Abstract
Biofilms, structured microbial consortia embedded in self-produced extracellular matrices, pose significant challenges across the medical, industrial, and environmental sectors due to their resistance to antimicrobial therapies and ability to evade the immune system. Their resilience is driven by multifaceted mechanisms, including matrix-mediated drug sequestration, metabolic dormancy, and quorum sensing (QS)-regulated virulence, which collectively sustain persistent infections and contribute to the amplification of antimicrobial resistance (AMR). This review critically examines the potential of plant-derived essential oils (EOs) as innovative agents for biofilm control. EOs exhibit broad-spectrum antibiofilm activity through multi-target mechanisms, including disrupting initial microbial adhesion, degrading extracellular polymeric substances (EPSs), suppressing QS pathways, and compromising membrane integrity. Their ability to act synergistically with conventional antimicrobials at sub-inhibitory concentrations enhances therapeutic efficacy while reducing the selection pressure for resistance. Despite their potential, EO applications face technical challenges, such as compositional variability due to botanical sources, formulation stability issues, and difficulties in standardization for large-scale production. Clinical translation is further complicated by biofilm stage- and strain-dependent efficacy, insufficient in vivo validation of therapeutic outcomes, and potential cytotoxicity at higher doses. These limitations underscore the need for optimized delivery systems, such as nanoencapsulation, to enhance bioavailability and mitigate adverse effects. Future strategies should include combinatorial approaches with antibiotics or EPS-degrading enzymes, advanced formulation technologies, and standardized protocols to bridge laboratory findings to clinical practice. By addressing these challenges, EOs hold transformative potential to mitigate biofilm-associated AMR, offering sustainable, multi-target alternatives for infection management and biofilm prevention in diverse contexts.
1. Introduction
Biofilms represent a cornerstone of microbial survival, enabling bacteria and fungi to thrive in hostile environments by forming structured, surface-adherent communities encased within a self-produced extracellular polymeric matrix [1]. These multicellular consortia are not merely passive aggregates but dynamic systems conferring significant survival advantages, including enhanced resistance to antimicrobial agents, immune evasion, and resilience against environmental stressors [2]. The ability of biofilms to colonize biotic and abiotic surfaces—ranging from human tissues to medical devices and industrial systems—underscores their clinical and environmental relevance [3]. By facilitating persistent infections, biofilm formation is intricately linked to chronic diseases, recurrent infections, and the failure of conventional therapeutic interventions, positioning biofilms as a critical challenge in modern microbiology [4].
The resistance of biofilms to treatment arises from a complex interplay of structural, physiological, and genetic factors. The extracellular matrix acts as a physical barrier, impeding drug penetration and sequestering antimicrobial agents, while the heterogeneous metabolic states of embedded cells, including dormant persister phenotypes, further reduce therapeutic efficacy. Concurrently, biofilm-associated microorganisms exhibit upregulated efflux pump activity, altered gene expression, and enhanced horizontal gene transfer, accelerating the development and dissemination of multidrug resistance (MDR). These mechanisms collectively render traditional antibiotics and antifungals insufficient, necessitating innovative strategies to disrupt biofilm integrity and restore antimicrobial susceptibility [5,6,7].
In response to these challenges, the scientific community has shifted its focus toward exploring alternative antimicrobial agents that can target biofilms through multifaceted mechanisms [8,9]. Plant-derived essential oils (EOs) have emerged as a promising frontier. These complex phytochemical mixtures exhibit broad-spectrum antimicrobial activity, disrupting biofilms through multiple pathways: destabilizing microbial membranes; inhibiting QS—a critical communication system that regulates biofilm development; and degrading extracellular matrix components [10,11]. Furthermore, EOs often exhibit synergistic effects when combined with conventional antimicrobials, thereby enhancing drug efficacy while minimizing the development of resistance [12]. Their ability to act at sub-inhibitory concentrations, natural origin, and low-toxicity profiles position them as viable candidates for standalone and adjunct therapies.
The chemical composition and antimicrobial properties of EOs are significantly influenced by geographic origin, climate conditions, and harvest-time air temperatures [13]. Studies have shown that plants’ EOs vary in chemical profile depending on whether they are grown in mountain, coastal, or inland regions [14]. This geographic variation is linked to differences in the production of key secondary metabolites, including monoterpenes, linalool, and eugenol, which contribute to aroma and bioactivity [13]. Environmental factors such as altitude, solar radiation, and water availability further affect the concentration and yield of EOs [15]. Seasonal temperature fluctuations, particularly temperature stress, have also been found to alter EO profiles [16,17]. These composition variations directly impact the oils’ antimicrobial efficacy, with certain chemotypes demonstrating enhanced activity against common pathogens like Escherichia coli and Staphylococcus aureus under specific environmental conditions [14,16].
This review article aims to analyze strategies for biofilm control using plant-derived bioactive molecules comprehensively. Given the exponential proliferation of studies in this domain over recent years, this work systematically synthesizes cutting-edge research to delineate emerging trends and evidence-based innovations. The review focuses on EOs as multifunctional antibiofilm agents. It elucidates their mechanisms of action—including quorum sensing (QS) inhibition, extracellular matrix disruption, and efflux pump modulation—while evaluating their synergies. Furthermore, it critically assesses their applicability across clinical, industrial, and environmental contexts, addressing challenges such as bioavailability and standardization. By consolidating the most recent advancements, this analysis underscores the transformative potential of natural compounds in mitigating biofilm-associated infections and antimicrobial resistance, offering a timely update to guide future research and translational applications.
2. Overview of Biofilms
2.1. Biofilm Formation
Biofilm formation is a dynamic, multistage process enabling microbial communities to adhere to biotic or abiotic surfaces, proliferate, and persist in diverse environments. The process is universally characterized by sequential phases, i.e., initial attachment, irreversible adhesion, colonization, maturation, and dispersal, with variations observed across microbial species and environmental contexts [2,18].
2.1.1. Initial Attachment and Adhesion
The biofilm lifecycle begins with the reversible attachment of planktonic cells to surfaces through weak interactions, including van der Waals forces, electrostatic interactions, and hydrophobic effects. Surface properties, such as roughness, charge, and hydrophobicity, significantly influence this phase [19]. For example, hydrophobic and positively charged surfaces enhance the adhesion of S. aureus and oral streptococci (Streptococcus mutans), while negatively charged surfaces deter attachment [4,20]. Bacterial appendages, including flagella, pili, and fimbriae, mediate surface contact and overcome repulsive forces. In Pseudomonas aeruginosa, the Pil-Chp surface sensing system triggers the accumulation of cyclic di-GMP (c-di-GMP), transitioning cells from reversible to irreversible adhesion by upregulating adhesins and extracellular polymeric substance (EPS) production [21]. Similarly, in Candida albicans, adhesins such as Als1, Als3, and Hwp1 facilitate rapid attachment to both biotic (e.g., epithelial tissue) and abiotic (e.g., catheters) surfaces within 60–90 min [22].
