LC-MS/MS Determination of Quorum Sensing Molecules in Plasma from Burn Patients with Septic Shock Sustained by Acinetobacter Baumannii
Abstract
1. Introduction
2. Results
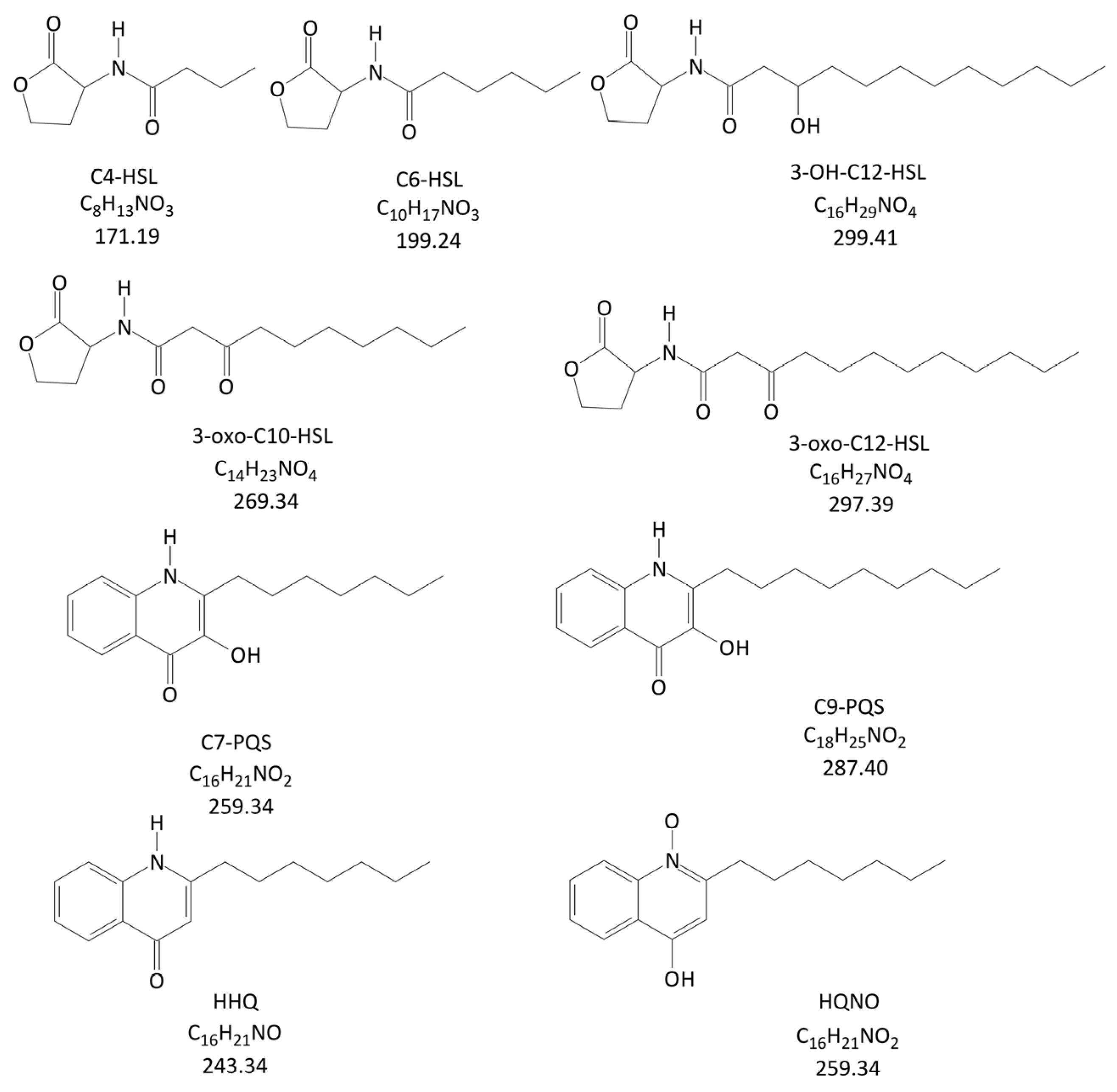
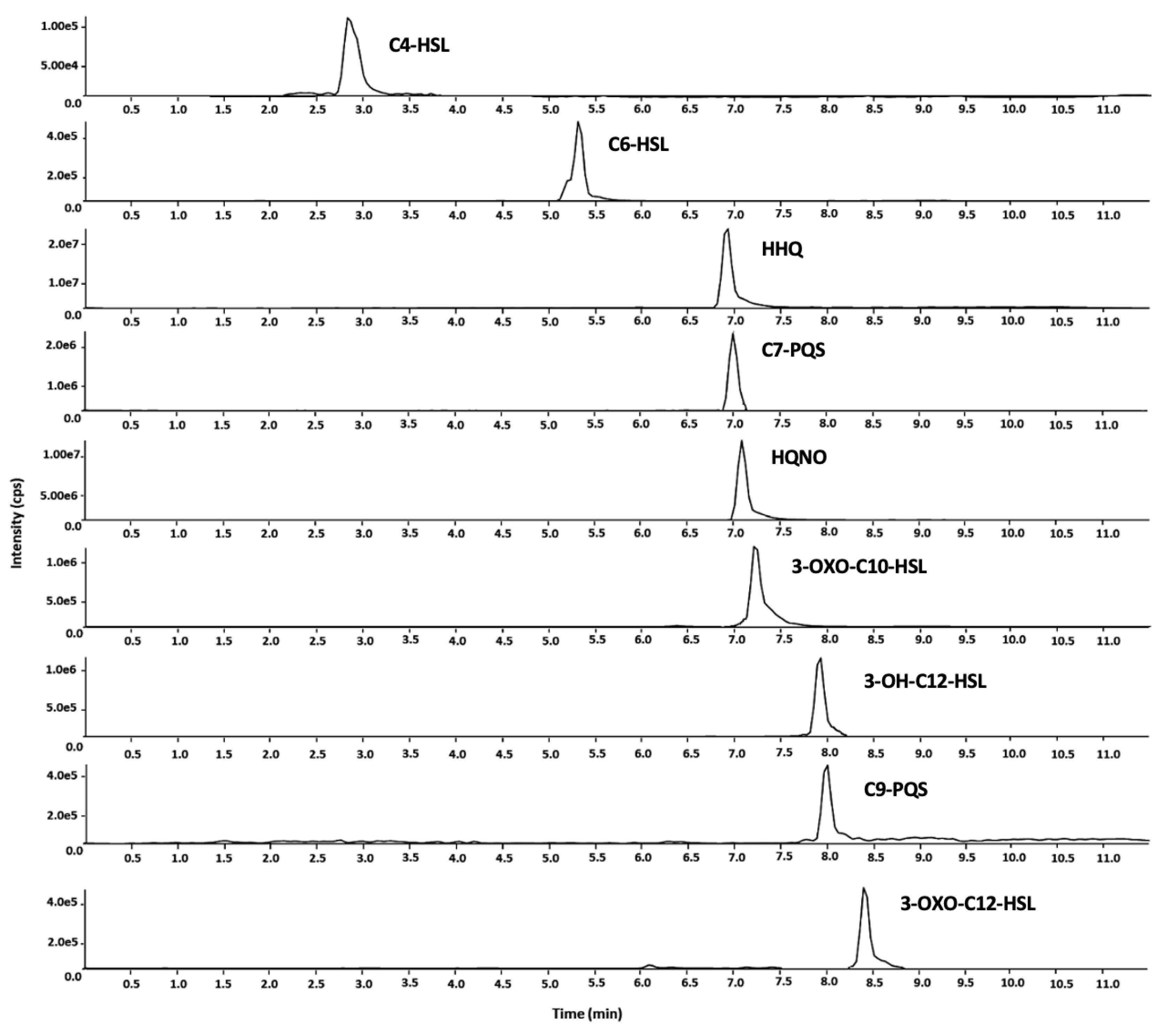
2.1. Method Development
2.2. Validation Results
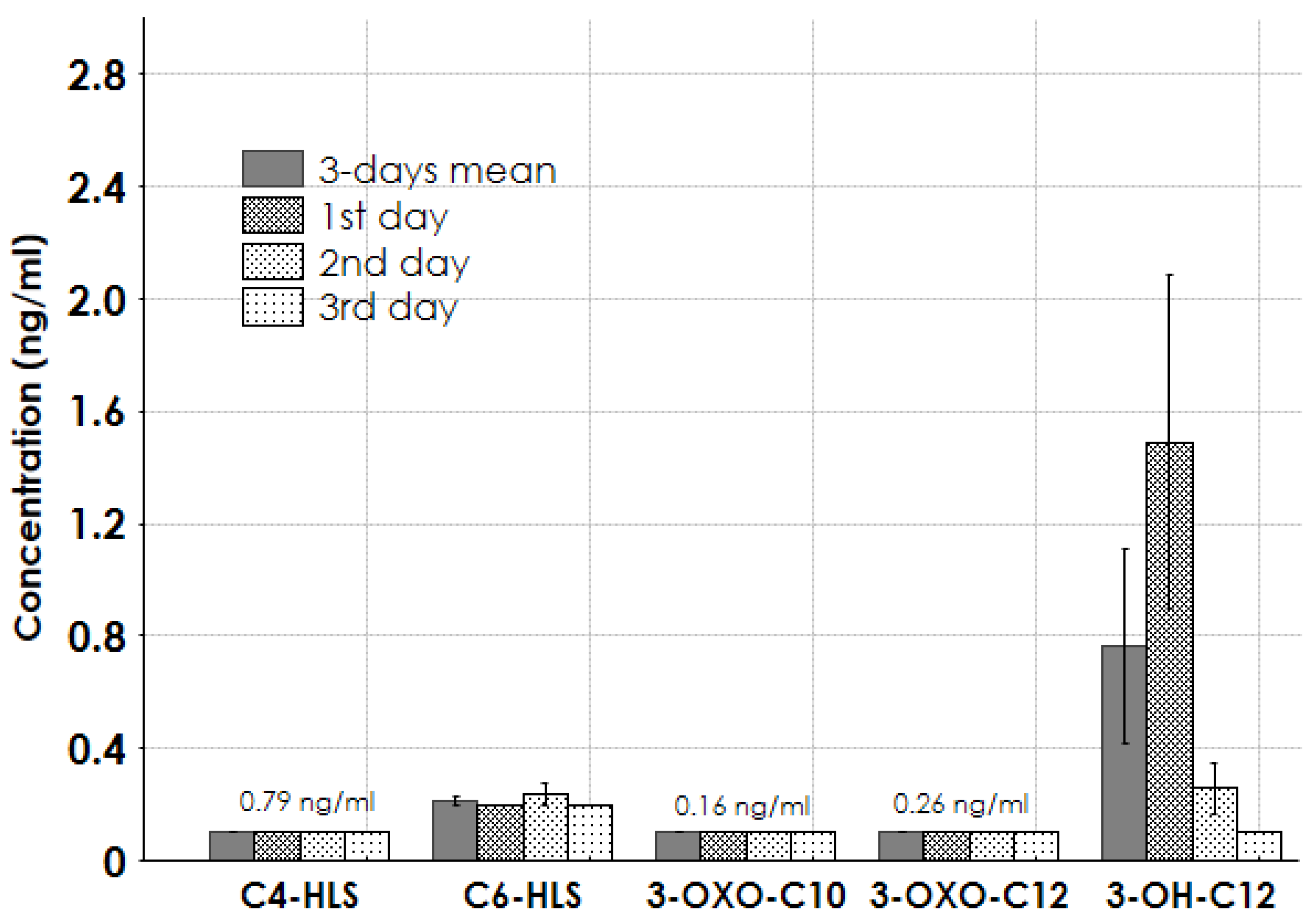
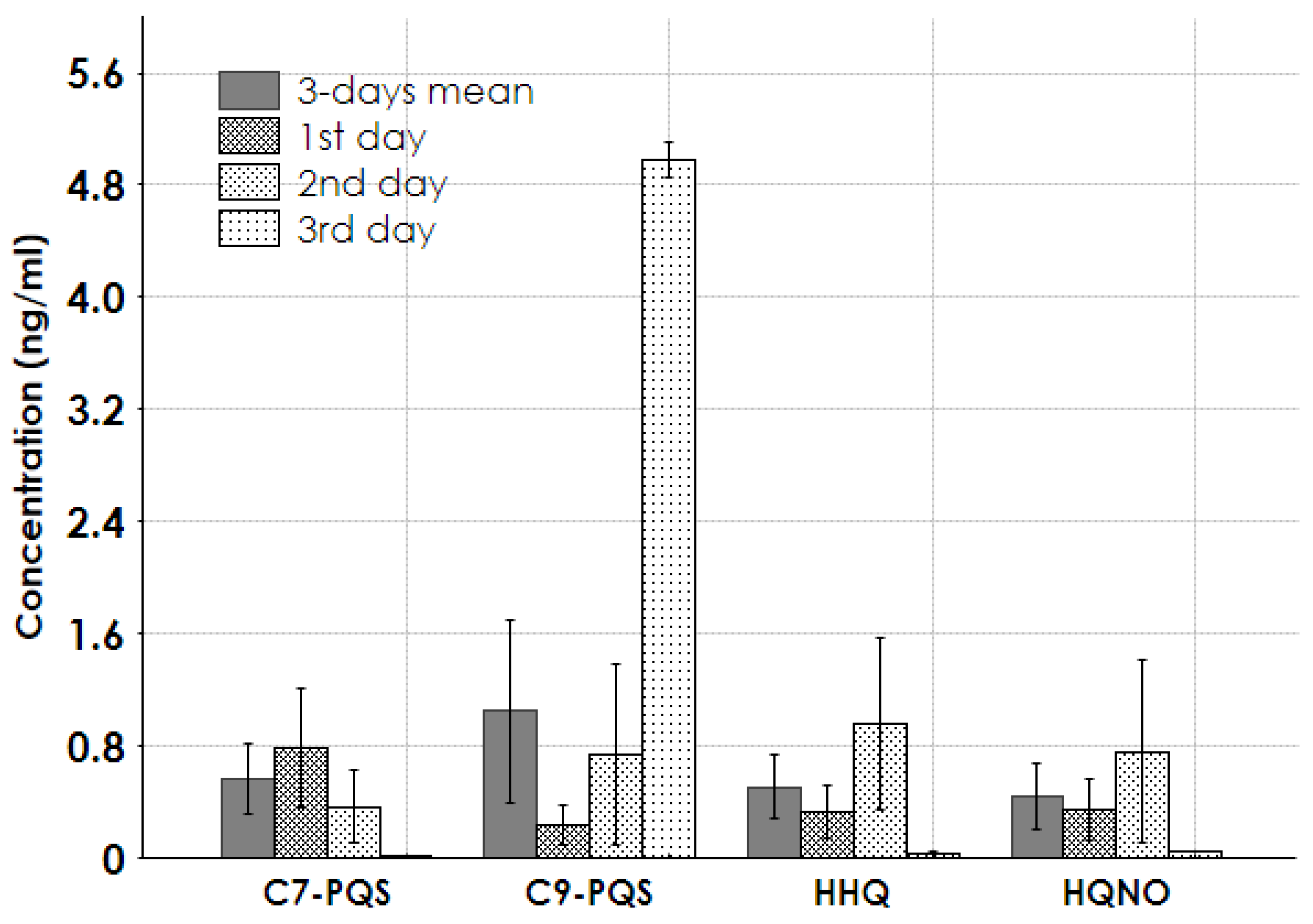
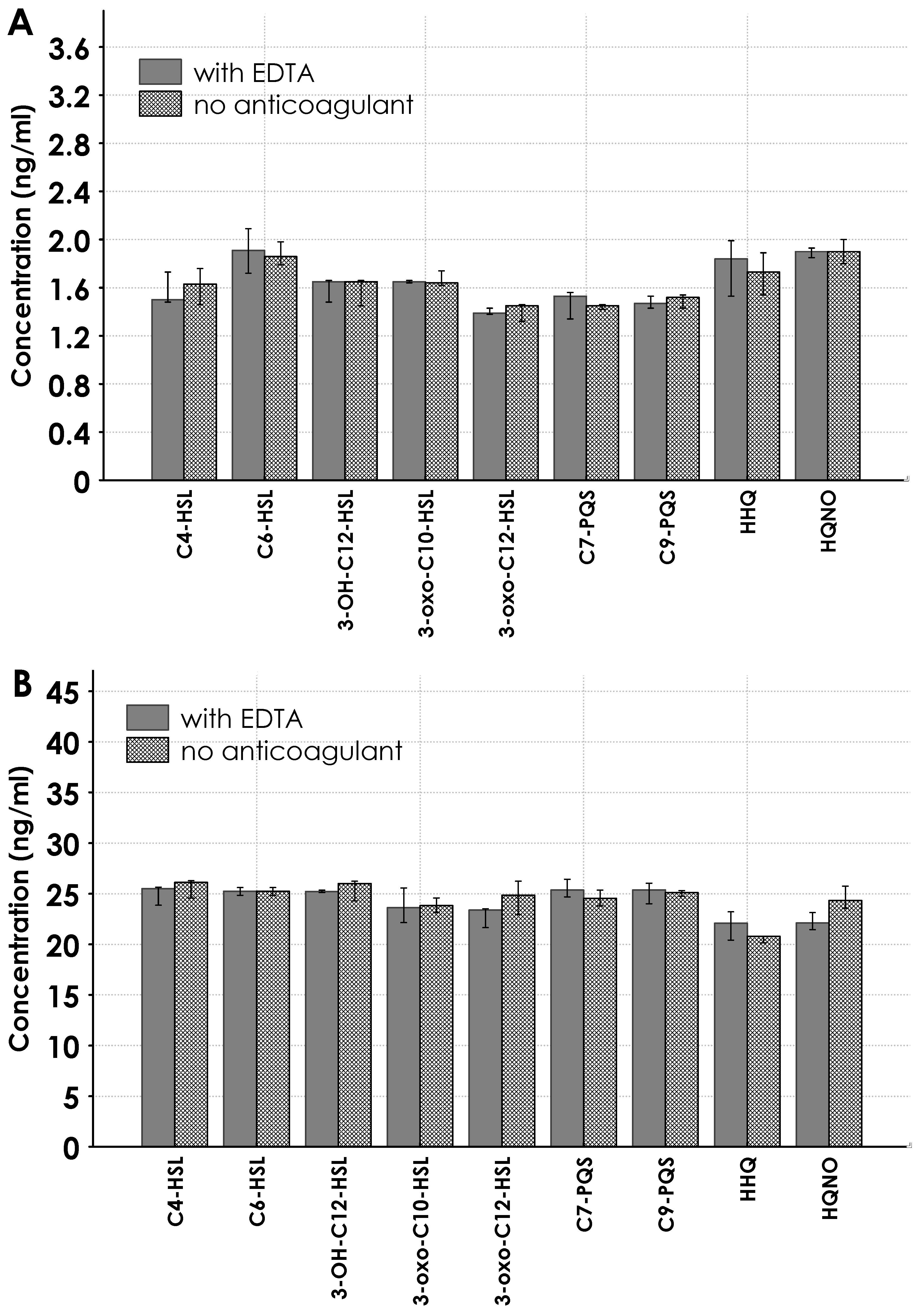
2.3. Results for Real Samples
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Standard and Working Solutions
4.3. Sample Preparation
4.4. Instrumental Analysis
4.5. Method Validation
4.6. Patients and Real Sampling Protocol
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AQ | 2-alkyl-4(1H)-quinolone metabolites |
| HSL | N-acyl-homoserine lactone |
| QS | Quorum Sensing |
References
- Acet, O.; Erdönmez, D.; Acet, B.O.; Odabaşı, M. N-acyl homoserine lactone molecules assisted quorum sensing: Effects, consequences and monitoring of bacteria talking in real life. Arch. Microbiol. 2021, 203, 3739–3749. [Google Scholar] [CrossRef]
