infect. 2011, 13, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Nallapareddy, S.R.; Sillanpää, J.; Ganesh, V.K.; Höök, M.; Murray, B.E. Inhibition of Enterococcus faecium Adherence to Collagen by Antibodies against High-Affinity Binding Subdomains of Acm. Infect. Immun. 2007, 75, 3192–3196. [Google Scholar] [CrossRef]
- Top, J.; Paganelli, F.L.; Zhang, X.; van Schaik, W.; Leavis, H.L.; van Luit-Asbroek, M.; van der Poll, T.; Leendertse, M.; Bonten, M.J.M.; Willems, R.J.L. The Enterococcus faecium Enterococcal Biofilm Regulator, EbrB, Regulates the Esp Operon and Is Implicated in Biofilm Formation and Intestinal Colonization. PLoS ONE 2013, 8, e65224. [Google Scholar] [CrossRef]
- Lebreton, F.; van Schaik, W.; Sanguinetti, M.; Posteraro, B.; Torelli, R.; Le Bras, F.; Verneuil, N.; Zhang, X.; Giard, J.-C.; Dhalluin, A.; et al. AsrR Is an Oxidative Stress Sensing Regulator Modulating Enterococcus faecium Opportunistic Traits, Antimicrobial Resistance, and Pathogenicity. PLoS Pathog. 2012, 8, e1002834. [Google Scholar] [CrossRef]
- Goudarzi, M.; Mobarez, A.M.; Najar-Peerayeh, S.; Mirzaee, M. Prevalence of Biofilm Formation and Vancomycin-Resistant Genes among Enterococcus faecium Isolated from Clinical and Environmental Specimens in Lorestan Hospitals. Iran. J. Microbiol. 2018, 10, 74–81. [Google Scholar]
- Novais, C.; Tedim, A.P.; Lanza, V.F.; Freitas, A.R.; Silveira, E.; Escada, R.; Roberts, A.P.; Al-Haroni, M.; Baquero, F.; Peixe, L.; et al. Co-Diversification of Enterococcus faecium Core Genomes and PBP5: Evidences of Pbp5 Horizontal Transfer. Front. Microbiol. 2016, 7, 1581. [Google Scholar] [CrossRef]
- Miller, W.R.; Murray, B.E.; Rice, L.B.; Arias, C.A. Resistance in Vancomycin-Resistant Enterococci. Infect. Dis. Clin. N. Am. 2020, 34, 751–771. [Google Scholar] [CrossRef]
- Rahim, S.; Pillai, S.K.; Gold, H.S.; Venkataraman, L.; Inglima, K.; Press, R.A. Linezolid-Resistant, Vancomycin-Resistant Enterococcus faecium Infection in Patients without Prior Exposure to Linezolid. Clin. Infect. Dis. 2003, 36, E146–E148. [Google Scholar] [CrossRef]
- Kinnear, C.L.; Hansen, E.; Morley, V.J.; Tracy, K.C.; Forstchen, M.; Read, A.F.; Woods, R.J. Daptomycin Treatment Impacts Resistance in Off-Target Populations of Vancomycin-Resistant Enterococcus faecium. PLoS Biol. 2020, 18, e3000987. [Google Scholar] [CrossRef]
- Ranotkar, S.; Kumar, P.; Zutshi, S.; Prashanth, K.S.; Bezbaruah, B.; Anand, J.; Lahkar, M. Vancomycin-Resistant Enterococci: Troublemaker of the 21st Century. J. Glob. Antimicrob. Resist. 2014, 2, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Courvalin, P. Vancomycin Resistance in Gram-Positive Cocci. Clin. Infect. Dis. 2006, 42, S25–S34. [Google Scholar] [CrossRef]
- Freitas, A.R.; Tedim, A.P.; Novais, C.; Coque, T.M.; Peixe, L. Distribution of Putative Virulence Markers in Enterococcus faecium: Towards a Safety Profile Review. J. Antimicrob. Chemother. 2018, 73, 306–319. [Google Scholar] [CrossRef]
- Almeida-Santos, A.C.; Novais, C.; Peixe, L.; Freitas, A.R. Vancomycin-Resistant Enterococcus faecium: A Current Perspective on Resilience, Adaptation, and the Urgent Need for Novel Strategies. J. Glob. Antimicrob. Resist. 2025, 41, 233–252. [Google Scholar] [CrossRef]
