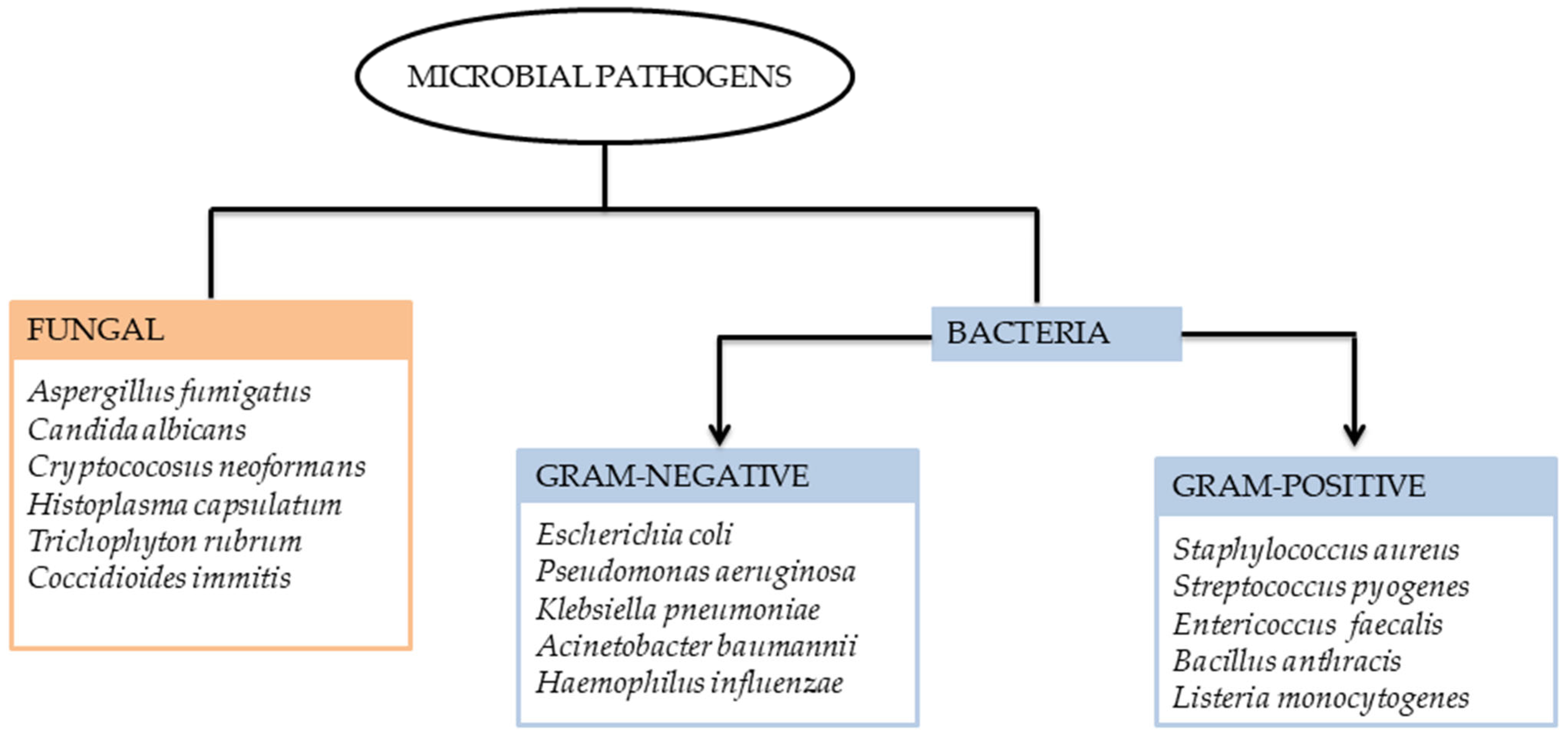
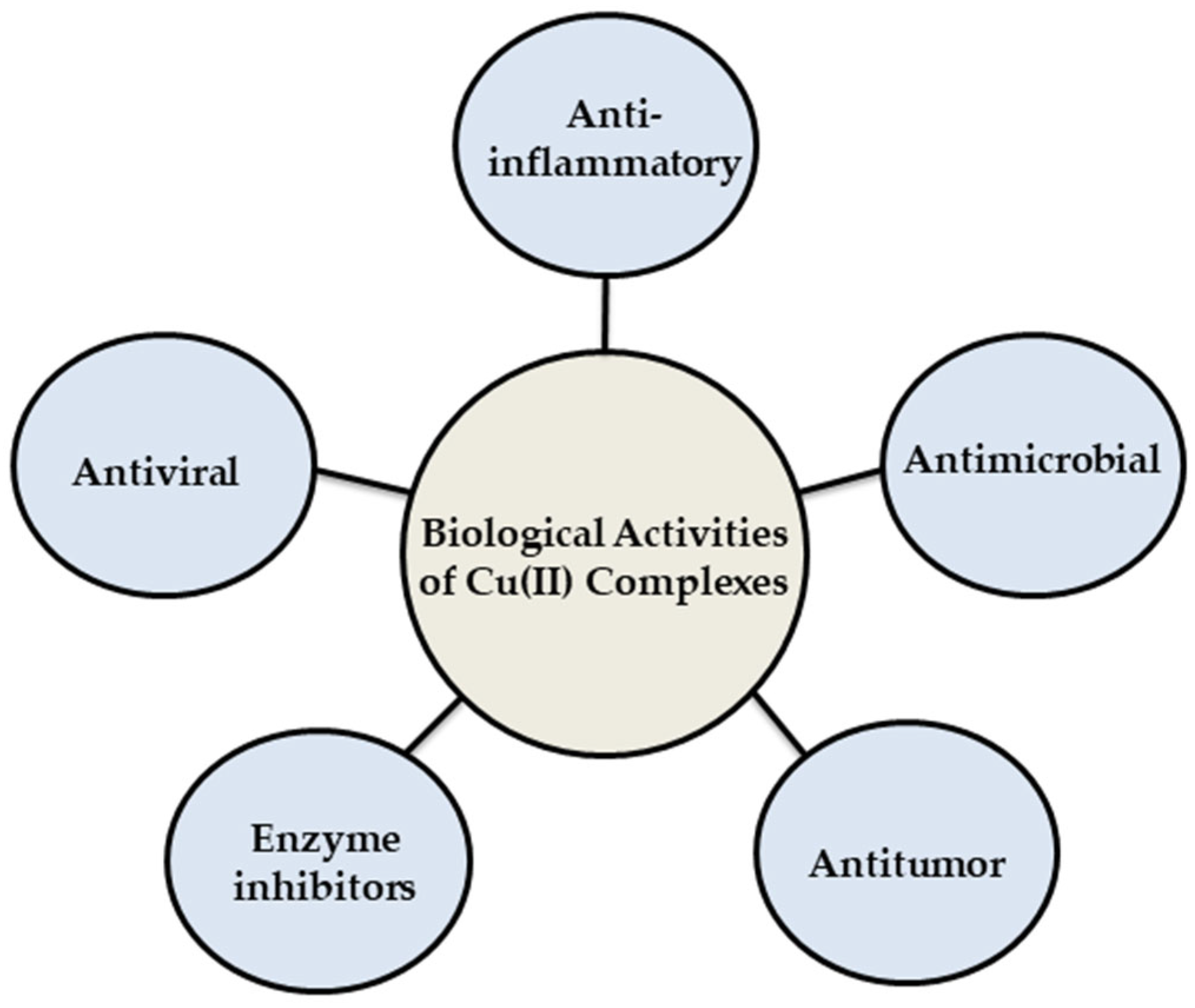
In recent times, there has been growing interest in developing metal-based drugs with pharmacological potential. The thermodynamic and kinetic properties of these complexes play a crucial role in determining the biological activity of bio-metals [
35]. Additionally, developing chelates in vivo can enhance the lipophilicity of drugs, significantly improving their ability to penetrate the target site, thereby increasing their effectiveness [
36]. Copper complexes have garnered significant attention in coordination chemistry, technical applications, and catalysis. This interest stems from their unique spectroscopic properties, anion selectivity, and notable biological significance [
36]. Copper(II) complexes represent a significant class in chemistry due to their intriguing coordination chemistry, which includes flexible redox properties, diverse geometries, and various oxidation states [
37,
38]. They have extensive applications across multiple fields, such as catalysts in oxidation [
39], reduction, and epoxidation reactions [
40]. Moreover, copper(II)-based compounds display a wide range of biological activities (as shown in
Figure 3) and have been extensively investigated in the medical field for their potential in treating various diseases [
41], which are intimately connected to their capacity to form metal complexes. For instance, when coordination occurs, the lipophilicity, which influences the rate of cellular entry, is altered. This phenomenon can be elucidated through forming coordination bonds, as Overtone’s concept and Tweedy’s chelation theory. During coordination formed by donor groups, the lipophilicity of the complexes is improved, which depends on the removal of π-electrons over the chelate ring system. This increased lipophilicity improves their ability to permeate the lipid layer of microorganisms, thereby leading to more efficient destruction [
42,
43]. This modification can reduce specific side effects and a novel range of bioactive properties not displayed by the free ligand [
44].
