Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications
Abstract
1. Introduction
2. Literature Review and Data Extraction
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Data Extraction
- Study identification: Authors, publication year, and title.
- Bacterial pigment: The pigment used in the investigation (e.g., prodigiosin) as well as the bacterial species from which it was derived.
- Method: It included extraction techniques as well as the method used for the application to textiles.
- Textile substrate: The type of material used (e.g., cotton, silk, polyamide).
- Results: A summary of the colour fastness results, along with the effectiveness of the bacterial pigment in inhibiting bacterial growth when applicable.
2.4. Synthesis of Results
2.5. Limitations of the Methodology
3. Molecular Pathways in Pigment Formation and Their Antibacterial Activity
4. Extraction Methods for Bacterial Pigments
4.1. Mechanical Methods
4.1.1. Bead Milling
4.1.2. High-Pressure Homogenisation (HPH)
4.1.3. Ultrasonication
4.2. Chemical Methods
4.2.1. Organic Solvent Extraction
4.2.2. Supercritical Fluid Extraction
4.2.3. Microwave-Assisted Extraction
4.2.4. Enzyme-Assisted Extraction
4.2.5. Ionic Liquid-Assisted Extraction
5. Bacterial Pigments in Textile Colouration
5.1. Carotenoids
5.2. Melanin
5.3. Violacein
5.4. Prodigiosin
6. The Antimicrobial Properties of Bacterial Pigments for Textile Functionalisation
7. Safety and Efficacy of Bacterial Pigments in Textiles
8. Challenges Associated with Bacterial Pigment Production and Application
9. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AHL | acyl-homoserine |
| AL2(SO4)3 | aluminium sulphate |
| Asm | acid sphingomyelinase |
| BBD | Box–Behnken design |
| BTCA | 1,2,3,4-butanetetracarboxylic acid |
| Ca(OH)2 | calcium hydroxide |
| COD | chemical oxygen demand |
| CO2 | carbon dioxide |
| CrtB | phytoene synthase |
| CrtI | phytoene desaturase |
| CrtY | lycopene cyclase |
| CrtZ | hydroxylases |
| CuSO4 | copper(II) sulphate |
| DAFS | dyeing after fermentation and sonication |
| DD | direct dyeing |
| EAE | enzyme-assisted extraction |
| FBR | fluidised bed reactor |
| FeSO4 | ferrous sulphate |
| FTIR | Fourier-transform infrared spectroscopy |
| GGDPS | GGPP synthase |
| GGPP | geranylgeranyl diphosphate |
| H2O2 | hydrogen peroxide |
| HPLC | high-performance liquid chromatography |
| HPH | high-pressure homogenisation |
| IL | ionic liquid |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| LB | Luria–Bertani |
| LCA | life cycle assessment |
| MAP | Methyl-3-n-Amylpyrrole |
| MBC | 4-Methoxy-2,2′-Bipyrrole-5-Carbaldehyde |
| MEP | 2-C-methyl-D-erythritol-4-phosphate |
| MVA | mevalonate |
| NaCl | sodium chloride |
| NaHCO3 | sodium bicarbonate |
| O2·- | superoxide anions |
| PMS | paper mill sludge |
| PQS | Pseudomonas quinolone signal |
| ROS | reactive oxygen species |
| SFD | simultaneous fermentation and dyeing |
| SFE | supercritical fluid extraction |
| SM2 | soybean meal |
| TBAOH | tetrabutylammonium hydroxide |
| TLC | thin-layer chromatography |
| UAE | ultrasound-assisted extraction |
| Y2O3 | yttrium(III) oxide |
| ZrO2 | zicornium dioxide |
References
- Churi, H.; Palan, S. Identification and Characterization of Bioactive Pigments from Marine Bacteria Isolated from Coastal Region of Kelwe-Mahim, Palghar. J. Emerg. Technol. Innov. Res. 2020, 7, 24–31. Available online: https://api.semanticscholar.org/CorpusID:228932890 (accessed on 1 May 2023).
- Eskandari, S.; Etemadifar, Z. Biocompatibility and radioprotection by newly characterized melanin pigment and its production from Dietzia schimae NM3 in optimized whey medium by response surface methodology. Ann. Microbiol. 2021, 71, 17. [Google Scholar] [CrossRef]
- Devi, M.; Ramakrishnan, E.; Deka, S.; Parasar, D.P. Bacteria as a source of biopigments and their potential applications. J. Microbiol. Methods 2024, 219, 106907. [Google Scholar] [CrossRef]
- Geyik, M.S.; Efe, D.; Gormez, A. Identification of Bacteria Producing Red Pigments and Their Application in the Textile Industry. Arab. J. Sci. Eng. 2024, 50, 1–10. [Google Scholar] [CrossRef]
- Mohana, D.C.; Thippeswamy, S.; Abhishek, R.U. Antioxidant, antibacterial, and ultraviolet-protective properties of carotenoids isolated from Micrococcus spp. Radiat. Prot. Environ. 2013, 36, 168. [Google Scholar] [CrossRef]
- Qayyum, S.; Basharat, S.; Mian, A.H.; Qayum, S.; Ali, M.; Changsheng, P.; Shahzad, M.; Sultan, F. Isolation, identification and antibacterial study of pigmented bacteria. Appl. Nanosci. 2020, 10, 4495–4503. [Google Scholar] [CrossRef]
- Dawoud, T.M.; Alharbi, N.S.; Theruvinthalakal, A.M.; Thekkangil, A.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Sankar, K.; Innasimuthu, G.M.; Alanzi, K.F.; et al. Characterization and antifungal activity of the yellow pigment produced by a Bacillus sp. DBS4 isolated from the lichen Dirinaria agealita. Saudi J. Biol. Sci. 2020, 27, 1403–1411. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process. Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Metwally, R.A.; El Sikaily, A.; El-Sersy, N.A.; Ghozlan, H.A.; Sabry, S.A. Antimicrobial activity of textile fabrics dyed with prodigiosin pigment extracted from marine Serratia rubidaea RAM_Alex bacteria. Egypt. J. Aquat. Res. 2021, 47, 301–305. [Google Scholar] [CrossRef]
- Choksi, J.; Vora, J.; Shrivastava, N. Bioactive Pigments from Isolated Bacteria and Its Antibacterial, Antioxidant and Sun Protective Application Useful for Cosmetic Products. Indian J. Microbiol. 2020, 60, 379–382. [Google Scholar] [CrossRef]
- Gonçalves, T.; Vasconcelos, U. Colour me blue: The history and the biotechnological potential of pyocyanin. Molecules 2021, 26, 927. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Azmi, W. An overview on biosynthesis and applications of extracellular pyocyanin pigment and its role in Pseudomonas aeruginosa pathogenesis. Ann. Phytomedicine Int. J. 2019, 8, 28–42. [Google Scholar] [CrossRef]
- Managò, A.; Becker, K.A.; Carpinteiro, A.; Wilker, B.; Soddemann, M.; Seitz, A.P.; Edwards, M.J.; Grassmé, H.; Szabò, I.; Gulbins, E. Pseudomonas aeruginosa Pyocyanin Induces Neutrophil Death via Mitochondrial Reactive Oxygen Species and Mitochondrial Acid Sphingomyelinase. Antioxid. Redox Signal. 2015, 22, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; McDermott, C.; Anoopkumar-Dukie, S.; McFarland, A.J.; Forbes, A.; Perkins, A.V.; Davey, A.K.; Chess-Williams, R.; Kiefel, M.J.; Arora, D.; et al. Cellular Effects of Pyocyanin, a Secreted Virulence Factor of Pseudomonas aeruginosa. Toxins 2016, 8, 236. [Google Scholar] [CrossRef]