2.1.2. Colonization and Microcolony Development
Irreversible adhesion is consolidated through molecular interactions (e.g., hydrogen bonding) and EPS secretion [23]. In S. aureus, microbial surface components recognizing adhesive matrix molecules (MSCRAMMs), such as fibronectin-binding proteins (FnBPA/B) and clumping factors (ClfA/B), anchor cells to host tissues, while wall teichoic acids mediate attachment to abiotic surfaces [24]. EPSs, composed of polysaccharides, proteins, extracellular DNA (eDNA), and lipids, act as a structural scaffold. For instance, poly-β-(1–6)-N-acetylglucosamine (PNAG) in Staphylococcus epidermidis and galactosaminogalactan (GAG) in Aspergillus fumigatus stabilize microcolonies [25,26]. In Vibrio cholerae, cell-surface adhesion proteins (RbmA, RbmC, and Bap1) drive the transition from 2D monolayers to 3D clusters, with mechanical forces during cell division promoting vertical expansion [27,28].
2.1.3. Maturation and Structural Complexity
Maturation involves the development of a stratified 3D architecture with functional and metabolic heterogeneity [29]. In oral biofilms, stratification creates oxygen and nutrient gradients, allowing anaerobic pathogens like Porphyromonas gingivalis to thrive in the deeper layers [20]. As observed in P. aeruginosa and S. epidermidis biofilms, water channels within the EPS matrix facilitate the distribution of nutrients and the removal of waste [25,30]. Fungal biofilms, such as those of C. albicans, exhibit spatial organization with yeast cells at the base and hyphae interspersed within a mannan–glucan ECM [31]. QS molecules, including acyl homoserine lactones (AHLs) and autoinducer-2 (AI-2), coordinate EPS production and metabolic specialization in multispecies communities [32,33].
2.1.4. Dispersal and Recolonization
Dispersal is triggered by environmental stressors such as nutrient depletion, hypoxia, or genetic regulation [34]. In P. aeruginosa, the enzymatic degradation of EPSs (e.g., proteases and nucleases) and surfactants, such as rhamnolipids, facilitates the release of motile cells [35,36]. C. albicans dispersal involves transcriptional regulators (Nrg1 and Pes1) that promote the release of adhesive, virulent yeast cells [31]. The dispersed cells often exhibit phenotypic variations, such as antibiotic-tolerant persister cells in S. aureus, which enhances their survival and recolonization potential [25].
2.2. Regulation of Biofilms
Biofilm regulation is a multifaceted process governed by genetic, biochemical, and environmental mechanisms, enabling microbial communities to adapt to diverse conditions [37]. Central to this regulation are QS systems and second-messenger molecules, which coordinate the development of biofilms across bacterial and fungal species [38]. QS facilitates cell-density-dependent communication through signaling molecules such as acyl-AHLs in Gram-negative bacteria, autoinducing peptides (AIPs) in Gram-positive bacteria, and universal autoinducer-2 (AI-2) [39]. For instance, in P. aeruginosa, hierarchical QS systems (Las, Rhl, PQS, and IQS) regulate virulence factors, EPS synthesis, and dispersal enzymes. At the same time, AI-1-mediated QS in Acinetobacter baumannii and AI-2 in Bifidobacterium longum modulate biofilm formation [40,41,42]. Similarly, in V. cholerae, HapR-driven QS controls biofilm dispersal [43].
Cyclic di-GMP (c-di-GMP) is a pivotal second messenger that promotes biofilm stability by upregulating adhesins, pili, and EPS production while suppressing motility [44]. Elevated c-di-GMP levels in Acidithiobacillus thiooxidans enhance biofilm formation in acidic environments via PilZ and PelD proteins [45]. Conversely, low c-di-GMP levels in P. aeruginosa and V. cholerae favor dispersal [46]. The interplay between QS and c-di-GMP is evident in Thermotoga maritima, where mutual regulation optimizes biofilm dynamics [47]. Cyclic AMP (cAMP) also modulates biofilm architecture; in P. aeruginosa, cAMP levels influence the formation of pellicle or bottom biofilms, depending on environmental conditions [48].
In fungal biofilms, such as those formed by C. albicans, transcriptional networks orchestrate adhesion, matrix production, and dispersal. Six master regulators (Efg1, Tec1, Bcr1, Ndt80, Brg1, and Rob1) form an interconnected circuit that controls over 1000 target genes, including adhesins (ALS1, ALS3, and HWP1) and matrix components [31,49]. Matrix biosynthesis is further regulated by Zap1 (negative) and Rlm1 (positive), while dispersal involves Nrg1, Pes1, and chromatin-modifying complexes [50].
Environmental factors such as pH, nutrient gradients, and oxygen availability fine-tune biofilm behavior. In oral streptococci, membrane-bound sensors detect changes in redox potential and pH, triggering adaptive genetic responses [51]. S. mutans dominates cariogenic biofilms through aciduricity and acidogenicity [52]. Hypoxia in P. aeruginosa biofilms induces antibiotic tolerance by limiting the uptake of reactive oxygen species (ROS) and the proton motive force (PMF)-dependent drug [53,54]. Mechanical forces, including cellular pressure and matrix phase separation, also influence the architecture of biofilms [55]. For example, V. cholerae biofilms use VPS polysaccharides and proteins (RbmA, RbmC, and Bap1) to form viscoelastic hydrogels. At the same time, mechanical stress in S. epidermidis triggers pH-dependent matrix reorganization [7].
Persister cells further reinforce biofilm resilience, which enters dormancy via a stringent response (ppGpp) and toxin–antitoxin systems (e.g., HipBA). These cells evade antibiotics and repopulate biofilms post-treatment [56,57]. In S. aureus, global regulators like Agr, SarA, and SigB balance adhesin expression, protease activity, and stress adaptation. Agr-mediated dispersal via phenol-soluble modulins (PSMs) contrasts with SarA’s promotion of polysaccharide intercellular adhesin (PIA) [58].
Synthetic biology approaches have advanced biofilm regulation in engineered systems. The overexpression of cytochromes and riboflavin pathways in Shewanella oneidensis enhances electron transfer efficiency in bioelectrochemical biofilms [36]. Polymicrobial interactions and functional redundancy further stabilize biofilm signaling under environmental stressors, though disruptions in microbial diversity can impair QS-mediated cross-kingdom communication [32,59].
2.3. Functional Significance of Biofilms
Biofilms serve as critical survival structures for bacteria, enabling resilience against environmental stressors, antimicrobial agents, and host immune responses [60]. The EPS matrix, composed of polysaccharides, proteins, eDNA, and lipids, forms a multifunctional barrier that shields embedded microbial communities from physical and chemical threats [61]. For instance, biofilms reduce UV penetration, allowing only 13% of UV-C, 31% of UV-B, and 33% of UV-A to reach cells, as demonstrated in P. aeruginosa, Listeria monocytogenes, and Deinococcus geothermalis [18,62]. Similarly, thermophiles (Sulfolobus acidocaldarius) and psychrophiles (Bacteriovorax) rely on EPSs to buffer extreme temperatures and resist freeze–thaw cycles, while acidophiles (Enterococcus faecalis) and halophiles (Halomonas stenophila) utilize matrix components, such as inositol and 3-O-methyl glucose, to mitigate pH fluctuations and salinity stress [63,64,65,66].