According to Zaki et al. [
45], metal-based compounds can be arranged into seven categories depending on the function of the metal and ligand moieties: (1) the metal complex is active in inert form, (2) the metal complex is active in its reactive form, (3) the metal serves as a radiation enhancer, (4) the compound contains a radioactive metal, (5) the metal or its biotransformation product is active, (6) a ligand is biologically active, and (7) only a fragment of the complex is active [
45]. Three-dimensional configurations of metal complexes formed by the coordination of organic ligands with metal allow the complexes to react more effectively with biological molecules. Therefore, in addition to the metal, the type of ligand has a significant effect on the chemical activities and subsequent medical applications of metal complexes, so the change of ligand can lead to changes in the photophysical, electrochemical, spectroscopic, and biological properties of the complexes [
46]. There are different mechanisms of action that depend on the geometry of the complexes and the nature of the ligand [
47]. They can act against microorganisms by occupying surface sites, which would typically be utilized in initiating the infection of the host cell, preventing the first step in the infection [
48]. Alternatively, other mechanisms involve the effects on the cell wall or membrane, interaction with DNA [
49], binding or inhibition of enzymes and membrane proteins [
50], and the generation of reactive oxygen species (ROS) (see
Figure 4) [
13]. This review delves into the antimicrobial capabilities of copper complexes. By compiling recent progress in copper complex research, we aim to shed light on the effectiveness and potential of supplementary treatments for tackling microbial infections.
3.1.1. Antibacterial Activity of Cu(II) Complexes
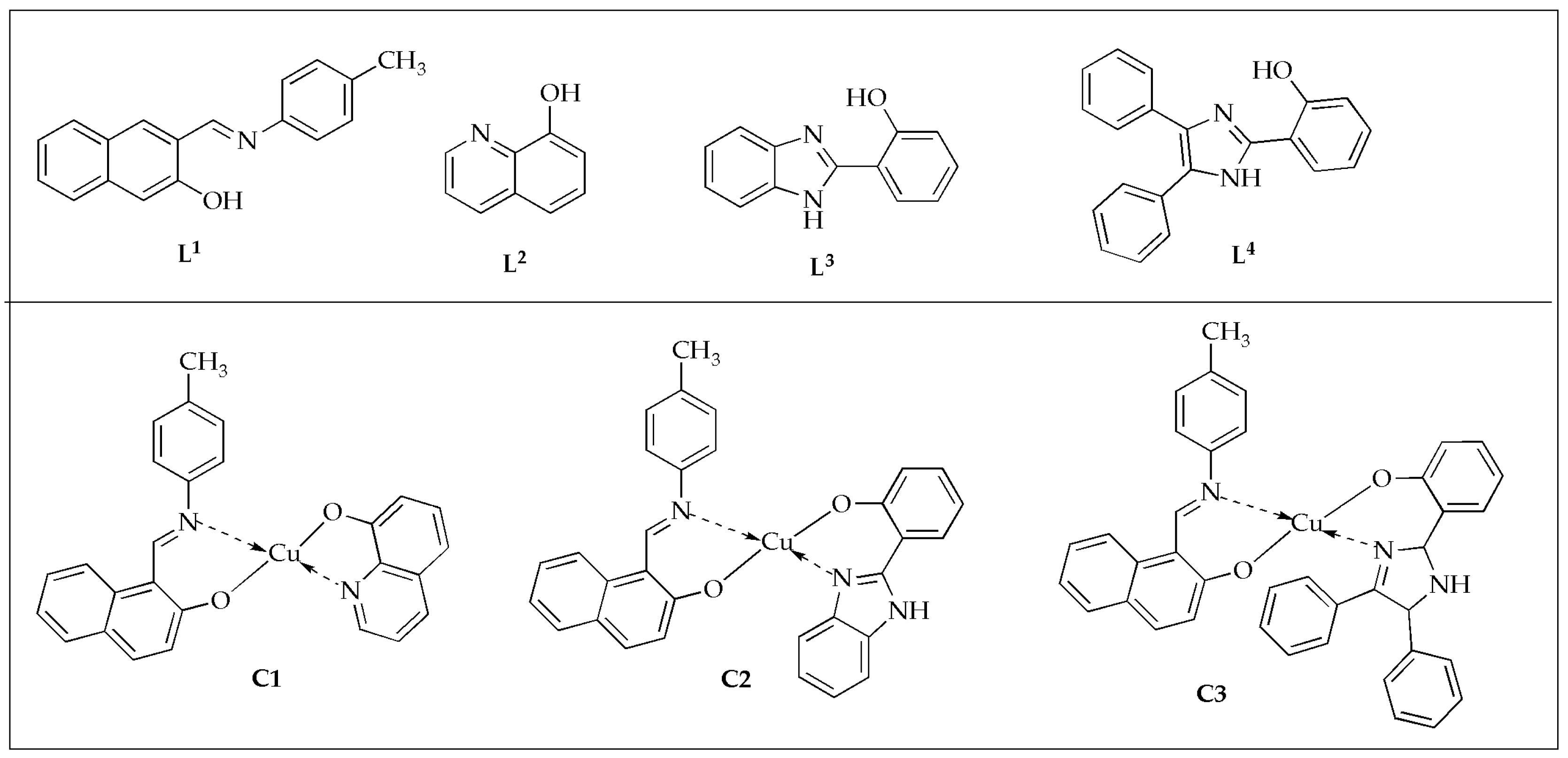
The antimicrobial activity of heteroleptic Cu(II) complexes with the general formula [Cu(
L1)(
Ln)] where
L1 is 1-{[(4-methylphenyl)imino]methyl}-2-naphthol as the primary ligand, and
Ln represents secondary ligands
L2 = 8-hydroxyquinoline,
L3 = 2-(1H-benzimidazol-2-yl)phenol, and
L4 = 2-(4,5-diphenyl-1H-imidazol-2-yl)phenol (
Figure 5) was evaluated by Ismael et al. against selected Gram-negative and Gram-positive bacteria, as well as fungal strains. [
51]. In their study, they found that the complexes outperformed the bare equivalent ligands in terms of their antimicrobial activity against specific bacteria and fungi by around 3.37 (2.99) times for complex
C1 (Cu
L2) and more significant than the bare
L1 ligand (
L2 co-ligand) against
A. fumigatus at 100 µg/mL (
Table 1). The inhibition zone diameters increased in the following order:
L1 <
L4 <
L2 <
L3 <
C3 <
C2 <
C1. The activity was further assessed using minimum inhibitory concentrations (MIC) measurements. In these evaluations, the synthesized complexes demonstrated enhanced potency with lower MIC values than the free ligands. Complex
C2 demonstrated the lowest MIC with values ranging between 20.50 and 22.50 ppm against
E. coli,
B. cereus, and
A. fumigatus. The condensation of p-toluidine with 2-hydroxy-naphthaldehyde yielded the primary ligand (
L1). Incorporating 8-hydroxy-quinoline (
L2) significantly enhanced the antimicrobial activity of the Cu(II) mixed-ligand complex (Cu
L2) [
51].