- Liang, M.-H.; Zhu, J.; Jiang, J.-G. Carotenoids biosynthesis and cleavage related genes from bacteria to plants. Crit. Rev. Food Sci. Nutr. 2018, 58, 2314–2333. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.; Mitra, M.; Shah, F.; Tirkey, S.R.; Mishra, S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J. Funct. Foods 2020, 67, 103867. [Google Scholar] [CrossRef]
- Wang, H.; Yang, A.; Zhang, G.; Ma, B.; Meng, F.; Peng, M.; Wang, H. Enhancement of carotenoid and bacteriochlorophyll by high salinity stress in photosynthetic bacteria. Int. Biodeterior. Biodegrad. 2017, 121, 91–96. [Google Scholar] [CrossRef]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C.-W. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef]
- Kusmita, L.; Tatsa, Y.A.; Franyoto, Y.D.; Sabdono, A.; Trianto, A.; Radjasa, O.K. Antibacterial Activity of Carotenoid from Bacterial Symbiont Virgibacillus salarius Strain 19.PP.Sc.1.6 against MDR E. coli and MRSA. Egypt. J. Aquat. Biol. Fish. 2021, 25, 147–157. Available online: https://ejabf.journals.ekb.eg/ (accessed on 3 May 2025). [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef]
- Pavan, M.E.; López, N.I.; Pettinari, M.J. Melanin biosynthesis in bacteria, regulation and production perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 1357–1370. [Google Scholar] [CrossRef]
- Di Salvo, E.; Vecchio, G.L.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent Advances and Progress on Melanin: From Source to Application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef]
- Choi, K.-Y. Bioprocess of Microbial Melanin Production and Isolation. Front. Bioeng. Biotechnol. 2021, 9, 765110. [Google Scholar] [CrossRef]
- Eliato, T.R.; Smith, J.T.; Tian, Z.; Kim, E.-S.; Hwang, W.; Andam, C.P.; Kim, Y.J. Melanin pigments extracted from horsehair as antibacterial agents. J. Mater. Chem. B 2020, 9, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.; Tran, N.T.H.; Pham, V.N.T.; Van-Thi, N.-D.; Tran, H.T.M. Anti-ultraviolet, antibacterial, and biofilm eradication activities against Cutibacterium acnes of melanins and melanin derivatives from Daedaleopsis tricolor and Fomes fomentarius. Front. Microbiol. 2024, 14, 1305778. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mordini, D.; Montalti, M. Melanin as a Photothermal Agent in Antimicrobial Systems. Int. J. Mol. Sci. 2024, 25, 8975. [Google Scholar] [CrossRef]
- Sahoo, S.R.; Pradhan, A.K. Prodigiosin: An In-depth Exploration of a Bioactive Compound from Serratia sp. Curr. Bioact. Compd. 2024, 20. [Google Scholar] [CrossRef]
- Couturier, M.; Bhalara, H.D.; Chawrai, S.R.; Monson, R.; Williamson, N.R.; Salmond, G.P.C.; Leeper, F.J. Substrate Flexibility of the Flavin-Dependent Dihydropyrrole Oxidases PigB and HapB Involved in Antibiotic Prodigiosin Biosynthesis. ChemBioChem 2019, 21, 523–530. [Google Scholar] [CrossRef]
- Yip, C.-H.; Yarkoni, O.; Ajioka, J.; Wan, K.-L.; Nathan, S. Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl. Microbiol. Biotechnol. 2019, 103, 1667–1680. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Koli, S.H.; Hallsworth, J.E.; Patil, S.V. Antimicrobial activity of prodigiosin is attributable to plasma-membrane damage. Nat. Prod. Res. 2017, 31, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Danevčič, T.; Vezjak, M.B.; Zorec, M.; Stopar, D. Prodigiosin—A Multifaceted Escherichia coli Antimicrobial Agent. PLoS ONE 2016, 11, e0162412. [Google Scholar] [CrossRef] [PubMed]
- Shouman, H.; Said, H.S.; Kenawy, H.I.; Hassan, R. Molecular and biological characterization of pyocyanin from clinical and environmental Pseudomonas aeruginosa. Microb. Cell Factories 2023, 22, 166. [Google Scholar] [CrossRef]
- Hamad, M.N.F.; Marrez, D.A.; El-Sherbieny, S.M.R. Toxicity Evaluation and Antimicrobial Activity of Purified Pyocyanin from Pseudomonas aeruginosa. Biointerface Res. Appl. Chem. 2020, 10, 6974–6990. [Google Scholar] [CrossRef]
- Yip, C.-H.; Mahalingam, S.; Wan, K.-L.; Nathan, S. Prodigiosin inhibits bacterial growth and virulence factors as a potential physiological response to interspecies competition. PLoS ONE 2021, 16, e0253445. [Google Scholar] [CrossRef]
- Rajendran, P.; Somasundaram, P.; Dufossé, L. Microbial pigments: Eco-friendly extraction techniques and some industrial applications. J. Mol. Struct. 2023, 1290, 135958. [Google Scholar] [CrossRef]
- Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R.G.; Koubaa, M. Mechanical cell disruption technologies for the extraction of dyes and pigments from microorganisms: A review. Fermentation 2021, 7, 36. [Google Scholar] [CrossRef]
- Agarwal, H.; Bajpai, S.; Mishra, A.; Kohli, I.; Varma, A.; Fouillaud, M.; Dufossé, L.; Joshi, N.C. Bacterial Pigments and Their Multifaceted Roles in Contemporary Biotechnology and Pharmacological Applications. Microorganisms 2023, 11, 614. [Google Scholar] [CrossRef]
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of bead milling parameters for the cell disruption of microalgae: Process modeling and application to Porphyridium cruentum and Nannochloropsis oculata. Bioresour. Technol. 2015, 196, 339–346. [Google Scholar] [CrossRef]
- Zinkoné, T.R.; Gifuni, I.; Lavenant, L.; Pruvost, J.; Marchal, L. Bead milling disruption kinetics of microalgae: Process modeling, optimization and application to biomolecules recovery from Chlorella sorokiniana. Bioresour. Technol. 2018, 267, 458–465. [Google Scholar] [CrossRef]
- Safi, C.; Camy, S.; Frances, C.; Varela, M.M.; Badia, E.C.; Pontalier, P.-Y.; Vaca-Garcia, C. Extraction of lipids and pigments of Chlorella vulgaris by supercritical carbon dioxide: Influence of bead milling on extraction performance. J. Appl. Phycol. 2014, 26, 1711–1718. [Google Scholar] [CrossRef]
- Zhang, R.; Grimi, N.; Marchal, L.; Lebovka, N.; Vorobiev, E. Effect of ultrasonication, high pressure homogenization and their combination on efficiency of extraction of bio-molecules from microalgae Parachlorella kessleri. Algal Res. 2019, 40, 101524. [Google Scholar] [CrossRef]
- Kumar, G.; Upadhyay, S.; Yadav, D.K.; Malakar, S.; Dhurve, P.; Suri, S. Application of ultrasound technology for extraction of color pigments from plant sources and their potential bio-functional properties: A review. J. Food Process. Eng. 2023, 46, e14238. [Google Scholar] [CrossRef]
- Sun, S.-Q.; Wang, Y.-J.; Xu, W.; Zhu, C.-J.; Liu, X.-X. Optimizing ultrasound-assisted extraction of prodigiosin by response surface methodology. Prep. Biochem. Biotechnol. 2014, 45, 101–108. [Google Scholar] [CrossRef]
- López, G.-D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef]
- Gu, Y.; Jérôme, F. Bio-based solvents: An emerging generation of fluids for the design of eco-efficient processes in catalysis and organic chemistry. Chem. Soc. Rev. 2013, 42, 9550–9570. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of pigments from microalgae and cyanobacteria-a review on current methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Poddar, K.; Padhan, B.; Sarkar, D.; Sarkar, A. Purification and optimization of pink pigment produced by newly isolated bacterial strain Enterobacter sp. SN Appl. Sci. 2021, 3, 105. [Google Scholar] [CrossRef]