A hallmark of biofilms is their ability to confer antibiotic tolerance and resistance. The EPS matrix impedes drug diffusion, binds antimicrobial agents, and facilitates enzymatic inactivation, such as that caused by β-lactamases in Klebsiella pneumoniae [1,67]. Additionally, reduced metabolic activity in deeper biofilm layers diminishes antibiotic efficacy, while efflux pumps and persister cells enhance survival [68,69,70]. Sub-inhibitory antibiotic concentrations further induce biofilm thickening, as observed in S. aureus and polymicrobial communities involving C. albicans and E. coli [71,72].
In clinical settings, biofilms drive persistent infections by evading immune recognition. EPSs mask pathogen-associated molecular patterns (PAMPs), reduce phagocytosis, and neutralize antimicrobial peptides [73]. For example, P. aeruginosa biofilms utilize alginate to block complement deposition, whereas S. epidermidis modulates immune responses through surface proteins [74,75]. Biofilms on medical devices (e.g., catheters and implants) act as reservoirs for chronic infections, necessitating device removal due to resistance to treatment [76].
Ecologically, biofilms facilitate horizontal gene transfer (HGT), promoting genetic adaptability. High cell density and matrix stability enhance conjugation, transformation, and transduction [77]. In marine systems, biofilms release metabolites that induce larval settlement in corals and macroalgae, though climate change alters these interactions [32].
Biofilms also dominate food processing environments, where pathogens such as L. monocytogenes and Salmonella enterica resist disinfectants through persister cells and EPS-mediated nutrient retention [46]. Similarly, oral biofilms (S. mutans and P. gingivalis) drive dental caries and periodontitis through acidic microenvironments and dysbiotic shifts [20].
3. Antibiofilm Efficacy of EOs
3.1. Factors Influencing Variability
Efficacy disparities in biofilm reduction by EOs arise from multiple interconnected factors, including microbial strain susceptibility, biofilm maturity, compositional oil differences, assay methodologies, and environmental conditions. Strain-specific responses are evident in examples such as Tea Tree EO (TTEO), which reduced Xanthomonas oryzae initial biofilm biomass to 18% at the minimum inhibitory concentration (MIC) of 18 mg/mL [78], and Coconut Oil, which exhibited starkly divergent activity: 65.48% reduction against C. albicans but only 15% against S. aureus [79]. Similarly, P. aeruginosa and S. mutans display differential resistance to the same EOs due to inherent genetic or structural adaptations [79].
Biofilm maturity critically impacts outcomes, with pre-formed biofilms often requiring higher doses for eradication than nascent biofilms. For instance, Crithmum maritimum EO achieved ~95% inhibition against methicillin-resistant S. aureus (MRSA) at ½ × MIC (MIC= 5.644 mg/mL), likely targeting metabolic pathways without bactericidal effects [80].
Methodological variability further complicates cross-study comparisons. Assay techniques such as Crystal Violet staining (measuring biomass) and log CFU reduction (viability) yield divergent results, as observed in studies of a Cinnamon/Cardamom EO combination tested against E. coli and Bacillus subtilis [81]. The Crystal Violet staining assay, wherein EOs are typically tested against mature biofilms formed in multi-well microtiter plates, is widely employed to quantify biofilm biomass. This method involves staining the biofilm matrix and measuring absorbance to evaluate the extent of biofilm reduction [82,83]. This assay is often used with the viable cell count method, which involves plating biofilm samples on agar plates post-treatment to count the surviving cells, providing insights into the EO’s effect on biofilm viability. This method allows researchers to assess both the reduction in biofilm mass and the viability of the cells within the biofilm [82,84].
Additionally, confocal laser scanning microscopy (CLSM) allows for the high-resolution imaging of biofilms, which in turn allows for the visualization of biofilm architecture and the penetration of EOs into biofilm layers. This method is handy for evaluating the spatial distribution and activity of EOs within the biofilm matrix, helping researchers assess how effectively the EO permeates and disrupts biofilm structure [85,86].
Furthermore, the microtiter plate assay has become a high-throughput method for assessing antibiofilm activity. In this method, biofilm formation is induced in a 96-well plate, and the EO is applied to the biofilm, with results being quantified via absorbance measurements or viable cell counts. The microtiter plate assay provides a convenient and efficient way to assess EO activity against biofilms in a controlled environment and has been widely applied in various studies [82,87].
Environmental matrices, including food models or meat substrates, alter EO activity [88]; for example, matrix effects on meat reduced EO efficacy against Yersinia enterocolitica [89]. The influence of the matrix on EO efficacy is typically assessed by using standard biofilm assays with or without the presence of complex matrices. For example, in food models, biofilm-forming bacteria are exposed to EOs under conditions mimicking real-world environments [89,90,91].
Nanoformulations and hybrid delivery systems enhance bioavailability and potency, exemplified by cinnamon EO–colistin nanoliposomes [92] and thymol combined with biogenic silver nanoparticles (bioAgNPs) [93], which synergistically disrupt biofilm integrity. Experimental conditions such as nanoemulsions or magnetite conjugation (e.g., Eucalyptus globulus EO) [94] and food-model assays (e.g., Citrus limon EO on kohlrabi surfaces) [91] further influence reported efficacy. These techniques enhance the penetration and effectiveness of EOs against biofilms by increasing their stability, solubility, and delivery to biofilm cells.
3.2. Antibiofilm Activity of EOs from Different Medicinal Plants
EOs derived from medicinal plants demonstrate significant antibiofilm activity across bacterial and fungal pathogens, with efficacy being influenced by concentration, formulation strategies, and synergistic combinations (Table 1 and Table S1 in Supplementary Data). Thyme (Thymus spp.), oregano (Origanum vulgare), and cinnamon (Cinnamomum zeylanicum) EOs exhibit potent activity against clinically relevant biofilms, including those formed by multidrug-resistant (MDR) strains. Innovative delivery systems, such as nanoparticle encapsulation or combinatorial therapies, further enhance their effectiveness in medical, industrial, and food safety applications.

Table 1.
Antibiofilm activity of some EOs.
4. Phytochemical Composition–Activity Correlation
EOs exhibit antibiofilm activity primarily due to dominant phytochemicals, synergism between compounds, and advanced delivery systems. Table 2 and Table S2 (Supplementary Data) summarize the key phytochemicals, their sources, inhibitory effects, and mechanisms. Phenolic monoterpenes, such as thymol and carvacrol, are among the most potent agents, disrupting microbial membranes, inhibiting QS, and degrading EPSs. For instance, T. vulgaris EO (75.46% thymol) achieves complete biofilm eradication in Cutibacterium acnes via membrane lysis and oxidative stress [103]. Synergistic combinations, such as carvacrol–p-cymene in O. vulgare EO, enhance penetration and gene suppression [80]. Non-phenolic terpenes, including 1,8-cineole and limonene, show moderate efficacy but require higher concentrations or formulation improvements (e.g., magnetite conjugation in E. globulus EO) [94]. Lower activity in oils like Sage EO (33% thujones) correlates with limited mechanistic disruption [104]. Nanoemulsions and combinatorial therapies (e.g., colistin nanoliposomes [92]) significantly amplify efficacy, underscoring the importance of delivery optimization.

Table 2.