The evaluation of the copper(II) thiosemicarbazone complexes (
C4–
C6) (
Figure 6) against selected G
+/G
− bacterial strains by Qi et al. [
52] displayed promising findings. These Cu(II) complexes demonstrated greater antimicrobial effectiveness against G
− bacteria comparable to G
+ bacteria. The complexity of the cell wall structure played a significant role in influencing the bacterial potency of the complexes. Compound
C4 was the most potent antibacterial agent against all tested G
+/G
− bacterial strains, and the enhanced antibacterial effect was attributed to the presence of two methyl groups substituted on the nitrogen, which resulted in a tertiary amine. Additionally, against
S. aureus, the ligands displayed no inhibition zones and exhibited inhibition zone diameters of 12.22 mm (
L6) and 13.74 mm (
L7) against
E. coli, respectively (
Table 2). The complexes showed improved inhibitions against
S. aureus and
E. coli with diameters between 14 and 25 mm. Notably, the complexes displayed excellent antibacterial properties against
E. coli. These findings are advantageous in advancing the development of novel Cu(II) complexes as potential antitumor drug candidates [
52].
The antimicrobial efficacy of pyrazole nucleating copper(II) (
Figure 7) complex
C7 was evaluated by Azam et al. [
53]. Antimicrobial analysis indicated that the pyrazole nucleating ligand (
L8) was less effective than its synthesized complex when tested against various pathogenic microorganisms. The studied compounds exhibit varying inhibition zones when tested against different microbial strains. Additionally, the results indicated that complex
C7 exhibited superior antibacterial activity against
S. sonnei,
B. subtilis, and
P. aeruginosa microbes, with an inhibition zone of 21 mm and an MIC value of 62.5 µg/mL (
Table 3). This performance surpassed that of the standard drug, Amoxicillin. Incorporating the metal ion into the pyrazole nucleating Schiff base ligand (
L8) significantly improved its antibacterial effectiveness. As a result, the new pyrazole nucleating Cu(II) complex
C7 emerges as a promising candidate for antimicrobial drugs [
53].
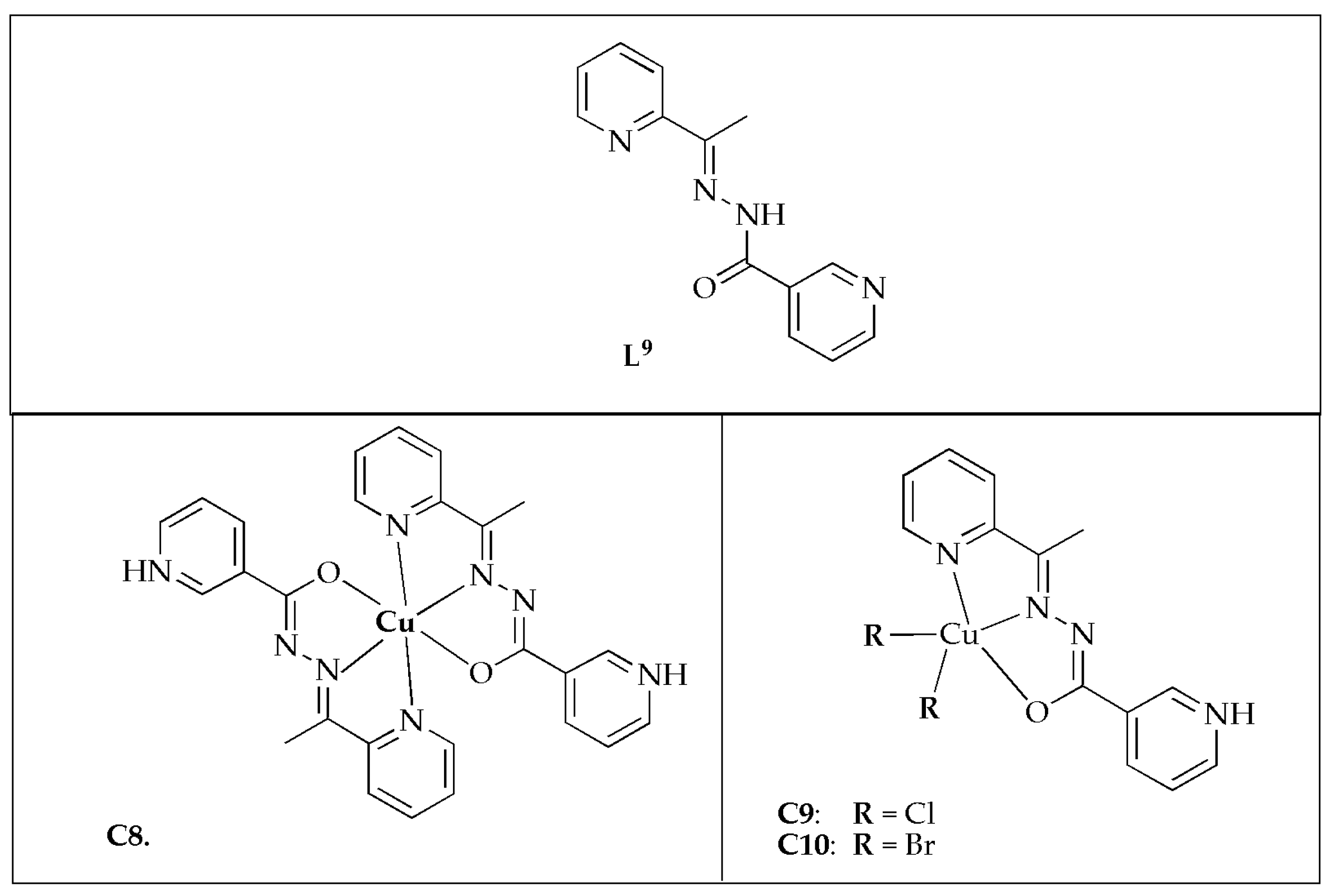
SanSantiago et al. [
54] synthesized copper(II) complexes with 2-cetylpyridinenicotinichydrazone (
L9) (
Figure 8) and assessed their antibacterial activity against various selected bacterial strains. The antibacterial effectiveness of free ligand
L9 demonstrated significant potency against
S. mutans and
S. salivarius, with MIC values of 1.56 µg/mL and 312 µg/mL, respectively. The Cu(II) complexes exhibited limited antibacterial potency against
E. faecalis, except for complexes
C9 and
C10, with MIC values of 100 µg/mL. However, they demonstrated moderate to good activity against other bacterial strains, with complex
C10 showing the best results, achieving a MIC value of 3.12 µg/mL against
S. mutans (
Table 4). Additionally, the hydrazone and its Cu(II) complexes exhibited superior MIC values comparable to the positive control. Generally, upon coordination with Cu(II) ion, the antibacterial potency of
L9 was enhanced. However, against
S. mutans and
S. salivarius, the hydrazone demonstrated comparable or superior effectiveness than the complexes. The observed enhancement might be attributed to the increased lipophilicity resulting from the sharing of the Cu
2+ charge with donor groups upon coordination and its reduced polarity. This enhances the complexes’ interchangeability with proteins comparable to free ligands. Additionally, the construction of ROS within the intracellular environment could contribute to the enhanced activity. The antibacterial effectiveness of complexes
C9 and
C10 surpassed that of complex
C8 against most of the investigated pathogens. The ESI-MS analysis disclosed that the emergence of cationic species [CuX(
L8)]
+ in solution might play a role in the antibacterial activity of complexes
C9 and
C10. The study concluded that the assessed compounds exhibited antibacterial efficacy, with the coordination of the Cu(II) atom significantly enhancing the potential of
L8 against most of the tested bacterial strains [
54].