- Azimi, S.; Klementiev, A.D.; Whiteley, M.; Diggle, S.P. Bacterial quorum sensing during infection. Annu. Rev. Microbiol. 2020, 74, 201–219. [Google Scholar] [CrossRef]
- Brewer, L.K.; Jones, J.W.; Blackwood, C.B.; Barbier, M.; Oglesby-Sherrouse, A.; Kane, M.A. Development and bioanalytical method validation of an LC-MS/MS assay for simultaneous quantitation of 2-alkyl-4(1H)-quinolones for application in bacterial cell culture and lung tissue. Anal. Bioanal. Chem. 2020, 412, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.A.; Bassler, B.L. A genetic analysis of the function of LuxO, a two-component response regulator involved in quorum sensing in Vibrio harveyi. Mol. Microbiol. 1999, 31, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Shin, D.; Kim, M.; Park, J.; Lim, S.; Ryu, S. LsrR-mediated quorum sensing controls invasiveness of Salmonella typhimurium by regulating SPI-1 and flagella genes. PLoS ONE 2012, 7, e37059. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Clemmer, K.M.; Bonomo, R.A.; Rather, P.N. Isolation and characterization of an autoinducer synthase from Acinetobacter baumannii. J. Bacteriol. 2008, 190, 3386–3392. [Google Scholar] [CrossRef]
- González, R.H.; Dijkshoorn, L.; Van den Barselaar, M.; Nudel, C. Quorum sensing signal profile of Acinetobacter strains from nosocomial and environmental sources. Rev. Argent. Microbiol. 2009, 41, 73–78. [Google Scholar]
- Bhargava, N.; Sharma, P.; Capalash, N. Quorum sensing in Acinetobacter: An emerging pathogen. Crit. Rev. Microbiol. 2010, 36, 349–360. [Google Scholar] [CrossRef]
- Saipriya, K.; Swathi, C.H.; Ratnakar, K.S.; Sritharan, V. Quorum-sensing system in Acinetobacter baumannii: A potential target for new drug development. J. Appl. Microbiol. 2019, 128, 15–27. [Google Scholar] [CrossRef]
- Montagut, E.J.; Marco, M.P. Biological and clinical significance of quorum sensing alkylquinolones: Current analytical and bioanalytical methods for their quantification. Anal. Bioanal. Chem. 2021, 413, 4599–4618. [Google Scholar] [CrossRef]