Key phytochemicals, EOs, and their antibiofilm activity.
5. Concentration-Dependent Efficacy: Thresholds and Nonlinear Responses in EO Activity
5.1. Thymus spp.: Sub-MIC Efficacy and Plateau Effects
Thymus serpyllum EO (Slovakia) demonstrated progressive biofilm inhibition, achieving a 72.93% reduction at MIC/2 (MIC50= 0.134 mg/mL) and more potent effects at higher doses [108]. T. vulgaris EO (India) reduced C. albicans biofilms by 80% at 0.5 × MIC (MIC= 25 µg/mL) to MIC [109], while its thymol chemotype variant (Spain) required 2 × MIC (MIC= 0.625 μL/mL) for 95.90% inhibition against unspecified strains [110]. Notably, T. vulgaris EO (Egypt) achieved complete biofilm eradication at 0.053 g/mL [103]. Thymus citriodorus (Portugal) exhibited high efficacy with Effective Concentration 50 (EC50) values as low as 0.062% [111], whereas T. capitatus EO required 0.03–0.13% v/v for total inhibition, highlighting formulation-dependent activity [100]. In contrast, T. mastichina (Portugal) exhibited inefficiency, requiring more than 2000% concentrations for partial biomass reduction [111]. Data gaps persist for oils like T. vulgaris EO (Pakistan), which reported 83.3% inhibition without detailed thresholds [112].
5.2. Origanum Vulgare
O. vulgare EO (Greece) exhibited dose-dependent biofilm reduction, increasing from 93.1% at ½ × MIC (MIC = 0.091 for MRSA strain and 0.182 mg/mL for MSSA strain) to 95.8% at 4 × MIC against Staphylococcus strains [80]. However, a plateau effect occurred beyond 4 mg/mL for the Kazakhstan variant [113]. Paradoxically, the Hungary-derived EO increased Neisseria gonorrhoeae biofilm biomass at high doses [114].
5.3. Cinnamomum spp.
C. cassia EO (China) demonstrated MIC values as low as 0.02% vol/vol against C. auris biofilms [99]. Cinnamon EO (Brazil) disrupted 90–100% of S. mutans and S. aureus biofilms at 4 × MIC (MIC= 0.056 mg/mL for S. mutans and 0.315 mg/mL for S. aureus) [115], while C. verum EO (Sri Lanka) inhibited C. albicans biofilms at 0.1 mg/mL [116]. Pre-formed L. monocytogenes biofilms required 2 × MIC (0.100 mg/mL) for eradication [117]. Sub-MIC cinnamaldehyde (Iran) reduced E. coli biofilm-related gene expression by 40% [118].
5.4. Tea Tree EOs (Maleuleka alternifolia)
TTEO achieved 100% inhibition against S. mutans at ≥0.5% concentration [119], while its primary component, terpinen-4-ol, showed >80% inhibition at 0.5 × MIC (MIC = 0.25% (v/v)) and near-complete eradication at 2–4 × MIC [120]. Sub-inhibitory TTEO doses reduced X. oryzae biofilm biomass but were less effective than MIC (18 mg/mL) [78]. Synergy with antibiotics enhanced efficacy at lower doses [121]. TTEO (Portugal) exhibited strain-specific sensitivity, with EC50 values ranging from 0.199% to 1.760% across the tested bacterial strains [111].
5.5. Eucalyptus-Derived EOs
Eucalyptus EO from China demonstrated dose-dependent inhibition against biofilm-forming bacteria, achieving a 77.53% reduction at 2× MIC (MIC= 15 mg/mL) [122]. Eucalyptol, a primary component of Eucalyptus EO, displayed potent sub-MIC activity, inhibiting biofilms at concentrations below MIC, suggesting non-bactericidal mechanisms [123]. E. globulus EO sustained biofilm inhibition for 24–72 h at the tested concentrations without requiring dose escalation, indicating prolonged efficacy [94]. In contrast, Eucalyptus griffithsii EO exhibited a linear dose–response relationship, achieving 93.73% inhibition against S. aureus at 20 µL/mL [124].
5.6. Citrus and Cymbopogon EOs
C. limon EO achieved 95% biofilm reduction against N. gonorrhoeae at 20–40 mg/mL [114], while Citrus limetta EO showed a linear dose–response relationship, with 0.5 mg/mL being sufficient for >90% inhibition [125]. C. flexuosus EO inhibited biofilms at 1 mg/mL but failed against mature biofilms at 16 mg/mL [101]. Cymbopogon martinii EO reduced planktonic and biofilm CFUs of C. albicans by 1.94 and 2.75 log10, respectively, at MIC/2 (MIC = 0.25 mg/mL) [126]. Conversely, Cymbopogon citratus EO fractions showed fungicidal effects only at MIC (0.16 mg/mL) levels, highlighting potential toxicity thresholds [127].
5.7. Clove and Other Notable EOs
Clove EO demonstrated optimal biofilm inhibition at 3% concentration, though higher doses did not enhance efficacy against unspecified bacterial strains [115]. Synergistic combinations, such as eugenol paired with mupirocin, improved biofilm reduction even at sub-MIC levels [128]. When formulated in poly-lactic acid-based nanoemulsions, carvacrol required 8% v/v for optimal activity against biofilm-forming pathogens [97].
5.8. Sub-MIC Efficacy and Nonlinear Responses
Eucalyptol and linalool (from Hedychium larsenii) inhibited Streptococcus pyogenes biofilms by 91% at 0.004% v/v, with effects plateauing beyond this concentration [129]. C. verum EO reduced S. aureus biofilms by 70% at 4×MIC (MIC= 0.048 mg/mL), with activity being detectable at MIC/16 [130]. However, diminishing returns were observed in citral, where 0.5% yielded ~80% biomass reduction but higher doses did not improve efficacy [131]. Similarly, lemongrass EO peaked at 0.3125% against dual-species biofilms, with no improvement at 10% [131].
5.9. Strain-Specific Variability
Manuka EO exhibited divergent effects of a 75.6% reduction in S. aureus biofilms at MIC/2 (MIC= 0.233 mg/mL) but no significant change against L. monocytogenes [132]. Sage EO showed inconsistent dose–response effects, with higher doses not constantly improving efficacy, likely due to strain-specific resistance or saturation [107]. Basil EO similarly displayed variable effects, with lower concentrations sometimes matching higher doses (e.g., 81.4–99.9% pre-adhesion inhibition) [107].
5.10. High-Potency and Threshold Variability
Rosemary EO from Ghana showed a clear dose–response relationship against Salmonella Typhi, with an IC50 of 2 µg/mL [133]. Garlic EO achieved 100% inhibition of L. monocytogenes biofilms at MIC and 2 × MIC (MIC= 0.100 mg/mL), with 68% eradication of pre-formed biofilms at MIC [117]. Aloysia rugosa EO required only 0.04 mg/mL for >90% inhibition [134], whereas Cuminum cyminum EO needed 50 mg/mL to reach 76.29% reduction [135]. Salvia sclarea EO achieved 87.34% biofilm reduction at 62.5 µL/L vapor concentration [136], while lavender EO required 2 × MIC for near-total biofilm removal [137].