- Padhan, B.; Poddar, K.; Sarkar, D.; Sarkar, A. Production, purification, and process optimization of intracellular pigment from novel psychrotolerant Paenibacillus sp. BPW19. Biotechnol. Rep. 2021, 29, e00592. [Google Scholar] [CrossRef]
- Ahmad, T.; Masoodi, F.A.; Rather, S.A.; Wani, S.M.; Gull, A. Supercritical Fluid Extraction: A Review. Journal of Biological and chemical Chronicles. J. Biol. Chem. Chronicles 2019, 5, 114–122. [Google Scholar] [CrossRef]
- Esquivel-Hernández, D.A.; Rodríguez-Rodríguez, J.; Cuéllar-Bermúdez, S.P.; García-Pérez, J.S.; Mancera-Andrade, E.I.; Núñez-Echevarría, J.E.; Ontiveros-Valencia, A.; Rostro-Alanis, M.; García-García, R.M.; Torres, J.A.; et al. Effect of supercritical carbon dioxide extraction parameters on the biological activities and metabolites present in extracts from Arthrospira platensis. Mar. Drugs 2017, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liu, W.; Wang, H.X.; Lv, C.H. The extraction of β-carotene from red yeast cells by supercritical carbon dioxide technique. Adv. Mater. Res. 2012, 554–556, 949–952. [Google Scholar] [CrossRef]
- Di Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Choi, S.-K.; Kim, J.-H.; Park, Y.-S.; Kim, Y.-J.; Chang, H.-I. An Efficient Method for the Extraction of Astaxanthin from the Red Yeast Xanthophyllomyces dendrorhous. J. Microbology Biotechnol. 2007, 17, 847–852. [Google Scholar]
- Łubek-Nguyen, A.; Ziemichód, W.; Olech, M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl. Sci. 2022, 12, 3232. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; Filho, J.G.d.O.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds From Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Gordon, M.H.; Niranjan, K. Aqueous enzyme assisted oil extraction from oilseeds andemulsion de-emulsifying methods: A review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- Alavarsa-Cascales, D.; Aliaño-González, M.J.; Palma, M.; Barbero, G.F.; Carrera, C. Optimization of an Enzyme-Assisted Extraction Method for the Anthocyanins Present in Açai (Euterpe oleracea Mart. Agronomy 2022, 12, 2327. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Silva, F.A.E.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past, Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- Syahmina, A.; Usuki, T. Ionic Liquid-Assisted Extraction of Essential Oils from Thujopsis dolobrata (Hiba). ACS Omega 2020, 5, 29618–29622. [Google Scholar] [CrossRef] [PubMed]
- Kiyonga, A.N.; Park, G.H.; Kim, H.S.; Suh, Y.-G.; Kim, T.K.; Jung, K. An efficient ionic liquid-mediated extraction and enrichment of isoimperatorin from Ostericum Ostericum koreanum (Max.) Kitagawa. Molecules 2021, 26, 6555. [Google Scholar] [CrossRef]
- Mesquita, L.M.d.S.; Martins, M.; Pisani, L.P.; Ventura, S.P.M.; de Rosso, V.V. Insights on the use of alternative solvents and technologies to recover bio-based food pigments. Compr. Rev. Food Sci. Food Saf. 2020, 20, 787–818. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K. Navigating the Pros and Cons: Advantages and Disadvantages of Ionic Liquids. Polym. Sci. 2023, 8, 19. [Google Scholar] [CrossRef]
- Ghadge, V.; Kumar, P.; Maity, T.K.; Prasad, K.; Shinde, P.B. Facile Alternative Sustainable Process for the Selective Extraction of Microbial Melanin. ACS Sustain. Chem. Eng. 2022, 10, 2681–2688. [Google Scholar] [CrossRef]
- Park, H.; Park, S.; Yang, Y.-H.; Choi, K.-Y. Microbial synthesis of violacein pigment and its potential applications. Crit. Rev. Biotechnol. 2021, 41, 879–901. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Giaroni, C.; Baj, A.; Bolognese, F. Bacterial pigments: A colorful palette reservoir for biotechnological applications. Biotechnol. Appl. Biochem. 2022, 69, 981–1001. [Google Scholar] [CrossRef]
- Kramar, A.; Kostic, M.M. Bacterial Secondary Metabolites as Biopigments for Textile Dyeing. Textiles 2022, 2, 252–264. [Google Scholar] [CrossRef]
- Clark, M. Fundamental principles of dyeing. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Volume 1, ch. 1; p. 126. [Google Scholar]
- Elmaaty, T.A.; Sayed-Ahmed, K.; Elsisi, H.; Magdi, M. Optimization of Extraction of Natural Antimicrobial Pigments Using Supercritical Fluids: A Review. Processes 2022, 10, 2111. [Google Scholar] [CrossRef]
- Pasdaran, A.; Zare, M.; Hamedi, A. A Review of the Chemistry and Biological Activities of Natural Colorants, Dyes, and Pigments: Challenges, and Opportunities for Food, Cosmetics, and Pharmaceutical Application. Chem. Biodivers. 2023, 20. [Google Scholar] [CrossRef]
- Alper, H.; Miyaoku, K.; Stephanopoulos, G. Characterization of lycopene-overproducing E. coli strains in high cell density fermentations. Appl. Microbiol. Biotechnol. 2006, 72, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Dieser, M.; Greenwood, M.; Foreman, C.M. Carotenoid pigmentation in Antarctic heterotrophic bacteria as a strategy to withstand environmental stresses. Arctic, Antarct. Alp. Res. 2010, 42, 396–405. [Google Scholar] [CrossRef]
- Arulselvi, P.I.; Umamaheswari, S. Screening of Yellow Pigment Producing Bacterial Isolates from Various Eco-climatic Areas and Analysis of the Carotenoid Produced by the Isolate. J. Food Process. Technol. 2013, 5, 292. [Google Scholar] [CrossRef]
- Kim, I.; Chhetri, G.; Kim, J.; Kang, M.; Seo, T. Lewinella aurantiaca sp. nov., a carotenoid pigment-producing bacterium isolated from surface seawater. Int. J. Syst. Evol. Microbiol. 2020, 70, 6180–6187. [Google Scholar] [CrossRef]
- Majumdar, S.; Priyadarshinee, R.; Kumar, A.; Mandal, D.T. Dasgupta Mandal. Exploring Planococcus sp. TRC1, a bacterial isolate, for carotenoid pigment production and detoxification of paper mill effluent in immobilized fluidized bed reactor. J. Clean. Prod. 2019, 211, 1389–1402. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Ahmad, W.Y.W.; Zakaria, Z.A.; Yusof, N.Z. Application of Bacterial Pigments as Colorant. In Application of Bacterial Pigments as Colorant: The Malaysian Perspective; Springer: Berlin/Heidelberg, Germany, 2012; pp. 57–74. [Google Scholar] [CrossRef]
- Bisht, G.; Srivastava, S.; Kulshreshtha, R.; Sourirajan, A.; Baumler, D.J.; Dev, K. Applications of red pigments from psychrophilic Rhodonellum psychrophilum GL8 in health, food and antimicrobial finishes on textiles. Process. Biochem. 2020, 94, 15–29. [Google Scholar] [CrossRef]
- Ki, I.; Thirumalai, M.A. Isolation and Characterization of Pigment Producing Bacteria and Their Possible Use in Textile Industry. 2021. Available online: https://sciensage.info/index.php/JASR/article/view/617 (accessed on 2 July 2024).