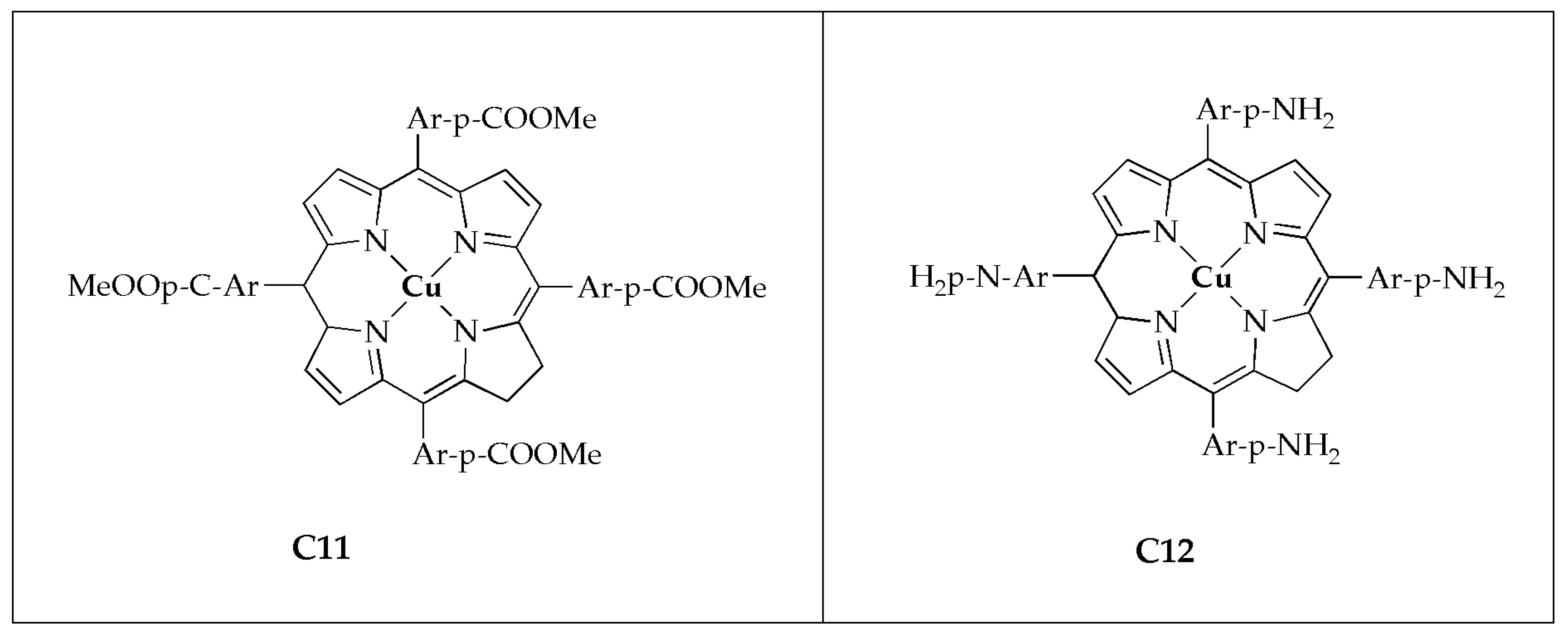
Beyene and Getaneh [
55] reported the antibacterial effectiveness of metalloporphyrins; 5, 20, 15, 20-tetrakis (para-X phenyl)porphyrinato Cu(II) (
Figure 9) and assessed their activity against selected G
−/G
+ bacteria. The studied complexes exhibited improved antibacterial growth inhibition potency comparable to the porphyrin ligand. The incorporation of the metal ion clearly suggests it is the ideal candidate for the inhibition of bacterial growth (
Table 5). The significant antibacterial effectiveness of transition metal complexes of porphyrins is attributed to two main factors: The Overtone concept and Tweedy’s chelation theory [
42,
43]. As the concentration of the complexes rises, their antimicrobial efficacy likewise enhances. Metal complex
C11 with the electron-withdrawing group (-COOMe) demonstrated superior activity than complex
C12 with the electron-donating group (-NH
2). Additionally, it was observed that metalloporphyrins exhibit greater bacterial growth inhibition comparable to metal salt or DMSO. It has been demonstrated that metalloporphyrins with an electron-withdrawing group at the para-positions exhibit superior antibacterial activity compared to those with an electron-donating group at the same positions. These findings suggest that metalloporphyrin derivatives hold significant promise as effective antibacterial agents [
55].