5.11. Synergistic Effects of EOs with Other Substances
Combining EOs with nanoparticles, antibiotics, or advanced delivery systems has emerged as a promising strategy to overcome biofilm resistance and reduce effective doses. For instance, niosome-loaded oregano EO exhibited 2–4× greater biofilm inhibition than free EO at sub-MIC levels, attributed to enhanced bioavailability and controlled release [130]. Similarly, O. vulgare EO paired with biogenic silver nanoparticles (bioAgNPs) achieved 80–99% reduction in K. pneumoniae and E. coli biofilms at low sub-MIC concentrations, leveraging nanoparticle penetration and EO-mediated membrane disruption [79]. Nanoformulations, such as Cinnamomum EO–colistin nanoliposomes, eradicated S. aureus biofilms within 12 h without cytotoxicity, even at high doses [84]. Synergy with conventional antimicrobials has also been demonstrated: eugenol paired with mupirocin reduced pre-formed L. monocytogenes biofilms by 76.82% at sub-MIC levels, suggesting complementary mechanisms of action [85]. These combinatorial approaches enhance efficacy and mitigate risks of resistance development, positioning them as critical tools in biofilm management [79,84,130].
6. Toxicity and Prolonged Exposure
The clinical translation of EOs for biofilm mitigation necessitates rigorous safety assessments, as their bioactive components may exhibit dose-dependent toxicity, organ-specific risks, and route-of-administration challenges. The current literature highlights critical gaps in standardized toxicological evaluations, contrasting safety profiles among EOs, and insufficient in vivo validation, which hinder their transition to clinical or industrial applications [138,139].
6.1. Cytotoxicity and Organ Toxicity
Several studies report cytotoxicity associated with EO components, particularly at concentrations exceeding MICs. For instance, clove EO (eugenol-rich) demonstrated cytotoxicity to human kidney cells and zebrafish embryos at MIC × 2 concentrations (46.0 µg/mL to 102.0 µg/mL), despite efficacy against Helicobacter pylori [140]. Similarly, cinnamon oil nanoemulsions (CEONs) caused cytotoxicity in bovine enamel models at 5% concentration, with potential allergic reactions noted [141]. Organ-specific risks are evident in E. globulus EO, which induced oxidative stress and mitochondrial dysfunction in C. albicans but lacked safety data for human mucosal tissues [123]. Notably, thymol and carvacrol—common in Lamiaceae EOs—exhibited membrane disruption in vitro but raised concerns about epithelial irritation in topical applications [80,142].
6.2. Dose Dependency and Administration Route Risks
Dose–response relationships often reveal a narrow therapeutic window. For example, O. vulgare EO reduced Salmonella biofilm by 93–95% at ½ × MIC (0.091 mg/mL)—4 × MIC (0.182) but showed inconsistent disc diffusion results at higher doses, suggesting potential tissue damage [80]. Volatile EOs, such as rosemary and lavender EOs, demonstrated reduced efficacy in vapor phases compared with liquid formulations, with unpredictable diffusion kinetics in clinical settings [142]. Sub-inhibitory doses of caraway EO (63.7% carvone) suppressed P. aeruginosa virulence but failed to eradicate biofilms, risking residual pathogenicity [143]. Oral administration risks are underscored by Syzygium aromaticum EO (clove), which caused cytotoxicity in salivary orthodontic models at MIC levels, necessitating short-contact formulations to mitigate mucosal damage [144].
6.3. Lack of Standardization in Safety Protocols
Variability in EO composition due to geographic, seasonal, and extraction factors complicates safety assessments. T. capitatus EO exhibited chemotype-dependent toxicity, with carvacrol-dominant variants showing higher cytotoxicity than thymol-rich counterparts [145]. Similarly, Cinnamomum verum EO safety varied by cinnamaldehyde content (52–80%), with no consensus on acceptable thresholds for dermal or oral use [99]. Few studies quantified lethal dose (LD50) or no-observed-adverse-effect levels (NOAELs), and protocols for biofilm-specific toxicity (e.g., MBEC/MBIC ratios) remain non-standardized [140,146].
6.4. Contrasting Safety Profiles Across EO Types
Phenolic-rich EOs (e.g., thyme and oregano) consistently showed higher cytotoxicity than sesquiterpene-dominant oils. For instance, T. vulgaris EO caused 57–92% biofilm reduction in S. aureus but required ethanol emulsification to reduce epithelial toxicity [142]. In contrast, C. limon EO (limonene > 60%) displayed low cytotoxicity in food models, making it preferable for surface sanitation [91]. Synergistic blends, such as cinnamaldehyde–eugenol combinations, improved safety margins by lowering effective doses (FICI 0.24–0.40) compared with individual components [147]. However, Artemisia dracunculus EO (estragole > 60%) raised metabolite safety concerns due to potential carcinogenicity [148].
7. Multimodal Mechanisms of Action: Disrupting Biofilm Integrity and Microbial Physiology
Biofilms, complex microbial communities embedded in protective extracellular matrices, pose significant challenges in clinical and industrial settings due to their resilience against conventional antimicrobial therapies. Their resistance stems from dynamic adhesion processes, matrix production, QS-mediated communication, and adaptive physiological responses. Overcoming biofilm-associated infections requires innovative strategies that target multiple stages of biofilm development and microbial survival mechanisms. EOs, derived from medicinal plants, have emerged as potent antibiofilm agents due to their multifaceted, synergistic actions that disrupt structural integrity and microbial physiology (Figure 1).
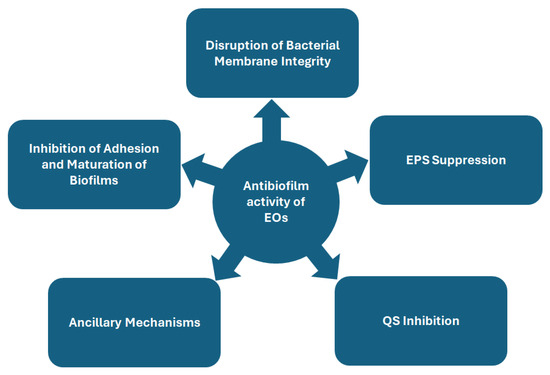
Figure 1.
Schematic overview of proposed mechanisms of antibiofilm activity of essential oils.
7.1. Inhibition of Adhesion and Maturation of Biofilms
7.1.1. Targeting Adhesion and Maturation: Surface Hydrophobicity, Motility, and Matrix Dynamics
EOs impede bacterial adhesion through multifaceted mechanisms, including the modulation of surface hydrophobicity, the suppression of motility-related genes, and the direct targeting of adhesion-specific proteins. Lavandula preparations reduced Campylobacter jejuni adhesion to polystyrene surfaces by ≥1 log10 CFU/mL at sub-inhibitory concentrations, which is linked to the downregulation of flagellar assembly genes (motA, flaG, flgG, flgI, fliQ, maf4, pseF, Cj0719c, and Cj1467) [137]. Eucalyptus spp. EOs inhibited the initial adhesion (58–95%) of A. baumannii, E. coli, L. monocytogenes, P. aeruginosa, and S. aureus at 10–20 µL/mL [124].