- Gurkok, S. A novel carotenoid from Metabacillus idriensis LipT27: Production, extraction, partial characterization, biological activities and use in textile dyeing. Arch. Microbiol. 2022, 204, 296. [Google Scholar] [CrossRef] [PubMed]
- Tran-Ly, A.N.; Reyes, C.; Schwarze, F.W.M.R.; Ribera, J. Microbial production of melanin and its various applications. World J. Microbiol. Biotechnol. 2020, 36, 170. [Google Scholar] [CrossRef]
- Singh, S.; Nimse, S.B.; Mathew, D.E.; Dhimmar, A.; Sahastrabudhe, H.; Gajjar, A.; Ghadge, V.A.; Kumar, P.; Shinde, P.B. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol. Adv. 2021, 53, 107773. [Google Scholar] [CrossRef]
- Amal, A.M.; Abeer, K.A.; Samia, H.M.; Nadia, A.H.E.-N. Selection of Pigment (Melanin) Production in Streptomyces and Their Application in Printing and Dyeing of Wool Fabrics. 2011. Available online: www.isca.in (accessed on 2 July 2024).
- Ahn, S.-Y.; Jang, S.; Sudheer, P.D.V.N.; Choi, K.-Y. Microbial Production of Melanin Pigments from Caffeic Acid and L-Tyrosine Using Streptomyces glaucescens and FCS-ECH-Expressing Escherichia coli. Int. J. Mol. Sci. 2021, 22, 2413. [Google Scholar] [CrossRef]
- Tsukamot, T.; Yasuj, H.; Hata, T.; Hayasaka, S.; Kato, H. Isolation of Bacteria Producing Bluish-Purple Pigment and Use for Dyeing. JARQ 2000, 34, 131–140. [Google Scholar]
- Cheng, K.-C.; Hsiao, H.-C.; Hou, Y.-C.; Hsieh, C.-W.; Hsu, H.-Y.; Chen, H.-Y.; Lin, S.-P. Improvement in Violacein Production by Utilizing Formic Acid to Induce Quorum Sensing in Chromobacterium violaceum. Antioxidants 2022, 11, 849. [Google Scholar] [CrossRef]
- Gohil, N.; Bhattacharjee, G.; Gayke, M.; Narode, H.; Alzahrani, K.J.; Singh, V. Enhanced production of violacein by Chromobacterium violaceum using agro-industrial waste soybean meal. J. Appl. Microbiol. 2021, 132, 1121–1133. [Google Scholar] [CrossRef]
- Kanelli, M.; Mandic, M.; Kalakona, M.; Vasilakos, S.; Kekos, D.; Nikodinovic-Runic, J.; Topakas, E. Microbial Production of Violacein and Process Optimization for Dyeing Polyamide Fabrics With Acquired Antimicrobial Properties. Front. Microbiol. 2018, 9, 1495. [Google Scholar] [CrossRef]
- Venil, C.K.; Yusof, N.Z.; Aruldass, C.A.; Ahmad, W.-A. Application of violet pigment from Chromobacterium violaceum UTM5 in textile dyeing. Biologia 2016, 71, 121–127. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Kumaran, S.; Kandeel, M.; Muralitharan, G.; Silviya, J.; Adhimoolam, G.L.; Panagal, M.; Pugazhvendan, S.R.; Suresh, G.; Aruni, A.W.; et al. Applications of Natural Violet Pigments from Halophilic Chromobacterium violaceum PDF23 for Textile Dyeing with Antimicrobial and Antioxidant Potentials. J. Nanomater. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Khaksar, F.; Rigi, G.; Mirdamadian, S.H. Creation of a violacein pigment hybrid with silver and titanium dioxide nanoparticles to produce multifunctional textiles with antimicrobial properties. Nanomed. Res. J. 2021, 6, 60–72. [Google Scholar] [CrossRef]