- Crouzet, L.; Rigottier-Gois, L.; Serror, P. Potential Use of Probiotic and Commensal Bacteria as Non-Antibiotic Strategies against Vancomycin-Resistant Enterococci. FEMS Microbiol. Lett. 2015, 362, fnv012. [Google Scholar] [CrossRef]
- Salgado, C.D. The Risk of Developing a Vancomycin-Resistant Enterococcus Bloodstream Infection for Colonized Patients. Am. J. Infect. Control. 2008, 36, S175.e5–S175.e8. [Google Scholar] [CrossRef]
- Tornieporth, N.G.; Roberts, R.B.; John, J.; Hafner, A.; Riley, L.W. Risk Factors Associated with Vancomycin-Resistant Enterococcus faecium Infection or Colonization in 145 Matched Case Patients and Control Patients. Clin. Infect. Dis. 1996, 23, 767–772. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Chuang, Y.-C.; Wang, J.-T.; Sheng, W.-H.; Chen, Y.-C.; Chang, S.-C. Predictors for Vancomycin Resistant Enterococcus faecium Transforming from Colonization to Infection: A Case Control Study. Antimicrob. Resist. Infect. Control. 2019, 8, 196. [Google Scholar] [CrossRef]
- Donskey, C.J.; Hanrahan, J.A.; Hutton, R.A.; Rice, L.B. Effect of Parenteral Antibiotic Administration on Persistence of Vancomycin-Resistant Enterococcus faecium in the Mouse Gastrointestinal Tract. J. Infect. Dis. 1999, 180, 384–390. [Google Scholar] [CrossRef]
- Fridkin, S.K.; Edwards, J.R.; Courval, J.M.; Hill, H.; Tenover, F.C.; Lawton, R.; Gaynes, R.P.; McGowan, J.E.; for the Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Project and the National Nosocomial Infections Surveillance (NNIS) System Hospitals. The Effect of Vancomycin and Third-Generation Cephalosporins on Prevalence of Vancomycin-Resistant Enterococci in 126 U.S. Adult Intensive Care Units. Ann. Intern. Med. 2001, 135, 175–183. [Google Scholar] [CrossRef]
- Peset, V.; Tallón, P.; Sola, C.; Sánchez, E.; Sarrión, A.; Pérez-Bellés, C.; Vindel, A.; Cantón, E.; Gobernado, M. Epidemiological, Microbiological, Clinical, and Prognostic Factors of Bacteremia Caused by High-Level Vancomycin-Resistant Enterococcus Species. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 742–749. [Google Scholar] [CrossRef]
- Zaas, A.K.; Song, X.; Tucker, P.; Perl, T.M. Risk Factors for Development of Vancomycin-Resistant Enterococcal Bloodstream Infection in Patients with Cancer Who Are Colonized with Vancomycin-Resistant Enterococci. Clin. Infect. Dis. 2002, 35, 1139–1146. [Google Scholar] [CrossRef]
- Bonten, M.J.; Hayden, M.K.; Nathan, C.; van Voorhis, J.; Matushek, M.; Slaughter, S.; Rice, T.; Weinstein, R.A. Epidemiology of Colonisation of Patients and Environment with Vancomycin-Resistant Enterococci. Lancet 1996, 348, 1615–1619. [Google Scholar] [CrossRef]
- de Bruin, M.A.; Riley, L.W. Does Vancomycin Prescribing Intervention Affect Vancomycin-Resistant Enterococcus Infection and Colonization in Hospitals? A Systematic Review. BMC Infect. Dis. 2007, 7, 24. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, S.I.; Kim, Y.R.; Lee, J.Y.; Park, Y.J.; Kang, M.W. Risk Factors for Vancomycin-Resistant Enterococci Infection and Mortality in Colonized Patients on Intensive Care Unit Admission. Am. J. Infect. Control. 2012, 40, 1018–1019. [Google Scholar] [CrossRef]