Molecular docking studies reveal that specific EO components directly interfere with adhesion proteins. For instance, eugenol (from clove EO), cinnamaldehyde (cinnamon EO), and 1,8-cineole (rosemary EO) bind strongly to the C. albicans Als3 adhesion protein, with eugenol exhibiting the highest binding affinity (−9.38 kcal/mol), thereby blocking fungal attachment to host surfaces [149]. In E. coli, heneicosane (from Jatropha intigrimma EO) disrupts FimH-mediated adhesion by interacting with residues Ile52 and Tyr48, reducing biofilm formation [150]. Litsea cubeba EO downregulates adhesion-related genes (ompW, VP0952, and VP0962) in Vibrio parahaemolyticus, impairing initial biofilm formation [151]. Similarly, Ocimum basilicum and Salvia officinalis EOs hinder surface colonization by reducing bacterial adhesion [107]. Thymol, a key component of thyme EO, penetrates exopolysaccharide matrices and inhibits adhesion proteins, synergizing with other compounds to block microbial attachment [152].
EOs also suppress microbial motility by targeting flagellar assembly, swarming, and QS-regulated pathways. T. vulgaris EO impaired motility in P. aeruginosa, H. influenzae, and Haemophilus parainfluenzae, inhibiting biofilm formation by 64.88–72.93% at MIC/2 (0.156 mg/mL against H. influenzae and H. parainfluenzae and 1.5–1.75 mg/mL against P. aeruginosa) [153]. Plectranthus barbatus EO (2.5% v/v) inhibits swarming and twitching motility in P. aeruginosa PAO1 by downregulating QS-dependent motility genes (pil and fli/flh) [154]. L. cubeba EO further impairs motility in V. parahaemolyticus by suppressing flagellar genes (flgM, flgL, flaA, and flaE) critical to movement [151]. O. vulgare EO reduces P. aeruginosa PAO1 swarming motility by 29.17% via QS interference, while Coridothymus capitatus EO (61% carvacrol) abolishes motility in P. aeruginosa at sub-inhibitory concentrations, likely through lasR suppression [155,156]. O. basilicum and S. officinalis EOs reduce swimming (up to 97%), twitching (up to 96.4%), and swarming motility (up to 84.9%) in bacterial strains, further hindering biofilm dispersal [107].
Additional studies highlight motility suppression in other pathogens. TTEO diminished X. oryzae biofilm biomass to 18 ± 1.9% by hindering swimming and swarming motility [78]. Thymus EOs reduced S. mutans hydrophobicity by 42.21% and inhibited biofilm formation by 98.11%, correlating with the downregulation of adhesion genes (gbpB and spaP) [110]. Laurel EO suppressed V. parahaemolyticus adhesion by reducing surface hydrophobicity and auto-aggregation while inhibiting swimming motility by 72% [151]. Thyme EOs reduced swimming and swarming motility in E. faecalis at 64–256 mg/mL, downregulating the ebpABC (pili formation) and epa (polysaccharide synthesis) genes, leading to diminished biofilm thickness [157]. A. rugosa EO reduced S. mutans adhesion to saliva-coated hydroxyapatite by 94% at 0.04 mg/mL, accompanied by decreased expression of gbpB and spaP [134].
QS-regulated swarming motility in P. aeruginosa PAO1 was significantly impaired by EOs. I. verum EO inhibited swarming by 38% at 100 µg/mL, exceeding trans-anethole activity [130]. Anethum graveolens EO reduced swarming by 33.33% at 100 µg/mL, outperforming limonene (28.9%) [158]. Carum carvi EO and its major component, carvone, exhibited dose-dependent inhibition, with 79.01% and 79.62% suppression at 2.5 mg/mL, respectively [159]. C. cyminum L. EO and cuminaldehyde demonstrated broad-spectrum anti-QS activity by inhibiting QS-regulated flagellar motility in P. aeruginosa. At 500 mg/mL, these compounds reduced motility by up to 90.12% (cumin EO) and 89.77% (cuminaldehyde), indicating their dual role in targeting both pigment production and motility [159].
7.1.2. Maturation Disruption via Matrix Degradation and QS Interference
EOs disrupt biofilm maturation by degrading extracellular matrices and interfering with QS. P. barbatus EO inhibited P. aeruginosa PAO1 biofilm formation (27.87–63.6% reduction) and disrupted architecture at 2.5% v/v by suppressing swarming motility and AHL-mediated QS [154]. Thymus spicata EO reduced P. aeruginosa PAO1 biofilm biomass by 88.13% at MIC/2 (12.5 mg/mL), with carvacrol being identified as the primary component responsible for matrix disruption and QS suppression [160]. Caraway EO reduced P. aeruginosa PAO1 biofilm formation by 60–72% and eradicated 72–73% of mature biofilms [143].
Cinnamon-derived compounds exhibited potent maturation inhibition. C. verum EO eradicated 99.75% of C. striatum biofilms at sub-inhibitory concentrations [96], while cinnamon oil nanoemulsion (5% v/v) suppressed multispecies oral biofilm maturation, reducing red/green fluorescence ratios (0.91 ± 0.10 vs. control 1.18 ± 0.07) [141]. Clove EO reduced Alicyclobacillus acidoterrestris biofilm biomass by 25–65% on glass and PVC surfaces, altering spatial organization [161]. O. vulgare EO dispersed 70% of S. suis biofilms by disrupting cell–cell interactions [114], and Origanum majorana EO inhibited C. albicans adhesion (MBIC₉₀: 1.93 µg/mL) [162]. C. aurantium EO degraded Stenotrophomonas maltophilia and B. subtilis biofilms over 14 days, showing progressive structural disintegration via MALDI-TOF MS [163].
Carvacrol-loaded nanoemulsions demonstrated efficacy against mature K. pneumoniae biofilms by penetrating matrices and disrupting structural integrity [97]. Nigella sativa EO and T. vulgaris methanolic extract inhibited biofilm maturation in S. aureus (94.1% inhibition) and P. mirabilis (83.3% inhibition), respectively [112].
7.1.3. Structural Alterations in Biofilms
EOs induce structural changes in microbial cells and biofilm architecture, disrupting their integrity and viability. Immortelle EO causes elongated cell forms in H. influenzae, suggesting interference with cell division [164]. Clove EO triggers cell elongation, surface roughness, and spore formation in A. acidoterrestris biofilms on glass and PVC surfaces [161]. Sub-inhibitory concentrations of oregano EO and carvacrol induce shrinkage and wrinkling in E. coli EAEC, indicative of metabolic stress [93]. TTEO and eugenol-rich Eucalyptus EO cause structural damage in multispecies biofilms and lead to biomass reduction and cellular scattering in E. faecalis and MRSA [121]. Trans-cinnamaldehyde triggers bacterial cell filamentation, forming non-viable, entangled networks [165].
Specific EOs destabilize biofilm matrices through compositional changes. Cinnamon EO modifies protein composition in Salmonella biofilms, weakening structural stability [163]. Lippia origanoides EO (thymol chemotype) disrupts Salmonella matrix synthesis by reducing glutamine, glutamate, and uridine diphosphate levels, impairing EPS production [166]. Linalool inhibits S. pyogenes biofilm formation by 91% through microcolony disruption [129]. Centipeda minima EO and its components (thymol and carvacrol) alter S. aureus cell morphology and adhesion at sub-MIC concentrations [167].