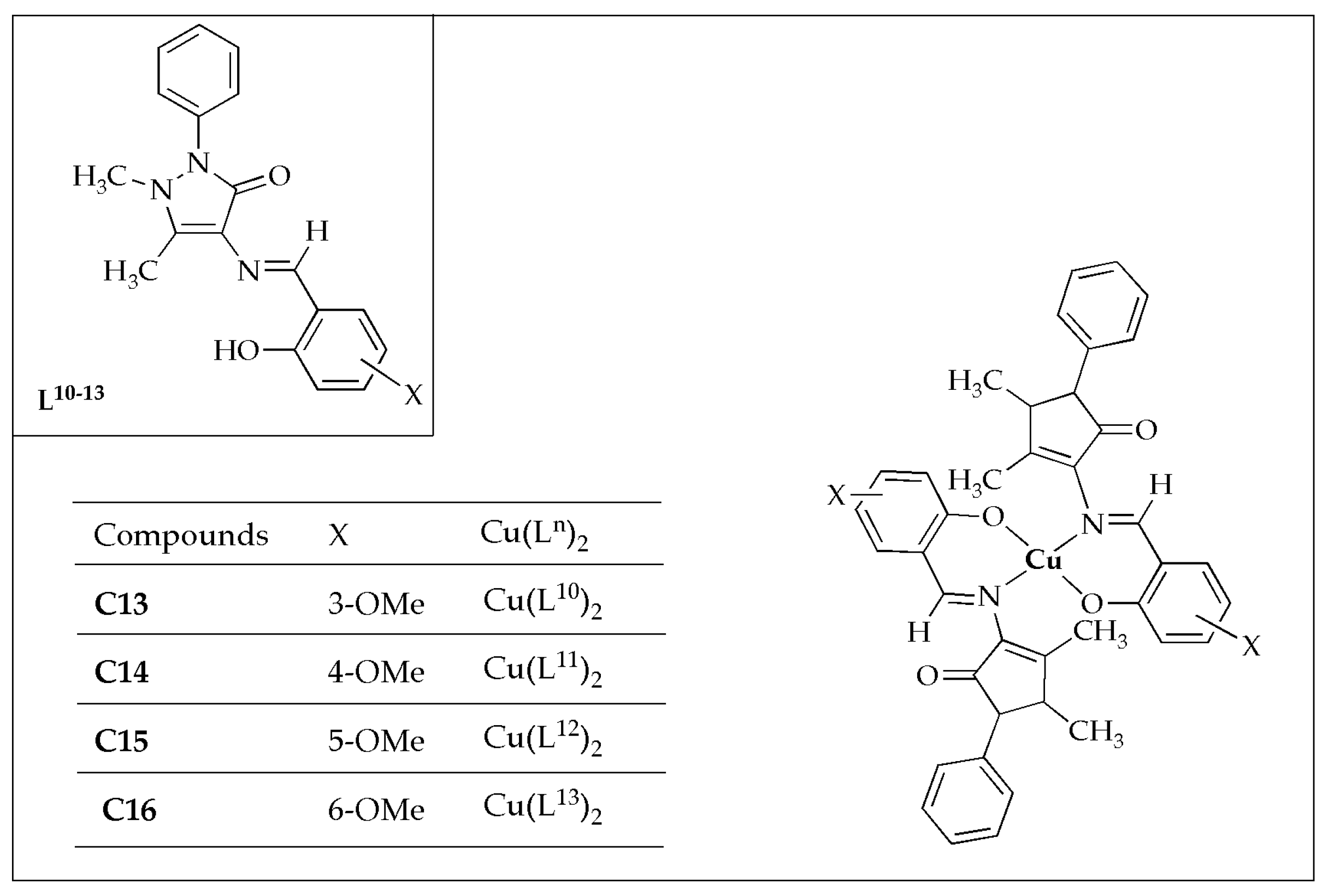
The biological potency of a novel series of Cu(II) complexes (
C13–
C16) with four Schiff-based ligands (
Figure 10), synthesized by Kargar et al. [
56], was evaluated against selected bacterial microbes. All the complexes investigated exhibited considerable antibacterial efficacy against
S. aureus and
E. coli, whereas the free ligands demonstrated only moderate efficacy (
Table 6). All assessed complexes exhibited superior antibacterial potency against G
+ strains compared to G
− strains. This difference is attributed to the thicker peptidoglycan layer in G
+ bacteria, which enables them to be readily absorbed. However, G-bacteria are shielded from certain physical invasions since they do not absorb the surrounding foreign materials. Altering only the methoxy site, the synthesized Schiff-based ligands exhibited a wide range of inhibitory effects on the growth of the strains (MIC values ranging from 64 to 512 µg/mL against
E. coli and
S. aureus), thus indicating a significant influence of modifications on antibacterial efficacy. The complexes exhibit superior bactericidal activity and inhibitory effects compared to their ligands, within a narrow range: MBC and MIC values are 128 and 64 µg/mL against
E. coli, and 64 and 32 µg/mL against
S. aureus, respectively. The Cu(II) complexes demonstrate a significant antibacterial effectiveness comparable to the parent Schiff base ligands [
56].
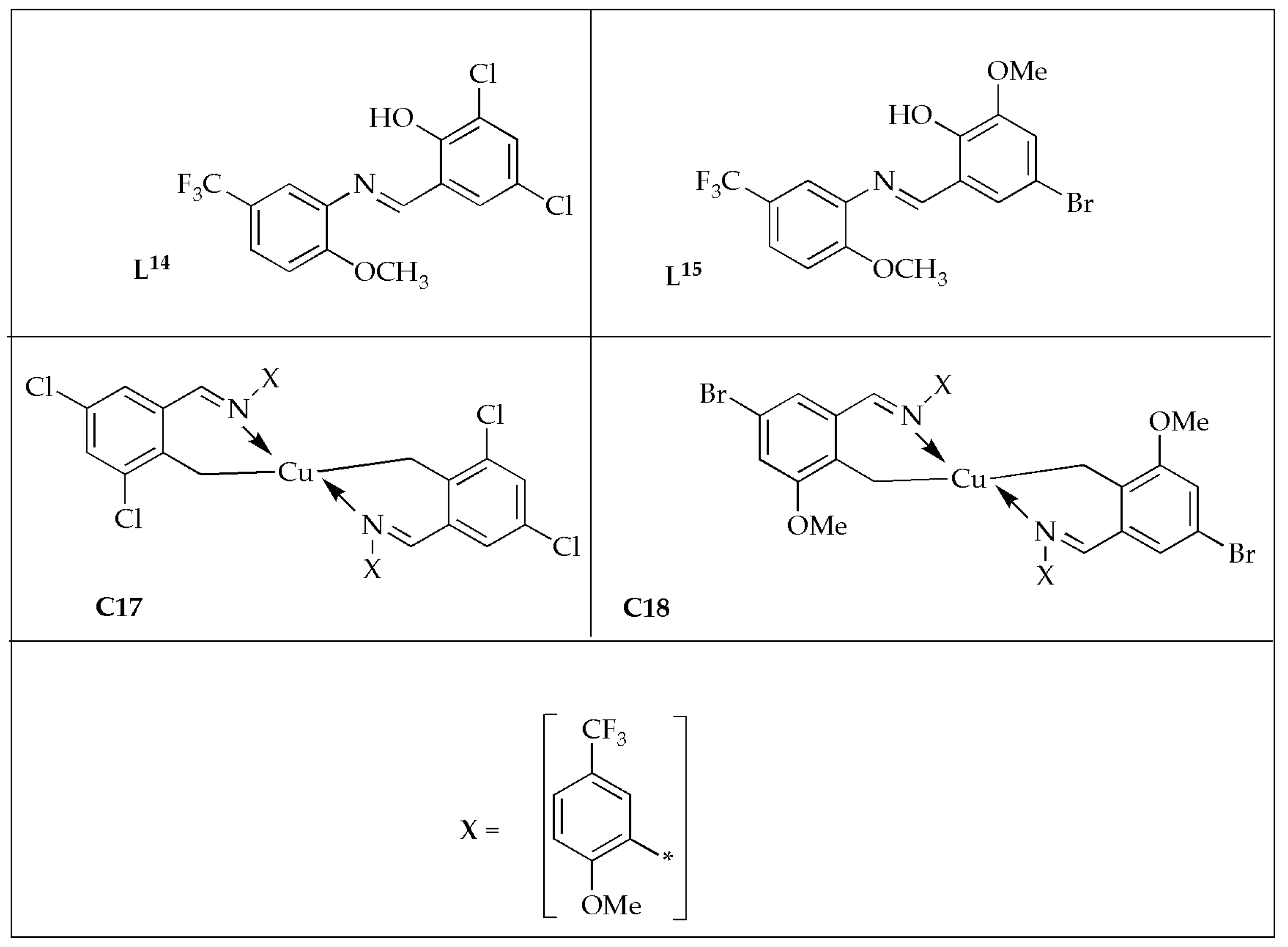
Jyothi et al. [
57] formulated novel [Cu(
L14)
2] (
C17) [Cu(
L15)
2] (
C18) complexes (
Figure 11) and reported their antibacterial effectiveness against selected bacterial strains. The free ligands exhibited moderate effectiveness against bacterial strains, whereas the Cu(II) complexes displayed good effectiveness comparable to the controls (
Table 7). The antibacterial potency was enhanced when the ligands were coordinated with the Cu(II) ion. The enhanced antibacterial effectiveness of the complexes may be attributed to the high lipophilicity of the metal complex [
55,
58]. Antibacterial results displayed that the complexes exhibited significantly more antibacterial potency than the corresponding Schiff bases but lower potency than the controls. Developing novel metal-based drugs is a potential path in treating bacterial infections.