- Bender, J.K.; Cattoir, V.; Hegstad, K.; Sadowy, E.; Coque, T.M.; Westh, H.; Hammerum, A.M.; Schaffer, K.; Burns, K.; Murchan, S.; et al. Update on Prevalence and Mechanisms of Resistance to Linezolid, Tigecycline and Daptomycin in Enterococci in Europe: Towards a Common Nomenclature. Drug Resist. Updates 2018, 40, 25–39. [Google Scholar] [CrossRef]
- Tran, T.T.; Munita, J.M.; Arias, C.A. Mechanisms of Drug Resistance: Daptomycin Resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 32–53. [Google Scholar] [CrossRef]
- Zeng, W.; Feng, L.; Qian, C.; Chen, T.; Wang, S.; Zhang, Y.; Zheng, X.; Wang, L.; Liu, S.; Zhou, T.; et al. Acquisition of Daptomycin Resistance by Enterococcus faecium Confers Collateral Sensitivity to Glycopeptides. Front. Microbiol. 2022, 13, 815600. [Google Scholar] [CrossRef]
- Prater, A.G.; Mehta, H.H.; Beabout, K.; Supandy, A.; Miller, W.R.; Tran, T.T.; Arias, C.A.; Shamoo, Y. Daptomycin Resistance in Enterococcus faecium Can Be Delayed by Disruption of the LiaFSR Stress Response Pathway. Antimicrob. Agents Chemother. 2021, 65, e01317-20. [Google Scholar] [CrossRef]
- Sinel, C.; Jaussaud, C.; Auzou, M.; Giard, J.-C.; Cattoir, V. Mutant Prevention Concentrations of Daptomycin for Enterococcus faecium Clinical Isolates. Int. J. Antimicrob. Agents 2016, 48, 449–452. [Google Scholar] [CrossRef]
- Swaney, S.M.; Aoki, H.; Ganoza, M.C.; Shinabarger, D.L. The Oxazolidinone Linezolid Inhibits Initiation of Protein Synthesis in Bacteria. Antimicrob. Agents Chemother. 1998, 42, 3251–3255. [Google Scholar] [CrossRef] [PubMed]
- Scheetz, M.H.; Knechtel, S.A.; Malczynski, M.; Postelnick, M.J.; Qi, C. Increasing Incidence of Linezolid-Intermediate or -Resistant, Vancomycin-Resistant Enterococcus faecium Strains Parallels Increasing Linezolid Consumption. Antimicrob. Agents Chemother. 2008, 52, 2256–2259. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A Novel Gene, optrA, That Confers Transferable Resistance to Oxazolidinones and Phenicols and Its Presence in Enterococcus faecalis and Enterococcus faecium of Human and Animal Origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; D’Andrea, M.M.; Brenciani, A.; Galeotti, C.L.; Morroni, G.; Pollini, S.; Varaldo, P.E.; Rossolini, G.M. Characterization of poxtA, a Novel Phenicol-Oxazolidinone-Tetracycline Resistance Gene from an MRSA of Clinical Origin. J. Antimicrob. Chemother. 2018, 73, 1763–1769. [Google Scholar] [CrossRef]
- Ruiz-Ripa, L.; Feßler, A.T.; Hanke, D.; Eichhorn, I.; Azcona-Gutiérrez, J.M.; Pérez-Moreno, M.O.; Seral, C.; Aspiroz, C.; Alonso, C.A.; Torres, L.; et al. Mechanisms of Linezolid Resistance Among Enterococci of Clinical Origin in Spain—Detection of optrA- and Cfr(D)-Carrying E. faecalis. Microorganisms 2020, 8, 1155. [Google Scholar] [CrossRef]