7.1.4. Proteomic Alterations and Enzymatic Inhibition in Biofilms
EOs induce proteomic shifts and inhibit enzymes critical to biofilm maturation and virulence. Cedar (Cedrus atlantica) EO modulates protein expression in Pseudomonas fluorescens and S. enterica biofilms, altering metabolic pathways over prolonged incubation [163]. Cinnamon EO nanoemulsion (5% v/v) suppresses C. albicans hyphal transition by downregulating HYR1, HWP1, ALS3, and the RAS1-cAMP-Efg1 pathway, reducing fungal pathogenicity [141,168]. Patchouli EO inhibits S. mutans glucosyltransferase activity, reducing insoluble glucan synthesis and biofilm cohesion [169].
Virulence factor inhibition is a key antibiofilm mechanism. E-cinnamaldehyde binds to V. cholerae biofilm-associated proteins (RbmA, RbmC, and FabH) and disrupts FtsZ, a cytoskeletal protein critical to cell division, leading to structural collapse [81]. C. cyminum L. EO and cuminaldehyde inhibit QS-regulated virulence enzymes in P. aeruginosa, suppressing elastase (62.12–63.14%) and protease (82.14–83.43%) production [159]. T. citriodorus EO reduces C. acnes biofilm biomass and metabolic activity, particularly in virulent phylotype IA1 strains, by altering proteomic profiles [111]. Black cardamom EO specifically targets Salmonella Typhimurium and E. coli O157:H7 biofilms (33.67–84.63% inhibition) without affecting planktonic cells, highlighting its biofilm-selective proteomic activity [170].
7.1.5. Dual-Phase Activity: Adhesion and Maturation Inhibition
Several EOs exhibit dual-phase activity, targeting both adhesion and maturation stages. Pimenta dioica EO and its component eugenol inhibited S. aureus biofilms by up to 70.25% on glass surfaces [171]. Ginger EO reduced multispecies (L. monocytogenes, S. Typhimurium, and P. aeruginosa) biofilm adhesion at 50–100 mg/mL while altering microbial composition [172]. Garlic EO completely inhibited S. Typhimurium biofilm formation at MIC/2 (1/128 µL/mL) across varying temperatures [173]. Cinnamon oil suppressed S. agalactiae biofilm adhesion by downregulating the pilA, pilB, and rogB genes (0.372–0.613-fold reduction), leading to sparse biofilm structures [95]. Oregano EO (O. vulgare), combined with C. ladaniferus or C. aurantium, achieved 68–80% biomass reduction in S. aureus biofilms at sub-synergistic concentrations (1/4 MIC; MIC= 0.03% v/v) [174].
7.2. Disruption of Bacterial Membrane Integrity
EOs derived from medicinal plants exhibit potent antibiofilm activity by targeting bacterial membrane integrity, a critical factor in biofilm viability. This mechanism involves the physical disruption of membrane structure, increased permeability, and interference with membrane-associated functions, resulting in cytoplasmic leakage, metabolic dysfunction, and cell death.
7.2.1. Membrane Structural Damage and Permeability Alterations
EOs induce structural damage and alter membrane permeability through diverse pathways. Pinus sylvestris and C. limon EOs caused drastic curvature changes and cytoplasmic leakage in N. gonorrhoeae, with 90% of treated cells appearing empty when analyzed with transmission electron microscopy (TEM). These effects were linked to lipopolysaccharide (LPS) targeting, as evidenced by the tenfold resistance of an LPS-deficient Neisseria meningitidis strain [114]. Similarly, Helichrysum italicum EO caused 96.1% and 79% membrane lysis in H. parainfluenzae and P. aeruginosa, respectively, at twice the MIC, accompanied by SEM-verified cell deformation [164]. Phenolic compounds, such as cinnamaldehyde in C. zeylanicum EO and eugenol in E. caryophyllata (clove) EO, directly damage bacterial cell walls, resulting in structural compromise and cellular contents leakage [115].
In S. aureus, C. minima EO and its monomers, thymol and carvacrol, elevated extracellular potassium ions (K+) and nucleic acids. At the same time, T. vulgaris EO disrupted C. acnes and S. epidermidis membranes, inducing phosphate (PO43−) and sulfur (S2−) ion leakage [103,167]. Dose-dependent effects were observed in Salmonella Enteritidis treated with L. origanoides thymol chemotype EO, where 2 × MIC (MIC = 20 µL/mL) caused nucleic acid and protein leakage [166]. Similarly, T. vulgaris EO induced hyper-permeabilization in Bacillus cereus, collapsing proton pumps and depleting intracellular ATP [152]. C. reticulata Blanco EO caused dose-dependent membrane rupture in L. monocytogenes, evidenced by collapsed cell surfaces and elevated propidium iodide uptake [106].
7.2.2. Hydrophobic Interactions and Lipid Bilayer Disruption
Hydrophobic EO components integrate into lipid bilayers, destabilizing membranes and triggering cytoplasmic leakage. Carvacrol in Thymus spicata EO disrupted S. aureus and Candida parapsilosis membranes. At the same time, D-limonene from C. limon EO exhibited rapid bactericidal activity against N. gonorrhoeae by increasing extracellular vesicle release within 15 min [114,160]. Carvacrol and thymol—major constituents of O. vulgare and T. vulgaris oils—alter membrane permeability, facilitating cytoplasmic leakage and impairing biofilm viability [115]. Thyme EO, rich in thymol, p-cymene, and γ-terpinene, induced hyper-permeabilization in B. cereus, collapsing proton pumps and depleting intracellular ATP [152].
C. citratus EO targeted ergosterol in fungal biofilms, increasing MIC values 31-fold in exogenous ergosterol, confirming membrane sterol binding as a key antifungal mechanism [127]. Similarly, C. verum leaf EO caused dose-dependent cell wall damage and cytoplasmic leakage in C. albicans, C. tropicalis, and Candida dubliniensis, corroborated by TEM showing vacuolation and scattered cytoplasmic content [116].
7.3. Inhibition of Biofilm Formation via EPS Suppression
EOs demonstrate potent antibiofilm activity by targeting EPS synthesis, a critical structural and functional component of biofilms. Their mechanisms include genetic regulation, enzymatic inhibition, structural disruption, biochemical depletion, and quantitative validation.
7.3.1. Genetic Regulation of EPS Biosynthesis
EOs disrupt EPS production by downregulating key polysaccharide synthesis and export genes. Lavandula EO impedes the capsule polysaccharide biosynthesis genes kpsM and kpsS in C. jejuni, compromising biofilm integrity [137]. In S. mutans, T. zygis and T. vulgaris suppress glucosyltransferase genes (gtfB, gtfC, and gtfD), while A. rugosa EO inhibits extracellular glucan synthesis at 0.01–0.04 mg/mL [110,134]. Shuanghuanglian EO targets wecB and wecC in MDR K. pneumoniae, reducing O-antigen polysaccharides [175]. Anise EO reduces EPSs in K. pneumoniae by 92% at 90 μg/mL, disrupting fimbria and flagellum systems [176].