- Venil, C.K.; Lakshmanaperumalsamy, P. An Insightful Overview on Microbial Pigment, Prodigiosin. Electron. J. Biol. 2009, 5, 49–61. [Google Scholar]
- Darshan, N.; Manonmani, H.K. Prodigiosin and its potential applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef]
- Alihosseini, F.; Ju, K.; Lango, J.; Hammock, B.D.; Sun, G. Antibacterial colorants: Characterization of prodiginines and their applications on textile materials. Biotechnol. Prog. 2008, 24, 742–747. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Applications of Prodigiosin Extracted from Marine Red Pigmented Bacteria Zooshikella sp. and Actinomycete Streptomyces sp. Microorganisms 2020, 8, 556. [Google Scholar] [CrossRef]
- Chauhan, K.; Dalsaniya, P.; Pathak, H. Optimization of Prodigiosin-type Biochrome Production and Effect of Mordants on Textile Dyeing to Improve Dye Fastness. Fibers Polym. 2015, 16, 802–808. [Google Scholar] [CrossRef]
- Mohammed, S.J.; Luti, K.J.K. A kinetic model for prodigiosin production by Serratia marcescens as a bio-colorant in bioreactor. In AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2020. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Velmurugan, P.; Malathi, M.; Lakshmanaperumalsamy, P. Extraction and Application of Pigment from Serratia marcescens SB08, an Insect Enteric Gut Bacterium, for Textile Dyeing. Textiles 2021, 1, 21–36. [Google Scholar] [CrossRef]
- Shete, P.; Sonawane, S.; Dhundhale, V.; Mulchandani, S. Isolation of Prodigiosin Producing Bacteria from Marine Ecosystem and Exploration of its Fabric Dyeing Potential. Int. J. Aquat. Sci. 2021, 12, 5010–5020. [Google Scholar]
- Ren, Y.; Fu, R.; Fang, K.; Xie, R.; Hao, L.; Chen, W.; Shi, Z. Clean dyeing of acrylic fabric by sustainable red bacterial pigment based on nano-suspension system. J. Clean. Prod. 2021, 281, 125295. [Google Scholar] [CrossRef]
- Ren, Y.; Gong, J.; Fu, R.; Zhang, J.; Fang, K.; Liu, X. Antibacterial dyeing of silk with prodigiosins suspention produced by liquid fermentation. J. Clean. Prod. 2018, 201, 648–656. [Google Scholar] [CrossRef]
- Azman, A.-S.; Mawang, C.-I.; Abubakar, S. Bacterial Pigments: The Bioactivities and as an Alternative for Therapeutic Applications. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef]
- Wu, J.; Fu, R.; Xiao, M.; Zheng, Q.; Wu, L.; Fang, K.; Ren, Y. Synergetic construction of color and multifunction for sustainable lyocell fabric by microbial nano pigment. Chem. Eng. J. 2023, 481, 148453. [Google Scholar] [CrossRef]
- Sundararajan, P.; Ramasamy, S.P. Development of sustainable, eco-friendly antimicrobial finishing of cotton fabric using prodigiosin of Serratia marcescens SP1. Prog. Org. Coatings 2024, 188, 108216. [Google Scholar] [CrossRef]