- Freitas, A.R.; Tedim, A.P.; Duarte, B.; Elghaieb, H.; Abbassi, M.S.; Hassen, A.; Read, A.; Alves, V.; Novais, C.; Peixe, L. Linezolid-Resistant (Tn6246::fexB-poxtA) Enterococcus faecium Strains Colonizing Humans and Bovines on Different Continents: Similarity without Epidemiological Link. J. Antimicrob. Chemother. 2020, 75, 2416–2423. [Google Scholar] [CrossRef]
- Egan, S.A.; Shore, A.C.; O’Connell, B.; Brennan, G.I.; Coleman, D.C. Linezolid Resistance in Enterococcus faecium and Enterococcus faecalis from Hospitalized Patients in Ireland: High Prevalence of the MDR Genes optrA and poxtA in Isolates with Diverse Genetic Backgrounds. J. Antimicrob. Chemother. 2020, 75, 1704–1711. [Google Scholar] [CrossRef]
- Misiakou, M.-A.; Hertz, F.B.; Schønning, K.; Häussler, S.; Nielsen, K.L. Emergence of Linezolid-Resistant Enterococcus faecium in a Tertiary Hospital in Copenhagen. Microb. Genom. 2023, 9, mgen001055. [Google Scholar] [CrossRef]
- Marshall, S.H.; Donskey, C.J.; Hutton-Thomas, R.; Salata, R.A.; Rice, L.B. Gene Dosage and Linezolid Resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob. Agents Chemother. 2002, 46, 3334–3336. [Google Scholar] [CrossRef]
- Turner, A.M.; Li, L.; Monk, I.R.; Lee, J.Y.H.; Ingle, D.J.; Portelli, S.; Sherry, N.L.; Isles, N.; Seemann, T.; Sharkey, L.K.; et al. Rifaximin Prophylaxis Causes Resistance to the Last-Resort Antibiotic Daptomycin. Nature 2024, 635, 969–977. [Google Scholar] [CrossRef]
- Turnidge, J.; Kahlmeter, G.; Cantón, R.; MacGowan, A.; Giske, C.G. Daptomycin in the Treatment of Enterococcal Bloodstream Infections and Endocarditis: A EUCAST Position Paper. Clin. Microbiol. Infect. 2020, 26, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Klare, I.; Badstübner, D.; Konstabel, C.; Böhme, G.; Claus, H.; Witte, W. Decreased Incidence of VanA-Type Vancomycin-Resistant Enterococci Isolated from Poultry Meat and from Fecal Samples of Humans in the Community after Discontinuation of Avoparcin Usage in Animal Husbandry. Microb. Drug Resist. 1999, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Nnadozie, C.F.; Odume, O.N. Freshwater Environments as Reservoirs of Antibiotic Resistant Bacteria and Their Role in the Dissemination of Antibiotic Resistance Genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef]
- Gouliouris, T.; Raven, K.E.; Moradigaravand, D.; Ludden, C.; Coll, F.; Blane, B.; Naydenova, P.; Horner, C.; Brown, N.M.; Corander, J.; et al. Detection of Vancomycin-Resistant Enterococcus faecium Hospital-Adapted Lineages in Municipal Wastewater Treatment Plants Indicates Widespread Distribution and Release into the Environment. Genome Res. 2019, 29, 626–634. [Google Scholar] [CrossRef]
- Arnold, K.E.; Laing, G.; McMahon, B.J.; Fanning, S.; Stekel, D.J.; Pahl, O.; Coyne, L.; Latham, S.M.; McIntyre, K.M. The Need for One Health Systems-Thinking Approaches to Understand Multiscale Dissemination of Antimicrobial Resistance. Lancet Planet. Health 2024, 8, e124–e133. [Google Scholar] [CrossRef]
- Cetinkaya, Y.; Falk, P.; Mayhall, C.G. Vancomycin-Resistant Enterococci. Clin. Microbiol. Rev. 2000, 13, 686–707. [Google Scholar] [CrossRef]
- CDC. Antibiotic Resistance Threats in the United States, 2013; CDC: Atlanta, GA, USA, 2013.