- Montagut, E.J.; Raya, J.; Martin-Gomez, M.T.; Vilaplana, L.; Rodriguez-Urretavizcaya, B.; Marco, M.P. An immunochemical approach to detect the Quorum sensing-regulated virulence factor 2-Heptyl-4-Quinoline N-Oxide (HQNO) produced by Pseudomonas aeruginosa clinical isolates. Microbiol. Spectr. 2022, 10, e01073-21. [Google Scholar] [CrossRef] [PubMed]
- Rothballer, M.; Uhl, J.; Kunze, J.; Schmitt-Kopplin, P.; Hartmann, A. Detection of the bacterial quorum-sensing signaling molecules N-acyl-homoserine lactones (HSL) and N-acyl-homoserine (HS) with an enzyme-linked immunosorbent assay (ELISA) and via ultrahigh-performance liquid chromatography coupled to mass spectrometry (UHPLC-MS). Methods Mol. Biol. 2018, 1673, 61–72. [Google Scholar] [PubMed]
- Chen, X.; Wang, C.; Zheng, Q.Y.; Hu, W.C.; Xia, X.H. Emerging advances in biosensor technologies for quorum sensing signal molecules. Anal. Bioanal. Chem. 2025, 417, 33–50. [Google Scholar] [CrossRef]