- Chaturwedi, S.B.; Mainali, S.; Chaudhary, R. Antibacterial Activity of Pigment Extracted from Pigment Producing Bacteria. BMC Res. Notes 2023. preprint. [Google Scholar] [CrossRef]
- Sengupta, S.; Bhowal, J. Characterization of a blue-green pigment extracted from Pseudomonas aeruginosa and its application in textile and paper dyeing. Environ. Sci. Pollut. Res. 2022, 30, 30343–30357. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ye, K.; Zhu, X.; Zhang, H.; Si, R.; Chen, J.; Chen, Z.; Song, K.; Yu, Z.; Han, B. an antimicrobial depsipeptide from marine-derived streptomyces cyaneofuscatus applied as a good natural dye for silk fabric. Mar. Drugs 2021, 20, 16. [Google Scholar] [CrossRef]
- Al-Tekreeti, A.R.A.; Luti, K.J.K. Utilization of an eco-friendly bioactive yellow pigment from Streptomyces thinghirensis AF7 for making colored antimicrobial fabrics. Iraqi J. Sci. 2023, 64, 4415–4426. [Google Scholar] [CrossRef]
- Pallath, N.; Johnson, S.; Paul, E.; Raslana, T.A. Prodigiosin pigment from Serratia marcescens MBM-17 from facial acne as antimicrobial agent. Environ. Qual. Manag. 2023, 33, 275–283. [Google Scholar] [CrossRef]
- Ibrahim, G.S.; Abdelhamid, S.A.; Elmansy, E.A.; Asker, M.M.; El Shall, F.N. Red pigment from isolated Serratia marcescens SEM: Structure, antimicrobial and antioxidant activity. Biocatal. Agric. Biotechnol. 2023, 54, 102932. [Google Scholar] [CrossRef]
- Janković, V.; Marković, D.; Nikodinovic-Runic, J.; Radetić, M.; Ilic-Tomic, T. Eco-friendly dyeing of polyamide and polyamide-elastane knits with living bacterial cultures of two Streptomyces sp. strains. World J. Microbiol. Biotechnol. 2022, 39, 32. [Google Scholar] [CrossRef]
- Gunasekaran, R.; Janarthanam, H.; Selvaraj, V. Biomedical exploration of Bacterial pigments extracted from Staphylococcus sp. and Pseudomonas sp. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 4060–4068. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Kolahreez, D.; Ramakrishna, S.; Williams, D. Key terminology in biomaterials and biocompatibility. Curr. Opin. Biomed. Eng. 2019, 10, 45–50. [Google Scholar] [CrossRef]
- Fatima, M.; Anuradha, K. Isolation, Characterization, and Optimization Studies of Bacterial Pigments. J. Pure Appl. Microbiol. 2022, 16, 1039–1048. [Google Scholar] [CrossRef]
- Widheden, J.; Ringström, E. 2.2—Life Cycle Assessment. In Handbook for Cleaning/Decontamination of Surfaces; Johansson, I., Somasundaran, P., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 695–720. [Google Scholar] [CrossRef]
- Jaiswal, A.; Koli, D.; Kumar, A.; Kumar, S.; Sagar, S. Pigments analysis of cyanobacterial strains. Int. J. Chem. Stud. 2018, 6, 1248–1251. [Google Scholar]
- Martins, M.; Mesquita, L.M.d.S.; Vaz, B.M.; Dias, A.C.; Torres-Acosta, M.A.; Quéguineur, B.; Coutinho, J.A.; Ventura, S.P. Extraction and Fractionation of Pigments from Saccharina latissima (Linnaeus, 2006) Using an Ionic Liquid + Oil + Water System. ACS Sustain. Chem. Eng. 2021, 9, 6599–6612. [Google Scholar] [CrossRef]