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33, e00048-19. [Google Scholar] [CrossRef]
- Ranjalkar, J.; Chandy, S.J. India’s National Action Plan for Antimicrobial Resistance—An Overview of the Context, Status, and Way Ahead. J. Fam. Med. Prim. Care 2019, 8, 1828–1834. [Google Scholar] [CrossRef]
- CDC. HAI Pathogens and Antimicrobial Resistance Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- Sörstedt, E.; Ahlbeck, G.; Snygg-Martin, U. Trends in Enterococcus faecium Bacteremia: Exploring Risk Factors with Emphasis on Prior Antibiotic Exposure. Microorganisms 2024, 12, 1932. [Google Scholar] [CrossRef]
- Huang, C.; Moradi, S.; Sholeh, M.; Tabaei, F.M.; Lai, T.; Tan, B.; Meng, J.; Azizian, K. Global Trends in Antimicrobial Resistance of Enterococcus faecium: A Systematic Review and Meta-Analysis of Clinical Isolates. Front. Pharmacol. 2025, 16, 1505674. [Google Scholar] [CrossRef]
- De Angelis, G.; Cataldo, M.A.; De Waure, C.; Venturiello, S.; La Torre, G.; Cauda, R.; Carmeli, Y.; Tacconelli, E. Infection Control and Prevention Measures to Reduce the Spread of Vancomycin-Resistant Enterococci in Hospitalized Patients: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2014, 69, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Cataldo, M.A. Vancomycin-Resistant Enterococci (VRE): Transmission and Control. Int. J. Antimicrob. Agents 2008, 31, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Vehreschild, M.J.G.T.; Haverkamp, M.; Biehl, L.M.; Lemmen, S.; Fätkenheuer, G. Vancomycin-Resistant Enterococci (VRE): A Reason to Isolate? Infection 2019, 47, 7–11. [Google Scholar] [CrossRef]
- Frakking, F.N.J.; Bril, W.S.; Sinnige, J.C.; van’t Klooster, J.E.; de Jong, B.A.W.; van Hannen, E.J.; Tersmette, M. Recommendations for the Successful Control of a Large Outbreak of Vancomycin-Resistant Enterococcus faecium in a Non-Endemic Hospital Setting. J. Hosp. Infect. 2018, 100, e216–e225. [Google Scholar] [CrossRef]
- Huang, S.S.; Septimus, E.; Kleinman, K.; Moody, J.; Hickok, J.; Avery, T.R.; Lankiewicz, J.; Gombosev, A.; Terpstra, L.; Hartford, F.; et al. Targeted versus Universal Decolonization to Prevent ICU Infection. N. Engl. J. Med. 2013, 368, 2255–2265. [Google Scholar] [CrossRef]
- Mac, S.; Fitzpatrick, T.; Johnstone, J.; Sander, B. Vancomycin-Resistant Enterococci (VRE) Screening and Isolation in the General Medicine Ward: A Cost-Effectiveness Analysis. Antimicrob. Resist. Infect. Control. 2019, 8, 168. [Google Scholar] [CrossRef]
- Humphreys, H. Controlling the Spread of Vancomycin-Resistant Enterococci. Is Active Screening Worthwhile? J. Hosp. Infect. 2014, 88, 191–198. [Google Scholar] [CrossRef]
- Hansen, S.G.K.; Klein, K.; Nymark, A.; Andersen, L.; Gradel, K.O.; Lis-Toender, J.; Oestergaard, C.; Chen, M.; Datcu, R.; Skov, M.N.; et al. Vancomycin-Resistant Enterococcus faecium: Impact of Ending Screening and Isolation in a Danish University Hospital. J. Hosp. Infect. 2024, 146, 82–92. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Trudeau, R.E.; Seville, M.T.; Chan, L. Impact of a Vancomycin-Resistant Enterococcus (VRE) Screening Result on Appropriateness of Antibiotic Therapy. Antimicrob. Steward. Healthc. Epidemiol. 2021, 1, e41. [Google Scholar] [CrossRef]