The antibacterial studies demonstrate that Cu(II) complexes consistently exhibit improved activity compared to their corresponding free ligands. This enhancement is attributed to factors such as increased lipophilicity, better membrane permeability, and the ability of copper to participate in redox reactions, potentially leading to ROS generation in microbial cells. Complexes C1–C3, incorporating mixed ligands, showed superior potency compared to individual components, highlighting the role of synergistic ligand interactions. Thiosemicarbazone-based complexes (C4–C6) were particularly effective against Gram-negative bacteria, with structural features such as methyl substitution enhancing their efficacy. Pyrazole-based complex C7 and hydrazone-derived complexes C8–C10 further emphasized that copper coordination improves antimicrobial action, likely due to increased bioavailability and interaction with bacterial biomolecules. Schiff base and porphyrin complexes (C11–C16) demonstrated that the electronic effects of substituents (e.g., -COOMe vs. -NH2) significantly impact antibacterial outcomes.
Overall, structural variations among ligands, combined with copper’s coordination properties, contribute to the observed antimicrobial trends. These findings support the development of Cu(II) complexes as promising antibacterial agents, though further in vivo and mechanistic investigations are needed to validate their therapeutic potential.
3.1.2. Antifungal Activity of Cu(II) Complexes
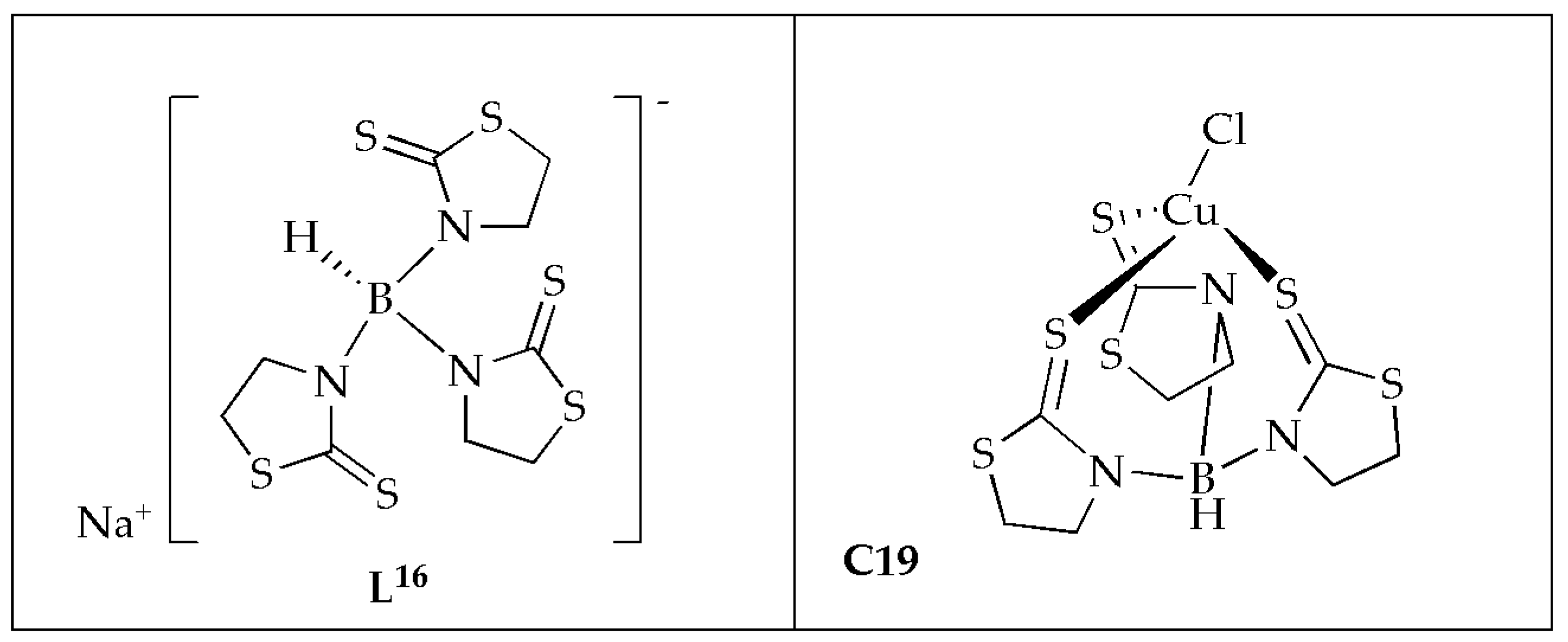
Ferreira et al. [
59] synthesized Cu(II) complex coordinated with scorpionate ligand in a tridentate mode (
Figure 12) and evaluated the antifungal activity against microorganisms of clinical importance. The MIC values ranging from 66 to 260 µmol/L
−1 demonstrate that complex
C19 exhibited the highest antifungal effectiveness. Complex
C19 demonstrated fungicidal potency against
C. albicans and
C. glabrata, with an MFC of 260 µmol/L
−1 (
Table 8). The Cu(II) complex exhibited superior antifungal activity, four times more effective than ketoconazole against
C. albicans. The inclusion of the transition metal enhanced the therapeutic index of antifungal substances in vitro. When assessing cytotoxicity in Vero cells, it showed a reduced potential for cellular injury, indicating a low risk of nephrotoxicity. The selectivity of Cu(II) complex ranged between 8 and 16 times more for
C. krusei and
C. albicans, comparable to Vero cells. These findings suggest that copper complexes are linked to enhanced antifungal activity and diminished toxicity. Given the challenge of synthesizing drugs that selectively target fungal strains due to their similarity to mammalian cells, Cu(II) coordination strategies appear favorable [
59].