- Ortori, C.A.; Dubern, J.F.; Chhabra, S.R.; Cámara, M.; Hardie, K.; Williams, P.; Barrett, D.A. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Anal. Bioanal. Chem. 2011, 399, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Readel, E.; Portillo, A.; Talebi, M.; Armstrong, D.W. Enantiomeric separation of quorum sensing autoinducer homoserine lactones using GC-MS and LC-MS. Anal. Bioanal. Chem. 2020, 412, 2927–2937. [Google Scholar] [CrossRef]
- Mariano, F.; Malvasio, V.; Risso, D.; Depetris, N.; Pensa, A.; Fucale, G.; Gennari, F.; Biancone, L.; Stella, M. Colistin therapy, survival and renal replacement therapy in burn patients: A 10-year single-center cohort study. Int. J. Gen. Med. 2022, 15, 5211–5221. [Google Scholar] [CrossRef]
- Sun, X.; Ni, Z.; Tang, J.; Ding, Y.; Wang, X.; Li, F. The abaI/abaR quorum sensing system effects on pathogenicity in Acinetobacter baumannii. Front. Microbiol. 2021, 12, 679241. [Google Scholar] [CrossRef]
- Dal Bello, F.; Zorzi, M.; Aigotti, R.; Medica, D.; Fanelli, V.; Cantaluppi, V.; Amante, E.; Orlandi, T.; Medana, C. Targeted and untargeted quantification of quorum sensing signalling molecules in bacterial cultures and biological samples via HPLC-TQ MS techniques. Anal. Bioanal. Chem. 2021, 413, 853–864. [Google Scholar] [CrossRef] [PubMed]
- EMA/CHMP/ICH/172948/2019; ICH Guideline M10 on Bioanalytical Method Validation. European Medicines Agency: Amsterdam, The Netherlands, 2019.
- U.S. Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation (Rev. 1); FDA: Silver Spring, MD, USA, 2013.
- EU Reference Laboratory. Guidance Document on the Estimation of LOD and LOQ for Measurements in the Field of Contaminants in Feed and Food; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Cantwell, H. (Ed.) Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 3rd ed.; 2025; Available online: https://www.eurachem.org/index.php/publications/guides/mv (accessed on 11 May 2025).