- Fazeli, H.; Esfahani, B.N.; Mirlohi, M. Efficacy of Probiotic for Inhibition of Intestinal Colonization by Vancomycin-Resistant Enterococci. Int. J. Infect. Dis. 2008, 12, e211–e212. [Google Scholar] [CrossRef]
- Li, X.; Song, L.; Zhu, S.; Xiao, Y.; Huang, Y.; Hua, Y.; Chu, Q.; Ren, Z. Two Strains of Lactobacilli Effectively Decrease the Colonization of VRE in a Mouse Model. Front. Cell. Infect. Microbiol. 2019, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, L.; Derrien, M.; Cherbuy, C.; Plancade, S.; Foulon, M.; Chalin, B.; van Hylckama Vlieg, J.E.T.; Grompone, G.; Rigottier-Gois, L.; Serror, P. Lactobacillus Paracasei CNCM I-3689 Reduces Vancomycin-Resistant Enterococcus Persistence and Promotes Bacteroidetes Resilience in the Gut Following Antibiotic Challenge. Sci. Rep. 2018, 8, 5098. [Google Scholar] [CrossRef] [PubMed]
- de Regt, M.J.A.; Willems, R.J.L.; Hené, R.J.; Siersema, P.D.; Verhaar, H.J.J.; Hopmans, T.E.M.; Bonten, M.J.M. Effects of Probiotics on Acquisition and Spread of Multiresistant Enterococci. Antimicrob. Agents Chemother. 2010, 54, 2801–2805. [Google Scholar] [CrossRef]
- Vidal, M.; Forestier, C.; Charbonnel, N.; Henard, S.; Rabaud, C.; Lesens, O. Probiotics and Intestinal Colonization by Vancomycin-Resistant Enterococci in Mice and Humans. J. Clin. Microbiol. 2010, 48, 2595–2598. [Google Scholar] [CrossRef]
- Manley, K.J.; Fraenkel, M.B.; Mayall, B.C.; Power, D.A. Probiotic Treatment of Vancomycin-Resistant Enterococci: A Randomised Controlled Trial. Med. J. Aust. 2007, 186, 454–457. [Google Scholar] [CrossRef]
- Montassier, E.; Valdés-Mas, R.; Batard, E.; Zmora, N.; Dori-Bachash, M.; Suez, J.; Elinav, E. Probiotics Impact the Antibiotic Resistance Gene Reservoir along the Human GI Tract in a Person-Specific and Antibiotic-Dependent Manner. Nat. Microbiol. 2021, 6, 1043–1054. [Google Scholar] [CrossRef]
- Kresken, M.; Klare, I.; Wichelhaus, T.A.; Wohlfarth, E.; Layer-Nicolaou, F.; Neumann, B.; Werner, G. Glycopeptide Resistance in Enterococcus Spp. and Coagulase-Negative Staphylococci from Hospitalised Patients in Germany: Occurrence, Characteristics and Dalbavancin Susceptibility. J. Glob. Antimicrob. Resist. 2022, 28, 102–107. [Google Scholar] [CrossRef]
- Sweeney, D.; Stoneburner, A.; Shinabarger, D.L.; Arhin, F.F.; Belley, A.; Moeck, G.; Pillar, C.M. Comparative in Vitro Activity of Oritavancin and Other Agents against Vancomycin-Susceptible and -Resistant Enterococci. J. Antimicrob. Chemother. 2017, 72, 622–624. [Google Scholar] [CrossRef]
- Kresken, M.; Körber-Irrgang, B.; Petrik, C.; Seifert, H.; Rodloff, A.; Becker, K. Temporal Trends of the in Vitro Activity of Tigecycline and Comparator Antibiotics against Clinical Aerobic Bacterial Isolates Collected in Germany, 2006–2014: Results of the Tigecycline Evaluation and Surveillance Trial (TEST). GMS Infect. Dis. 2016, 4, Doc07. [Google Scholar] [CrossRef]
- Gong, J.; Su, D.; Shang, J.; Yu, H.; Du, G.; Lin, Y.; Sun, Z.; Liu, G. Efficacy and Safety of High-Dose Tigecycline for the Treatment of Infectious Diseases: A Meta-Analysis. Medicine 2019, 98, e17091. [Google Scholar] [CrossRef]