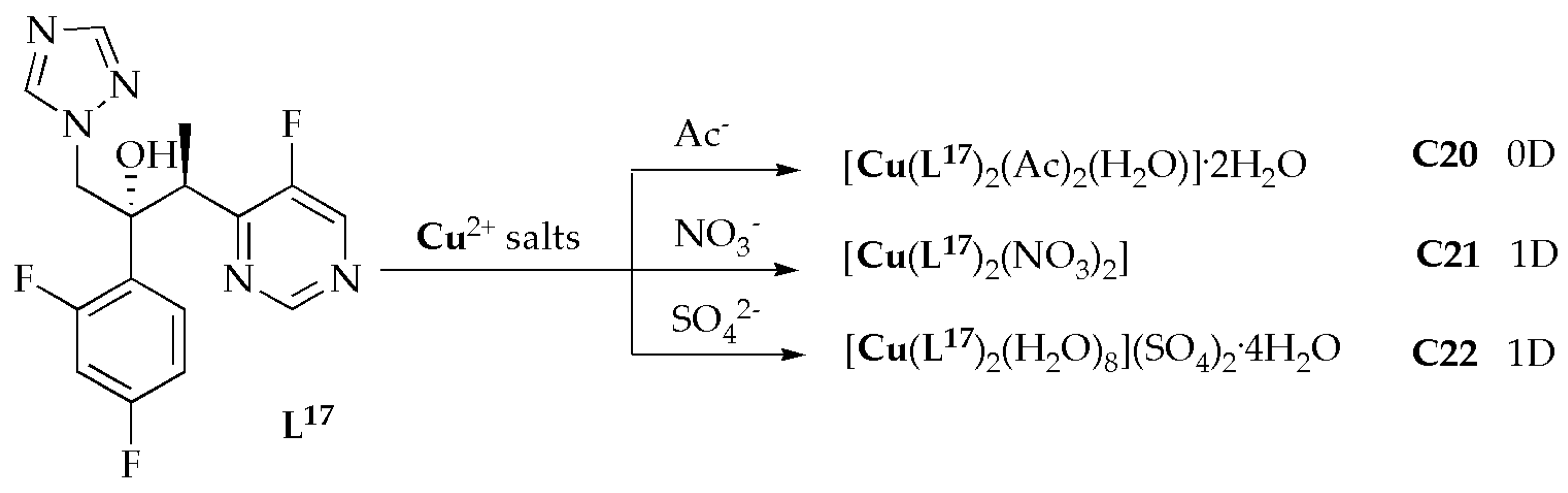
Copper(II)-based metal coordination complexes
C20–
C22 (
Figure 13) with voriconazole ligands (
L17) were synthesized and evaluated for their antimicrobial potency against selected fungal strains by Zhao et al. [
60]. The antifungal results for complexes
C20–
C22 demonstrated varying levels of efficacy in inhibiting different types of
Candida. Notably, they showed potent inhibitory activity against
C. glabrata and
C. neoformans. Amongst the complexes,
C20 notably displayed the highest effectiveness against
Candida spp., with exceptional MIC values ranging from 0.05 to 0.2 µg/mL (
Table 9). Furthermore, the free ligand
L17 had MIC values higher than most complexes, suggesting that the complexes exhibit greater efficacy against
Candida spp. comparable to the free ligand voriconazole. The antifungal assessments showed that complexes
C20 through
C22 are highly effective against a range of
Aspergillus spp. Notably, complex
C20 demonstrated the most potent inhibition of fungal effectiveness, with MIC values of 0.05, 0.05, 0.1, and 0.2 µg/mL for
A. niger,
A. flavus,
A. fumigatus, and
A. terreus, respectively. These values are either superior to or comparable to those of voriconazole. Significantly, the antifungal activities of complexes
C20–
C22 are either more potent or comparable to the free ligand. As previously mentioned, complex
C20, which contains CH
3COO
−, exhibited mononuclear motifs, whereas complex
C21, with a NO
3− ligand, and complex
C22, with a SO
42−, presented polymeric structures. Complex
C20 showed higher inhibitory efficiency than
C21 and
C22 complexes. Notably, three new Cu(II)-based metal complexes feature 0D and 1D motifs. They demonstrated exceptional inhibitory efficiency against the fungal species, indicating their potential applications in the antimicrobial field. The findings of this study serve as an outstanding example of constructing metal coordination complexes with biological activities [
60].
Ramesh et al. [
61] assessed the antifungal effectiveness of bivalent metal complexes [Cu(
L17)
2] (
C23) and [Cu(
L18)
2] (
C24) with newly synthesized Schiff bases
L18 = (1-(5-(4-flourophenyl)isoxazole-3-ylimino)methyl)napthalen-2-ol),
L19 = (2-(5-(4-flourophenyl)isoxazole-3-ylimino)methyl)-4-methylphenol) (
Figure 14), against selected fungal strains [
61]. Mancozeb was explored as a standard drug for antifungal activity. It was reported that Schiff base ligands displayed moderate effectiveness against fungal strains, while their corresponding Cu(II) complexes exhibited higher activity than the free Schiff base ligands. This enhanced activity of the complexes can be attributed to the chelate effect. Based on the inhibition zone diameter, complex
C23 demonstrated the highest activity against
Macrophomina phaseolina (
M. phaseolina) and
Sclerotium rolfsii (
S. rolfsii) when compared to complex
C24. However, it was less effective than the standard drug. Naphthalen-2-ol in complex
C23 contributed to its greater antifungal activity than
C24, which contained 4-methylphenol. The tabulated results indicate that Cu(II) complexes exhibited superior potential effectiveness against the specified fungal species (
Table 10). This is likely due to the atomic radius and electro-negativity of the Cu(II) ion. Additionally, factors such as dipole moment, solubility, complex geometry, stereochemistry, coordination sites, concentration, and hydrophobicity also contribute to the antifungal potency of these complexes. The antifungal activity of the Schiff base ligand and its metal complexes highlights that metal complexes have greater antifungal potency than the Schiff bases alone [
61].
Azam et al. [
53] prepared complex
C7.
Figure 7 containing a pyrazole nucleating ligand (
L8) with Cu(II) ion, and investigated the antifungal activity against selected fungal strains. The analysis demonstrated that the pyrazole nucleating ligand,
L8, exhibited lower activity against the tested pathogenic microorganisms compared to its synthesized complex
C7. Complex
C7 demonstrated superior efficacy, with inhibition zones ranging from 17 to 25 mm (MIC = 125 µg/mL), while the free ligand L
8 had inhibition zones of 8–13 mm (MIC = 500 µg/mL) (
Table 11). Additionally, complex
C7 exhibited good activity when compared to fluconazole, with inhibition zones of 20–28 mm (MIC = 125 µg/mL) against all the tested fungal pathogens. The complex effectively inhibited the growth of fungal strains
P. notatum,
A. niger, and
A. flavus, resulting in an expansion of the inhibition zone to approximately 23 mm (125 µg/mL). The Cu(II) complex exhibited superior antifungal potency against
A. flavus when compared to Fluconazole (control). The coordination of the ligand with the Cu(II) ion significantly improved their activity against fungal strains, showing the potential of copper complexes for antifungal applications [
53].