- Zlaugotne, B.; Sanchez, F.A.D.; Pubule, J.; Blumberga, D. Life Cycle Impact Assessment of Microalgae and Synthetic Astaxanthin Pigments. Environ. Clim. Technol. 2023, 27, 233–242. [Google Scholar] [CrossRef]
- Aldaghi, S.A.; Ubais, R.; Schmitt, I.; Wendisch, V.F.; Costamagna, M.; Perucca, M. Life Cycle Assessment of Bacterial, Algal, and Synthetic Approaches for Astaxanthin Production at a Laboratory Scale: Comparative Environmental Analysis and Sensitivity of Energy Sources. Processes 2023, 11, 2911. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Devi, P.R. Bacterial Pigments: Sustainable Compounds With Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 1–17. [Google Scholar] [CrossRef]
- Pailliè-Jiménez, M.E.; Stincone, P.; Brandelli, A. Natural Pigments of Microbial Origin. Front. Sustain. Food Syst. 2020, 4, 590439. [Google Scholar] [CrossRef]
- Rao, M.P.N.; Xiao, M.; Li, W.-J. Fungal and bacterial pigments: Secondary metabolites with wide applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Barreto, J.V.d.O.; Casanova, L.M.; Junior, A.N.; Reis-Mansur, M.C.P.P.; Vermelho, A.B. Microbial Pigments: Major Groups and Industrial Applications. Microorganisms 2023, 11, 2920. [Google Scholar] [CrossRef]
- Misko, G.G. Colorants in Food Packaging: FDA Safety Requirements|Food Safety. Available online: https://www.food-safety.com/articles/5010-colorants-in-food-packaging-fda-safety-requirements (accessed on 29 April 2025).
- Bandyopadhyay, K.; Dutta, P.; Ganguly, S.; Pramanik, A. A Review on Microbial-Pigment: A Good Source of Biocolour. Pharma Innov. J. 2019, 8, 416–420. [Google Scholar]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef]
- Salas-Villalobos, U.A.; Santacruz, A.; Castillo-Reyna, J.; Aguilar, O. An in-situ approach based in mineral oil to decrease end-product inhibition in prodigiosin production by Serratia marcescens. Food Bioprod. Process. 2022, 135, 217–226. [Google Scholar] [CrossRef]
- Kulkarni, V.M.; Dixit, A.S.; Patwardhan, A.V.; Bajwa, A.S. A Different Approach to Augment Pigment Production and its Extraction from Kocuria flava by Using Ultrasound Technique. J. Biol. Act. Prod. Nat. 2018, 8, 34–42. [Google Scholar] [CrossRef]
- Celedón, R.S.; Díaz, L.B. Natural pigments of bacterial origin and their possible biomedical applications. Microorganisms 2021, 9, 739. [Google Scholar] [CrossRef] [PubMed]
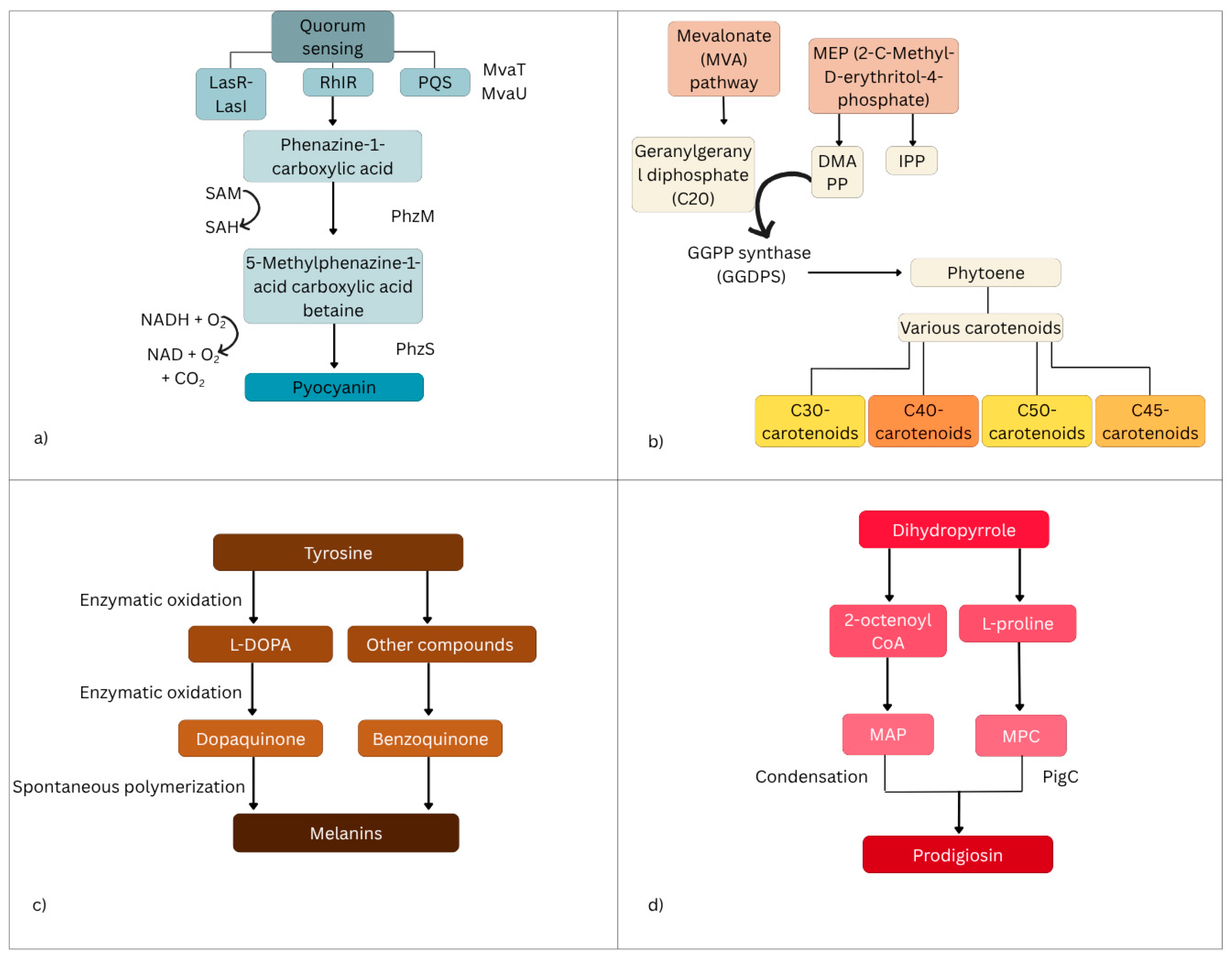

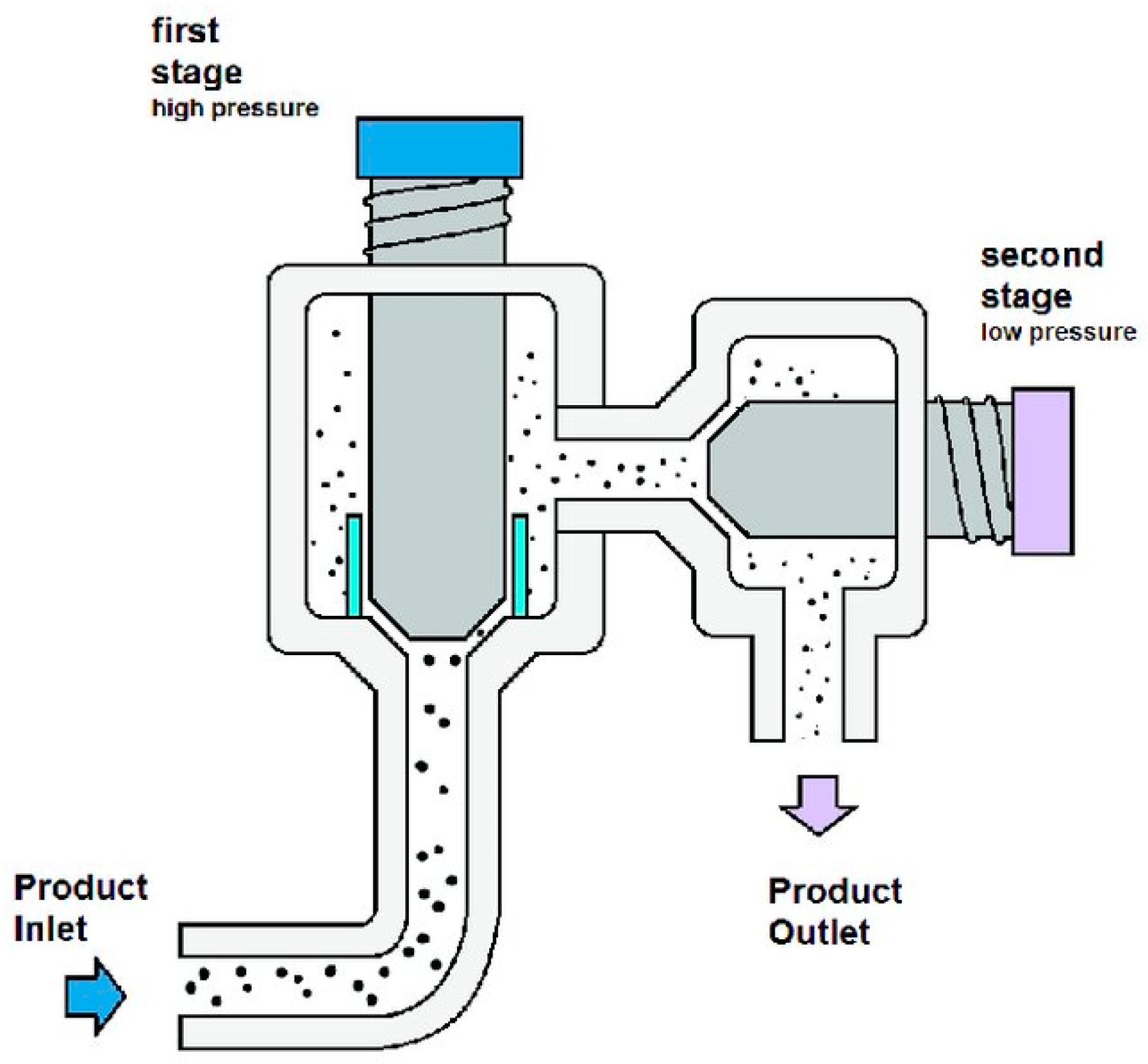

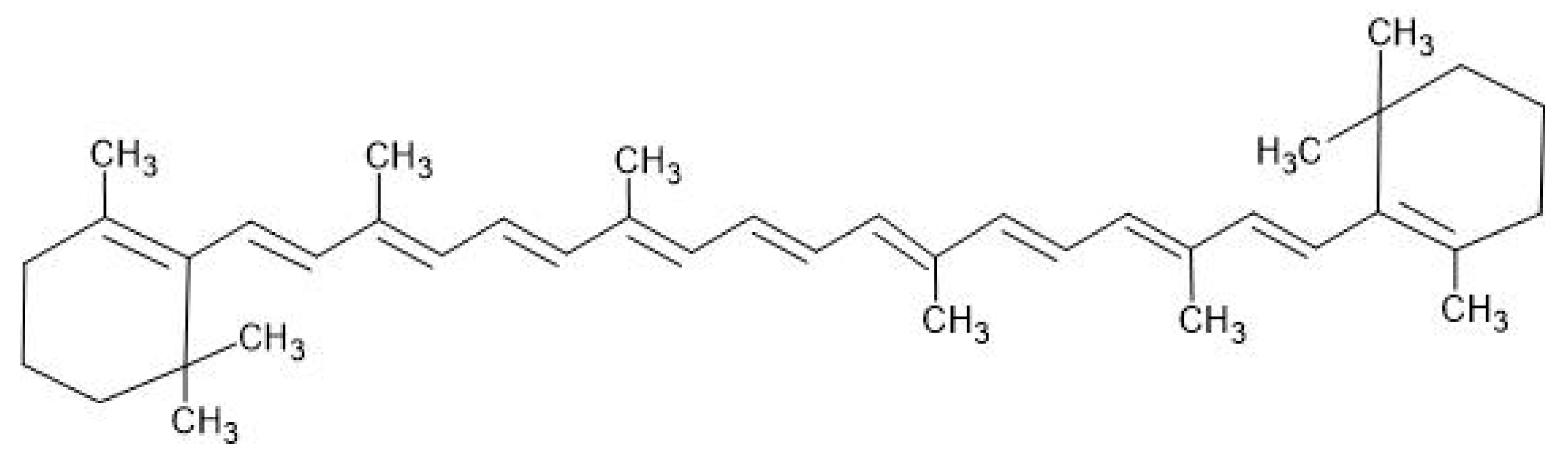

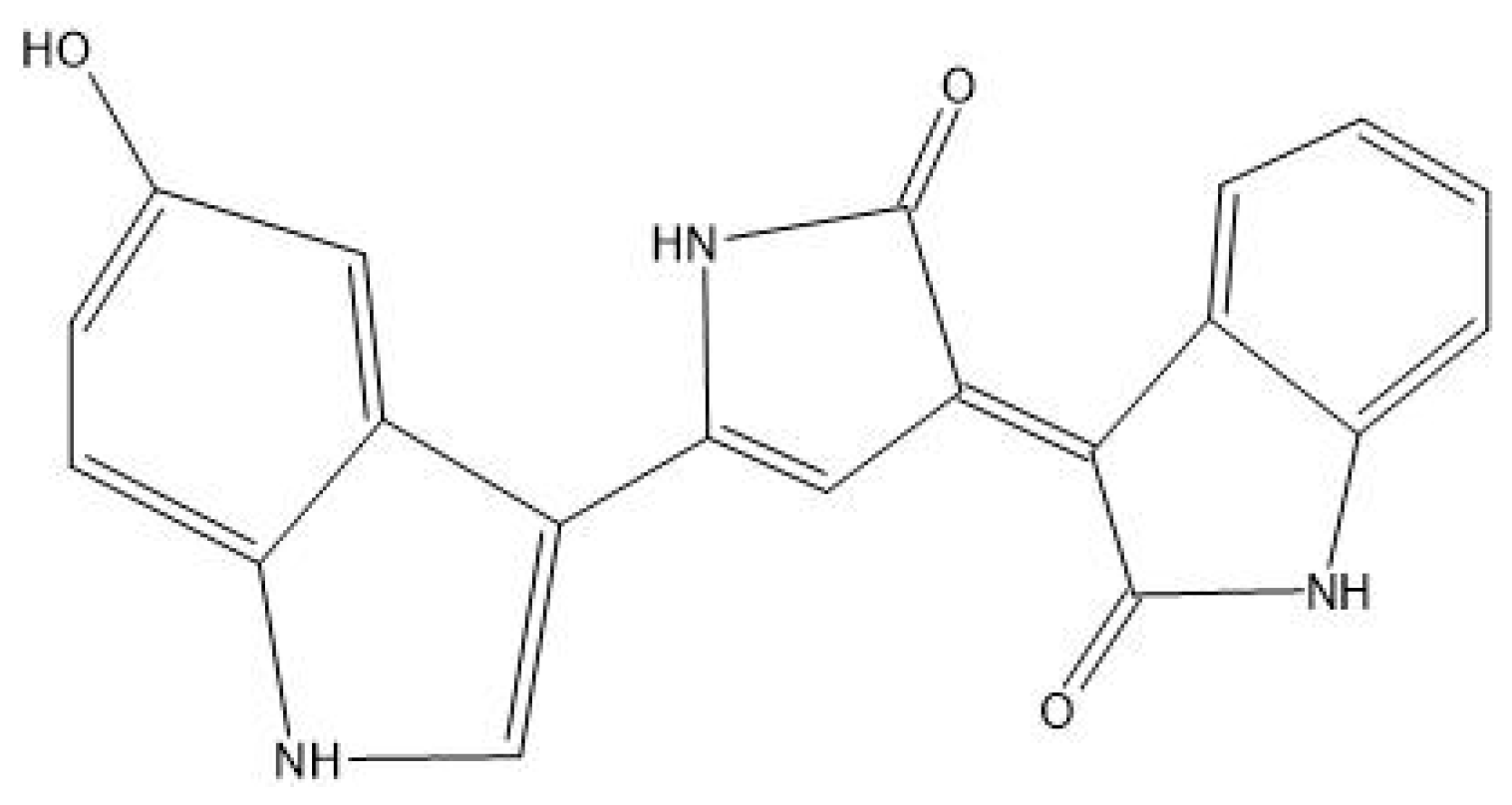

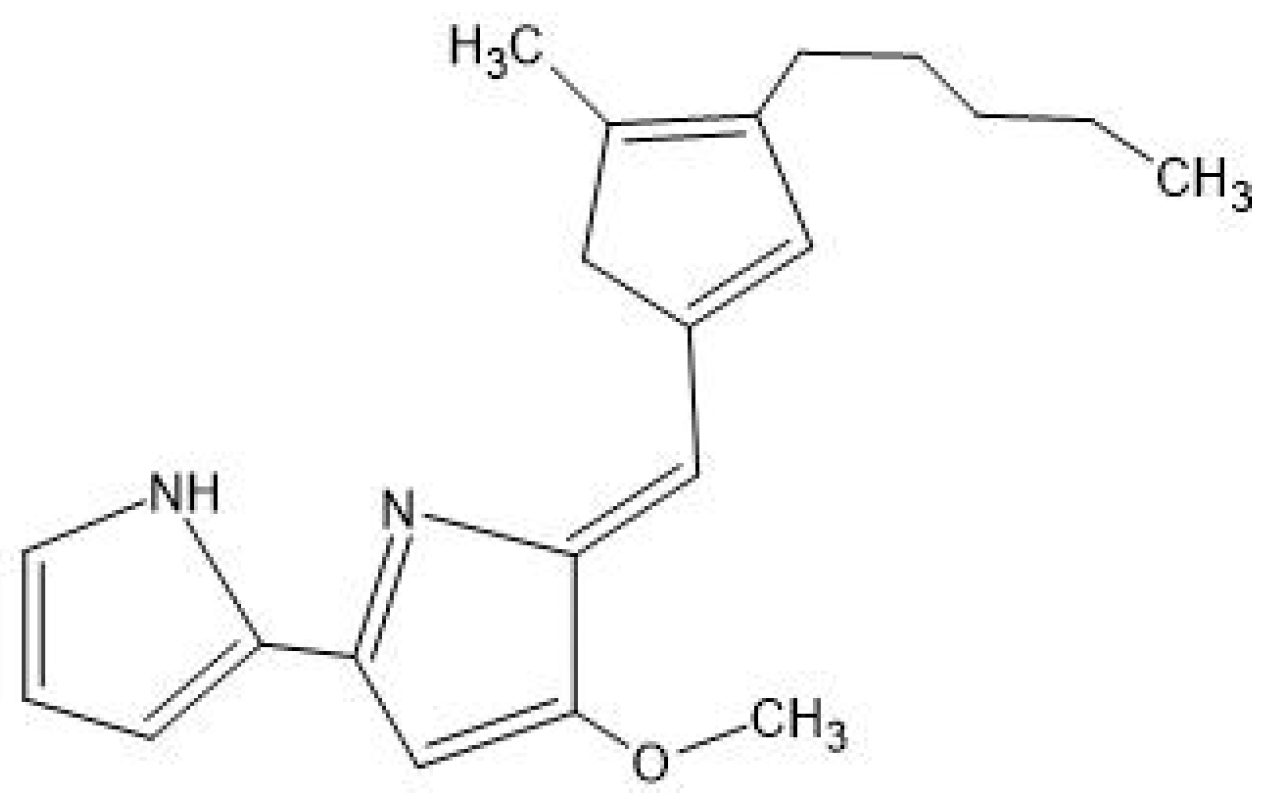
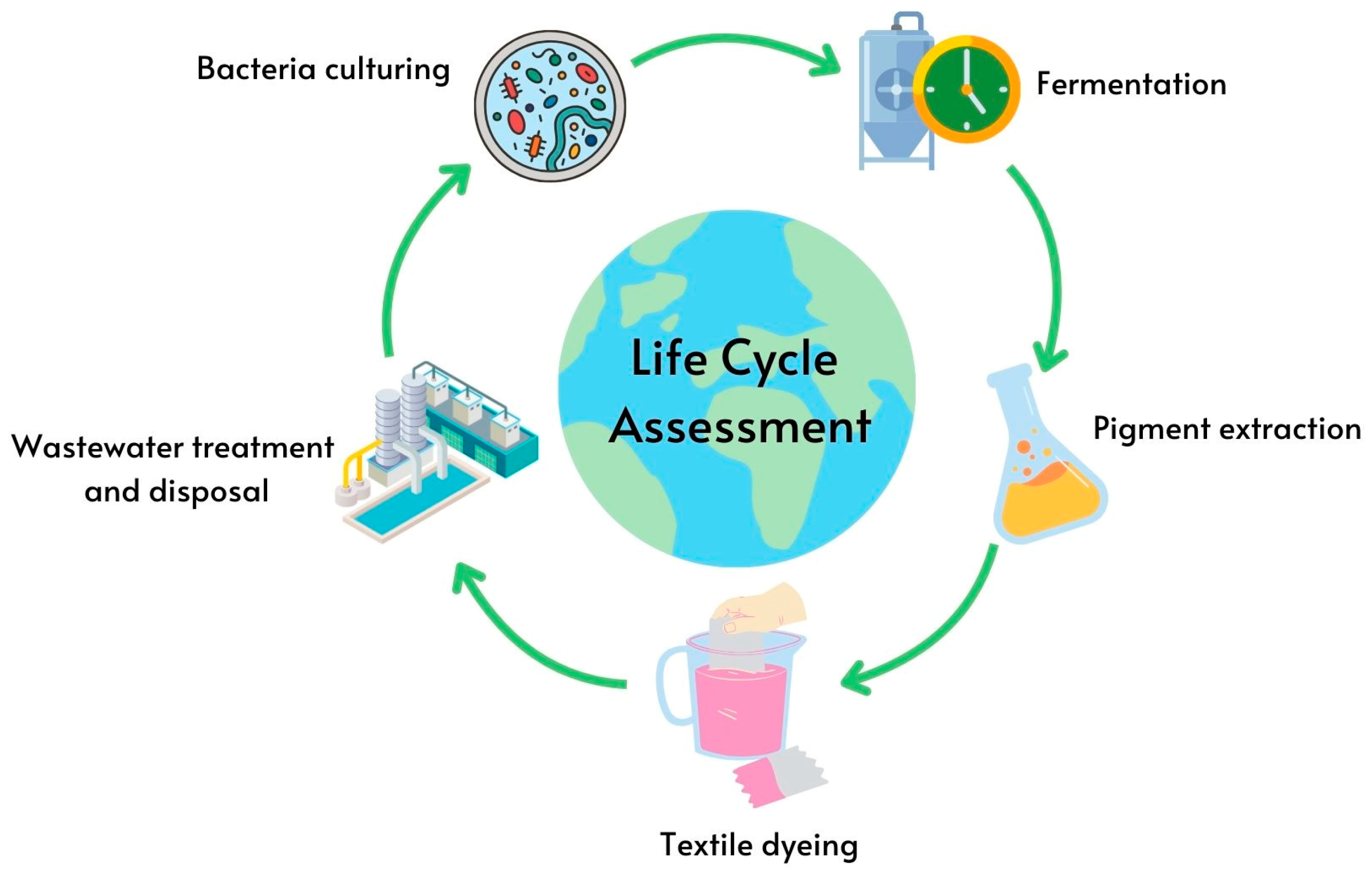
| Pigment | Antibacterial Mechanism | Effective Against | References |
|---|---|---|---|
| Pyocyanin | ROS generation | Gram-positive | [14,33,34] |