- Miller, C.; Gilmore, J. Detection of quorum-sensing molecules for pathogenic molecules using cell-based and cell-free biosensors. Antibiotics 2020, 9, 259. [Google Scholar] [CrossRef]
- Bredenbruch, F.; Geffers, R.; Nimtz, M.; Buer, J.; Häussler, S. The Pseudomonas aeruginosa quinolone signal (PQS) has an iron-chelating activity. Environ. Microbiol. 2006, 8, 1318–1329. [Google Scholar] [CrossRef]
- Turnpenny, P.; Padfield, A.; Barton, P.; Teague, J.; Rahme, L.G.; Pucci, M.J.; Zahler, R.; Rubio, A. Bioanalysis of Pseudomonas aeruginosa alkyl quinolone signalling molecules in infected mouse tissue using LC-MS/MS and its application to a pharmacodynamic evaluation of MvfR inhibition. J. Pharm. Biomed. Anal. 2017, 139, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Quan, C.; Wang, X.; Zhao, P.; Fan, S. Extraction, purification and identification of bacterial signal molecules based on N-acyl homoserine lactones. Microb. Biotechnol. 2011, 4, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Dubern, J.F.; Diggle, S.P. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol. BioSyst. 2008, 4, 882–888. [Google Scholar] [CrossRef]
- Barr, H.L.; Halliday, N.; Cámara, M.; Barrett, D.A.; Williams, P.; Forrester, D.L.; Simms, R.; Smyth, A.R.; Honeybourne, D.; Whitehouse, J.L.; et al. Pseudomonas aeruginosa quorum sensing molecules correlate with clinical status in cystic fibrosis. Eur. Respir. J. 2015, 46, 1046–1054. [Google Scholar] [CrossRef]
- Zeng, X.; Zou, Y.; Zheng, J.; Qiu, S.; Liu, L.; Wei, C. Quorum sensing-mediated microbial interactions: Mechanisms, applications, challenges and perspectives. Microbiol. Res. 2023, 273, 127414. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharma, P.; Capalash, N. Pyocyanin stimulates quorum sensing-mediated tolerance to oxidative stress and increases persister cell populations in Acinetobacter baumannii. Infect. Immun. 2014, 82, 3417–3425. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Kamaluddin, E.; Foster, D. Targeted rapid endotoxin adsorption: Can we bring precision medicine to sepsis? Blood Purif. 2025, 1–12. [Google Scholar] [CrossRef]
- Bowen, R.A.R.; Remaley, A.T. Interferences from blood collection tube components on clinical chemistry assays. Biochem. Medica 2014, 24, 31–44. [Google Scholar] [CrossRef]
| Analyte | R2 | LOQ (ng/mL) | Concentration (ng/mL) | Within-Run (n = 3) | Inter-Run (n = 3) | |
|---|---|---|---|---|---|---|
| Precision (CV%) | Accuracy (Bias%) | Precision (CV%) | ||||
| C4-HSL | 0.9984 | 0.79 | 1.56 | 8.2 | +1.3 | 9.6 |
| 6.25 | 6.3 | −1.0 | 2.0 | |||
| 25.0 | 6.0 | +1.3 | 0.1 | |||
| C6-HSL | 0.9995 | 0.15 | 1.56 | 11 | +16 | 11 |
| 6.25 | 1.0 | −2.2 | 6.8 | |||
| 25.0 | 0.6 | −0.1 | 12 | |||
| HHQ | 0.9957 | 0.15 | 1.56 | 9.6 | +3.2 | 15 |
| 6.25 | 9.1 | +4.2 | 9.4 | |||
| 25.0 | 10 | −2.4 | 10 | |||
| HQNO | 0.9911 | 0.02 | 1.56 | 13 | +23 | 25 |
| 6.25 | 10 | −15 | 17 | |||
| 25.0 | 17 | −7 | 15 | |||
| 3-oxo-C10-HSL | 0.9954 | 0.16 | 1.56 | 7.5 | +1.7 | 8.2 |
| 6.25 | 3.9 | −6.2 | 4.1 | |||
| 25.0 | 5.7 | −0.9 | 5.8 | |||
| C7-PQS | 0.9992 | 0.05 | 1.56 | 9.3 | −4.3 | 8.8 |
| 6.25 | 3.1 | −14 | 11 | |||
| 25.0 | 3.5 | +1.9 | 7.5 | |||