- Smith, J.R.; Barber, K.E.; Raut, A.; Aboutaleb, M.; Sakoulas, G.; Rybak, M.J. β-Lactam Combinations with Daptomycin Provide Synergy against Vancomycin-Resistant Enterococcus faecalis and Enterococcus faecium. J. Antimicrob. Chemother. 2015, 70, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Sakoulas, G.; Bayer, A.S.; Pogliano, J.; Tsuji, B.T.; Yang, S.-J.; Mishra, N.N.; Nizet, V.; Yeaman, M.R.; Moise, P.A. Ampicillin Enhances Daptomycin- and Cationic Host Defense Peptide-Mediated Killing of Ampicillin- and Vancomycin-Resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2012, 56, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.; Smith, J.R.; Rybak, M.J. Role of Combination Antimicrobial Therapy for Vancomycin-Resistant Enterococcus faecium Infections: Review of the Current Evidence. Pharmacotherapy 2017, 37, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Schutt, A.C.; Bohm, N.M. Multidrug-Resistant Enterococcus faecium Endocarditis Treated with Combination Tigecycline and High-Dose Daptomycin. Ann. Pharmacother. 2009, 43, 2108–2112. [Google Scholar] [CrossRef]
- Pontikis, K.; Pefanis, A.; Tsaganos, T.; Tzepi, I.-M.; Carrer, D.-P.; Giamarellou, H. Efficacy of Tigecycline Alone and in Combination with Gentamicin in the Treatment of Experimental Endocarditis Due to Linezolid-Resistant Enterococcus faecium. Antimicrob. Agents Chemother. 2013, 57, 3392–3394. [Google Scholar] [CrossRef]
- Jenkins, I. Linezolid- and Vancomycin-Resistant Enterococcus faecium Endocarditis: Successful Treatment with Tigecycline and Daptomycin. J. Hosp. Med. 2007, 2, 343–344. [Google Scholar] [CrossRef]
- Zinner, S.H.; Gilbert, D.; Lubenko, I.Y.; Greer, K.; Firsov, A.A. Selection of Linezolid-Resistant Enterococcus faecium in an in Vitro Dynamic Model:: Protective Effect of Doxycycline. J. Antimicrob. Chemother. 2008, 61, 629–635. [Google Scholar] [CrossRef]
- Sabol, K.; Patterson, J.E.; Lewis, J.S.; Owens, A.; Cadena, J.; Jorgensen, J.H. Emergence of Daptomycin Resistance in Enterococcus faecium during Daptomycin Therapy. Antimicrob. Agents Chemother. 2005, 49, 1664–1665. [Google Scholar] [CrossRef]
- Rigvava, S.; Kusradze, I.; Tchgkonia, I.; Karumidze, N.; Dvalidze, T.; Goderdzishvili, M. Novel Lytic Bacteriophage vB_GEC_EfS_9 against Enterococcus faecium. Virus Res. 2022, 307, 198599. [Google Scholar] [CrossRef]
- Stellfox, M.E.; Fernandes, C.; Shields, R.K.; Haidar, G.; Hughes Kramer, K.; Dembinski, E.; Mangalea, M.R.; Arya, G.; Canfield, G.S.; Duerkop, B.A.; et al. Bacteriophage and Antibiotic Combination Therapy for Recurrent Enterococcus faecium Bacteremia. mBio 2024, 15, e0339623. [Google Scholar] [CrossRef]
- Wei, Y.; Palacios Araya, D.; Palmer, K.L. Enterococcus faecium: Evolution, Adaptation, Pathogenesis and Emerging Therapeutics. Nat. Rev. Microbiol. 2024, 22, 705–721. [Google Scholar] [CrossRef] [PubMed]
- DeLisle, S.; Perl, T.M. Vancomycin-Resistant Enterococci: A Road Map on How To Prevent the Emergence and Transmission of Antimicrobial Resistance. Chest 2003, 123, 504S–518S. [Google Scholar] [CrossRef] [PubMed]
- Coombs, G.; Bell, J.; Blyth, C.; Bond, K.; Daley, D.; Cooley, L.; Gottlieb, T.; Iredell, J.; Warner, M.; Robson, J.; et al. Australian Group on Antimicrobial Resistance Surveillance Outcomes Programs. Bloodstream Infections: 2023 Report; ACSQHC: Sydney, Australia, 2024.
- UKHSA. Start Smart Then Focus: Antimicrobial Stewardship Toolkit for Inpatient Care Settings; UK Health Security Agency: London, UK, 2023.