| Carotenoid | Outer membrane disruption ROS generation | Gram-negative | [19,20] |
| Melanin | Outer membrane disruption ROS generation Photothermal effect | Gram-positive and Gram-negative species | [25,26,27] |
| Prodigiosin | Outer membrane disruption Inhibition of cellular processes | Gram-positive species | [31,32,35] |
| Bacteria | Pigment | Substrates | Fastness |
|---|---|---|---|
| Chryseobacterium sp. | Carotenoid | Natural silk, Dubai silk, linen, Japanese cotton, and Indian cotton | No information |
| Rhodonellum psychrophilum | Carotenoid | Cotton, glace cotton, silk, and rayon | No information |
| Metabacillus idrensis | Carotenoid | Cotton | No information |
| Streptomyces virginae | Melanin | Wool | Perspiration: 4–5 Light: 5–6 |
| Streptomyces glaucescens | Melanin | Cotton | Washing: 4–5 |
| Janthinobacterium lividum | Violacein | Silk, cotton, wool, polyester, acrylic, acetate, vinylon, and nylon | |
| Chromobacterium violaceum | Violacein | Polyamide 6.6 | Washing and perspiration: 5 |
| Chromobacterium violaceum | Violacein | Pure cotton, pure silk, pure rayon, rayon jacquard, silk satin, cotton, and polyester | Silk: Light: 1 Washing: 2 Perspiration: 3–4 Other fibres: No information |
| Prodigiosin | |||
| Vibrio sp. | Wool, nylon, acrylic, and silk | No information | |
| Serratia sp. | Prodigiosin | Cotton and wool | Washing: 4–5 and 3–4 Light: 4 and 2–3 |
| Serratia marcescens | Prodigiosin | Wool, cotton, silk, nylon, and polyester | Pure silk: Light: 4–5 Washing: 4–5 Rubbing: 4–5 China silk: Light: 4–5 |
| Staphylococcus aureus | Prodigiosin | Washing: 4–5 Rubbing: 4–5 Cotton: Washing: 4–5 Rubbing: 4–5 | |
| Acrylic | No information | ||
| Serratia rubidaea | Prodigiosin | Cotton linen, cotton baft, cotton gabardine, cotton jersey, chiffon, satin, Dacron, and polyester | Satin and chiffon: 1 Light: 5 Washing: 6 Linen, baft, gabardine, Dacron: Light: 2 Washing: 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, M.; Felgueiras, H.P.; Leite, B.R.; Soares, G.M.B. Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications. Antibiotics 2025, 14, 520. https://doi.org/10.3390/antibiotics14050520
Gomes M, Felgueiras HP, Leite BR, Soares GMB. Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications. Antibiotics. 2025; 14(5):520. https://doi.org/10.3390/antibiotics14050520
Chicago/Turabian StyleGomes, Micaela, Helena P. Felgueiras, Barbara R. Leite, and Graça M. B. Soares. 2025. "Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications" Antibiotics 14, no. 5: 520. https://doi.org/10.3390/antibiotics14050520
APA StyleGomes, M., Felgueiras, H. P., Leite, B. R., & Soares, G. M. B. (2025). Colourful Protection: Challenges and Perspectives of Antibacterial Pigments Extracted from Bacteria for Textile Applications. Antibiotics, 14(5), 520. https://doi.org/10.3390/antibiotics14050520