| 3-oxo-C12-HSL | 0.9959 | 0.26 | 1.56 | 13 | −4.3 | 11 |
| 6.25 | 2.0 | −11 | 6.4 | |||
| 25.0 | 11 | −6.6 | 9.6 | |||
| C9-PQS | 0.9932 | 0.03 | 1.56 | 3.0 | −0.1 | 24 |
| 6.25 | 8.0 | +0.3 | 8 | |||
| 25.0 | 15 | −0.1 | 9 | |||
| 3-OH-C12-HSL | 0.9992 | 0.62 | 1.56 | 9.3 | +5.8 | 0.2 |
| 6.25 | 10 | +11 | 13 | |||
| 25.0 | 6.6 | +4.6 | 1.6 | |||
| Analyte | tR (Min) | Precursor Ion | DP (V) | EP (V) | Product Ion | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| C4-HSL | 2.89 | 172.1 | 30 | 15 | 102.2 | 14 | 12 |
| 71.1 | 19 | 12 | |||||
| C6-HSL | 5.37 | 200.3 | 30 | 13 | 102.0 | 15 | 15 |
| 99.0 | 15 | 15 | |||||
| HHQ | 6.95 | 244.3 | 35 | 11 | 172.0 | 47 | 20 |
| 159.2 | 43 | 10 | |||||
| C7-PQS | 7.00 | 260.3 | 25 | 13 | 175.2 | 39 | 20 |
| 147.1 | 50 | 20 | |||||
| HQNO | 7.15 | 260.3 | 30 | 10 | 159.0 | 41 | 15 |
| 186.0 | 50 | 22 | |||||
| 3-oxo-C10-HSL | 7.24 | 270.3 | 25 | 13 | 169.0 | 18 | 11 |
| 102.1 | 18 | 10 | |||||
| 3-OH-C12-HSL | 7.97 | 300.2 | 25 | 6 | 282.2 | 13 | 20 |
| 102.1 | 17 | 14 | |||||
| C9-PQS | 8.02 | 288.4 | 40 | 14 | 175.3 | 41 | 14 |
| 147.0 | 52 | 20 | |||||
| 3-oxo-C12-HSL | 8.41 | 298.4 | 31 | 13 | 197.2 | 20 | 18 |
| 102.1 | 17 | 20 | |||||
| C6-HSL-d3 | 5.28 | 203.3 | 25 | 14 | 102.2 | 15 | 10 |
| C9-PQS-d4 | 7.99 | 292.2 | 35 | 5 | 179.3 | 45 | 12 |
| Age/Sex | Diagnosis | Day Samples | Hemoculture | Exitus | |
|---|---|---|---|---|---|
| 1. | 89/F | Burns 15% | 1-2-3 | yes/Acinetobacter baumanni/Candida parapsilosis/Enterococcus faecium | No |
| 2. | 43/M | Burns 45% | 1-2 | yes/Acinetobacter baumanni/Candida albicans | No |
| 3. | 19/F | Burns 85% | 1-2 | yes/Acinetobacter baumanni | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpenito, N.; Leporati, M.; Sciarrillo, A.; Pensa, A.; Gambino, R.; Musso, G.; Mella, A.; Biancone, L.; Risso, D.; Mariano, F.; et al. LC-MS/MS Determination of Quorum Sensing Molecules in Plasma from Burn Patients with Septic Shock Sustained by Acinetobacter Baumannii. Antibiotics 2025, 14, 517. https://doi.org/10.3390/antibiotics14050517
Carpenito N, Leporati M, Sciarrillo A, Pensa A, Gambino R, Musso G, Mella A, Biancone L, Risso D, Mariano F, et al. LC-MS/MS Determination of Quorum Sensing Molecules in Plasma from Burn Patients with Septic Shock Sustained by Acinetobacter Baumannii. Antibiotics. 2025; 14(5):517. https://doi.org/10.3390/antibiotics14050517
Chicago/Turabian StyleCarpenito, Nicolò, Marta Leporati, Alberto Sciarrillo, Anna Pensa, Roberto Gambino, Giovanni Musso, Alberto Mella, Luigi Biancone, Daniela Risso, Filippo Mariano, and et al. 2025. "LC-MS/MS Determination of Quorum Sensing Molecules in Plasma from Burn Patients with Septic Shock Sustained by Acinetobacter Baumannii" Antibiotics 14, no. 5: 517. https://doi.org/10.3390/antibiotics14050517
APA StyleCarpenito, N., Leporati, M., Sciarrillo, A., Pensa, A., Gambino, R., Musso, G., Mella, A., Biancone, L., Risso, D., Mariano, F., & Cosseddu, D. (2025). LC-MS/MS Determination of Quorum Sensing Molecules in Plasma from Burn Patients with Septic Shock Sustained by Acinetobacter Baumannii. Antibiotics, 14(5), 517. https://doi.org/10.3390/antibiotics14050517