- Ashiru-Oredope, D.; Sharland, M.; Charani, E.; McNulty, C.; Cooke, J.; ARHAI Antimicrobial Stewardship Group. Improving the Quality of Antibiotic Prescribing in the NHS by Developing a New Antimicrobial Stewardship Programme: Start Smart--Then Focus. J. Antimicrob. Chemother. 2012, 67 (Suppl. S1), i51–i63. [Google Scholar] [CrossRef]
- Ashiru-Oredope, D.; Budd, E.L.; Bhattacharya, A.; Din, N.; McNulty, C.A.M.; Micallef, C.; Ladenheim, D.; Beech, E.; Murdan, S.; Hopkins, S.; et al. Implementation of Antimicrobial Stewardship Interventions Recommended by National Toolkits in Primary and Secondary Healthcare Sectors in England: TARGET and Start Smart Then Focus. J. Antimicrob. Chemother. 2016, 71, 1408–1414. [Google Scholar] [CrossRef]
- Moore, M.; McNulty, C. European Antibiotic Awareness Day 2012: TARGET Antibiotics through Guidance, Education, and Tools. Br. J. Gen. Pract. 2012, 62, 621–622. [Google Scholar] [CrossRef]
- UKHSA. Antimicrobial Prescribing and Stewardship Competency Framework; UK Health Security Agency: London, UK, 2023.
- Wanat, M.; Santillo, M.; Galal, U.; Davoudianfar, M.; Bongard, E.; Savic, S.; Savic, L.; Porter, C.; Fielding, J.; Butler, C.C.; et al. Mixed-Methods Evaluation of a Behavioural Intervention Package to Identify and Amend Incorrect Penicillin Allergy Records in UK General Practice. BMJ Open 2022, 12, e057471. [Google Scholar] [CrossRef]
- UK Government. Confronting Antimicrobial Resistance 2024 to 2029; Department of Health & Social Care: London, UK, 2024.
- García Martínez de Artola, D.; Castro, B.; Ramos, M.J.; Díaz Cuevas, Z.; Lakhwani, S.; Lecuona, M. Outbreak of Vancomycin-Resistant Enterococcus on a Haematology Ward: Management and Control. J. Infect. Prev. 2017, 18, 149–153. [Google Scholar] [CrossRef]
- Brandl, K.; Plitas, G.; Mihu, C.N.; Ubeda, C.; Jia, T.; Fleisher, M.; Schnabl, B.; DeMatteo, R.P.; Pamer, E.G. Vancomycin-Resistant Enterococci Exploit Antibiotic-Induced Innate Immune Deficits. Nature 2008, 455, 804–807. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Gandra, S. How Can We Tackle the Overuse of Antibiotics in Low- and Middle-Income Countries? Expert Rev. Anti Infect. Ther. 2023, 21, 1189–1201. [Google Scholar] [CrossRef]
- Zaidi, S.-E.-Z.; Zaheer, R.; Zovoilis, A.; McAllister, T.A. Enterococci as a One Health Indicator of Antimicrobial Resistance. Can. J. Microbiol. 2024, 70, 303–335. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radford-Smith, D.E.; Anthony, D.C. Vancomycin-Resistant E. faecium: Addressing Global and Clinical Challenges. Antibiotics 2025, 14, 522. https://doi.org/10.3390/antibiotics14050522
Radford-Smith DE, Anthony DC. Vancomycin-Resistant E. faecium: Addressing Global and Clinical Challenges. Antibiotics. 2025; 14(5):522. https://doi.org/10.3390/antibiotics14050522
Chicago/Turabian StyleRadford-Smith, Daniel E., and Daniel C. Anthony. 2025. "Vancomycin-Resistant E. faecium: Addressing Global and Clinical Challenges" Antibiotics 14, no. 5: 522. https://doi.org/10.3390/antibiotics14050522
APA StyleRadford-Smith, D. E., & Anthony, D. C. (2025). Vancomycin-Resistant E. faecium: Addressing Global and Clinical Challenges. Antibiotics, 14(5), 522. https://doi.org/10.3390/antibiotics14050522