The antifungal effectiveness of water-soluble Cu(II) complex
C25, from bidentate-morpholine based ligand (L-morpholinopropylimino)methyl)-6-methoxyphenol), was synthesized and investigated against
C. albicans and
A. niger, by Senthilkumar et al. (
Figure 15) [
62]. The study demonstrated that the Cu(II) complex
C25 displayed greater antifungal activity than the ligand
L20. This enhanced activity was attributed to factors such as the size and charge distribution of the metal ion, as well as the shape and redox potential of the metal chelates. Based on the zone of inhibition values, it was reported that complex
C25 (23.7 ± 1.19 mm against
C. albicans and 19.8 ± 0.99 mm against
A. niger) was more potent than the ligand (13.3 ± 0.67 mm against
C. albicans and 11.7 ± 0.59 mm against
A. niger) (
Table 12). However, it was less active when compared to amphotericin (control), which had inhibition zones of 28.7 ± 1.44 mm against
C. albicans and 28.6 ± 1.43 mm against
A. niger. The observed inhibition zones for these species follow this order:
L20 <
C25 < amphotericin. As confirmed by the results, groove binding is the expected route for Cu(II) complex to interact with DNA. Molecular docking results indicated that the prepared ligand and the Cu(II) ion can interact with the DNA helix. This study confirmed that the synthesized Cu(II) complex
C25 exhibits enhanced antifungal efficacy compared to the free ligand [
62].
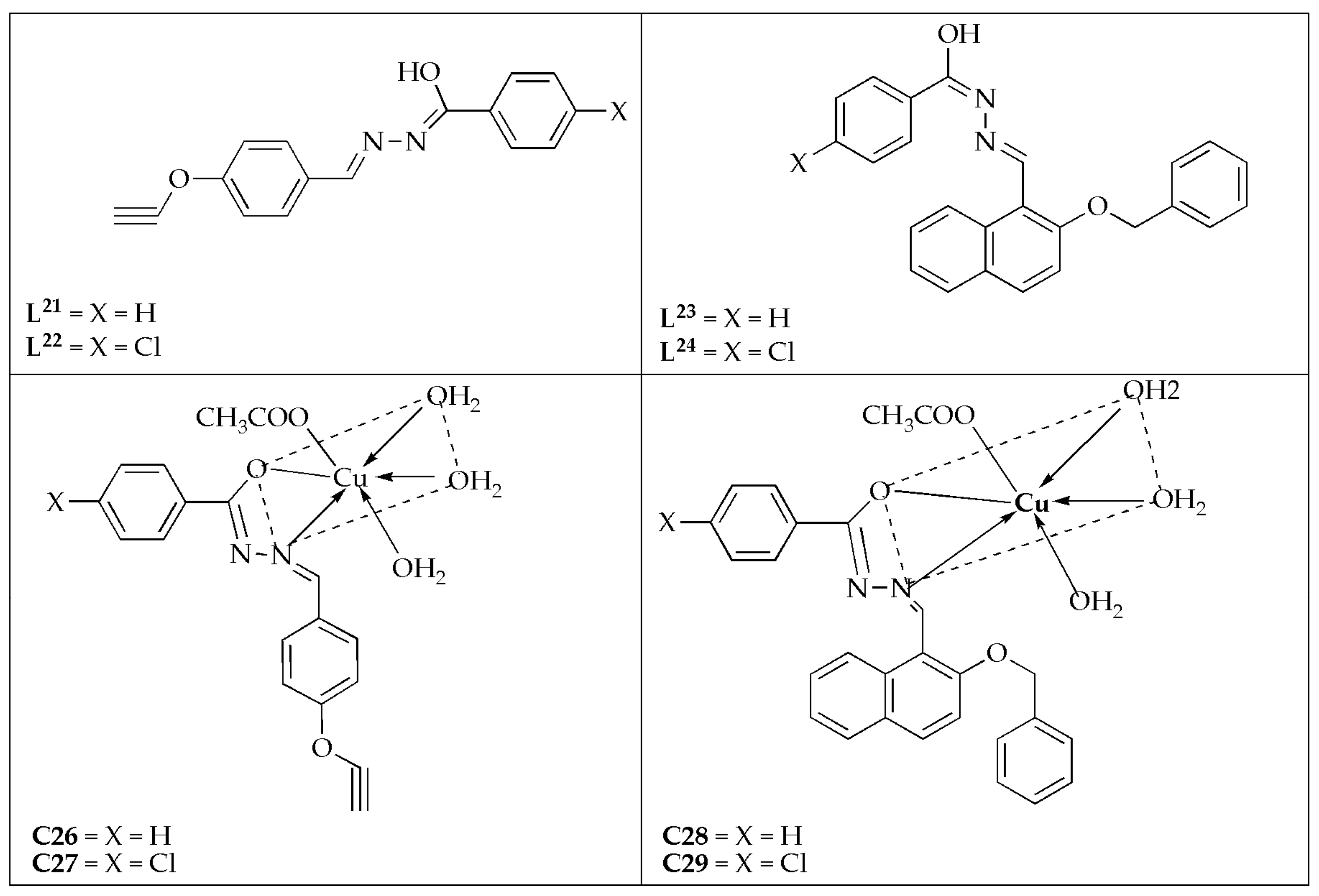
Yadav et al. [
63] synthesized a series of Cu(II) complexes prepared by four novel Schiff base ligands (
L21–
L24) (
Figure 16) and investigated their antifungal effectiveness against
C. albicans and
A. niger. The antifungal efficacy displayed by the Schiff bases follows the order:
L21 <
L22 <
L23 <
L24 against the fungal strains. This activity pattern is likely related to their molecular weights, which follow the same sequence. The synthesized complexes were more effective on
C. albicans with MIC values between 0.0052 and 0.0068 µM/mL compared to
A. niger (MIC values between 0.0102 and 0.0255 µM/mL) (
Table 13). Notably, complex
C29 (0.0052 µM/mL) was the most effective agent compared to its counterparts, exhibiting a comparable activity to the reference drug (fluconazole) with a MIC value of 0.0051 µM/mL against
C. albicans. The reactivity and inhibition trend against the tested microbes follows the sequence of
L21−24 <
C26 <
C27 <
C28 <
C29 ≤ fluconazole. The Cu(II) complexes exhibited the highest potency. Schiff base hydrazone ligands exhibited greater potency when coordinated with the Cu(II) ion [
63].
The antifungal studies reviewed reveal that coordination of bioactive ligands with Cu(II) significantly enhances antifungal potency. This effect is commonly attributed to increased lipophilicity, improved cell permeability, and possible interaction with fungal biomolecules or the generation of reactive oxygen species.
Complexes such as C19 and C20 demonstrated superior activity against Candida and Aspergillus species, often outperforming standard drugs like ketoconazole and fluconazole. The enhanced efficacy is linked to ligand structure, coordination geometry, and nuclearity of the complexes. Schiff base-derived complexes (C23–C29) and those with pyrazole or morpholine-based ligands also exhibited more potent activity than their corresponding free ligands, supporting the chelation theory.
These results suggest that Cu(II) complexes are promising candidates for antifungal drug development. However, further studies, particularly the mechanisms of action, toxicity, and in vivo efficacy, are essential to advance them toward clinical applications.