7.3.2. Structural Disruption of Biofilm Matrices
EOs physically destabilize biofilm matrices. Linalool reduces S. pyogenes EPSs by 75% at 0.004% MBIC (MBIC = 0.004% (v/v)), as visualized via SEM [129]. Patchouli EO (1.25% v/v) diminishes P. aeruginosa PAO1 EPSs by 63.46%, disrupting S. mutans biofilms dose-dependently [169]. Thyme EO (128–256 mg/mL) reduces matrix density in E. faecalis, while oregano EO, thymol, and bioAgNP combinations collapse EAEC matrices [93,157]. L. origanoides EO disperses S. Enteritidis biofilms [166]. Thymol at sub-MIC reduces extracellular matrix density in biofilms, as observed via light microscopy, though direct biochemical validation is needed [109].
7.3.3. Biochemical Depletion of EPS Components
C. reticulata peel EO (2% v/v) reduces L. monocytogenes polysaccharides, proteins, and eDNA by 54.74%, 47.77%, and 63.74%, respectively [106]. Eucalyptol decreases Candida glabrata carbohydrate content by 15.2–21%, alongside protein and eDNA reductions [123]. Lemongrass EO (0.3125%) and citral (0.5%) degrade biofilm matrices, reducing eDNA (86% and 63%, respectively), proteins (89% and 77%, respectively), and carbohydrates (68% and 44%, respectively) [131].
7.4. QS Inhibition
EOs and their bioactive constituents have demonstrated significant potential in disrupting QS-mediated biofilm formation and virulence in pathogenic bacteria. These effects are evidenced by the inhibition of QS-regulated phenotypes, such as violacein and pyocyanin production, swarming motility, and Agr system signaling, across diverse bacterial models.
Multiple EOs suppress QS-regulated violacein production in Chromobacterium violaceum. O. vulgare EO exhibited dose-dependent inhibition, achieving over 50% suppression at sub-inhibitory concentrations (MIC/4: 0.012 mg/mL) and 72.7% at MIC, outperforming its component terpinene-4-ol (42.29%) [155]. Similarly, oregano EO, carvacrol, thymol, and thymol combined with bioAgNPs reduced violacein production by 93%, 94%, 92%, and 95%, respectively, without affecting bacterial viability [93]. I. verum EO demonstrated 76.18% inhibition at MIC (10 mg/mL), whereas its major component, trans-anethole, showed weaker activity (48.78% at 1.25 mg/mL) [130]. A. graveolens EO inhibited violacein by 67.52% at MIC (10 mg/mL), outperforming its constituent limonene (18.68%) [158]. Carum carvi EO reduced violacein production in a concentration-dependent manner, achieving inhibition of 47.57% at MIC (10 mg/mL) and 25.28% at MIC/32 (0.312 mg/mL) [159].
Robusta coffee (Coffea canephora) extracts, derived from fresh and dried fruit peels, also inhibited QS in C. violaceum. At sub-inhibitory concentrations, these extracts reduced violacein synthesis by 44.53% to 47.48% after 24 h [177]. C. cyminum L. EO and its primary constituent, cuminaldehyde, suppressed violacein production in C. violaceum CV026 at 2 mg/mL. Cuminaldehyde exhibited stronger quantitative activity (VIC50 = 1.676 mg/mL) compared with the whole EO (VIC50 = 2.746 mg/mL) [159].
QS-regulated pyocyanin production in P. aeruginosa was markedly inhibited by EOs. O. basilicum EO reduced pyocyanin by 13.3–55.6%, while S. officinalis EO achieved up to 58.7% inhibition at ½ MIC (MIC ranged from 5 to 20 mg/mL) across multiple strains [107]. C. capitatus EO nearly abolished pyocyanin synthesis in 11 out of the 12 tested P. aeruginosa strains, with complete inhibition in strain 23P and 7% residual production in PA14 and 40P [156]. Although direct genetic evidence was lacking, these effects were attributed to QS interference due to the established QS control of pyocyanin [107,156].
Citral, a monoterpene aldehyde, specifically disrupted the Agr QS system in S. aureus. By downregulating agrA expression—the central regulator of the Agr system—by approximately 69.8%, citral suppressed downstream virulence pathways, including an 87.3% reduction in hla (alpha-toxin) expression. This inhibition highlights citral’s capacity to attenuate QS-mediated pathogenicity and biofilm stability [131].
7.5. Ancillary Mechanisms: Oxidative Stress, Genetic Regulation, and Host Immune Modulation
7.5.1. Genetic and Metabolic Regulation
EOs modulate gene expression and metabolic pathways to impair biofilm resilience. Thymus EOs downregulate brpA, vicR, and relA in S. mutans, disrupting biofilm robustness and acid tolerance [110]. Cinnamon EO suppresses S. agalactiae pilus biosynthesis by downregulating pilA, pilB, and rogB [95], while C. reticulata peel EO represses L. monocytogenes genes involved in DNA repair (recA and dnaE), cell wall biosynthesis, and signaling (dhaA and dltA), compromising cellular integrity [106]. Artemisia EOs inhibit enteropathogenic E. coli (EPEC) by downregulating the LEE operon, which is critical to type III secretion systems [178]. Sub-MICs of C. verum and O. majorana EOs reduce planktonic cell viability by >90%, limiting metabolic precursors for biofilms [96]. Lemon EO suppresses glycolysis in S. mutans by reducing lactate dehydrogenase (LDH) activity and ldh gene expression, decreasing acid production and enamel demineralization [179]. Artemisia EO formulation disrupts bacterial respiration, causing dose-dependent reductions in viability (88% at 90 μg/mL) and intracellular damage [176].
7.5.2. Oxidative Stress Induction and Apoptosis
EOs induce oxidative damage and apoptosis in microbial cells. Oregano EO elevates ROS and malondialdehyde levels in E. faecalis, causing lipid peroxidation [90]. TTEO triggers a 6-fold ROS increase in X. oryzae pv. oryzae, disrupting biofilms [78]. Eucalyptol induces ROS (a 3.1-fold increase in C. glabrata), disrupts mitochondrial membrane potential, and inhibits hyphal transition in C. albicans via HWP1 downregulation, leading to apoptosis [123]. Amomum villosum Lour EO induces apoptosis-like death in MRSA, reducing viable cells from 93% to 48.8% [180]. Thymol exhibits fungicidal activity against C. tropicalis, reducing hyphal production and CFUs within 12–24 h [109].
7.5.3. Enhanced Bioavailability via Delivery Systems
Nanoencapsulation improves EO bioavailability and antibiofilm activity. Poly(ε-caprolactone) nanocapsules (Th-NCs and Or-NCs) enable biofilm inhibition at non-cytotoxic doses [145]. Nano-gold/L. angustifolia composites (12.7 nm) enhance antibacterial activity and wound healing (96.78% closure), indirectly supporting biofilm control [98]. Niosome-loaded oregano EO suppresses biofilm-related genes more effectively than free oil [181]. Cinnamaldehyde-loaded nanofibers achieve rapid release (100% within 60 min) and prolonged antifungal activity against C. glabrata (58% CFU reduction) and C. albicans (49% reduction) [182].
7.5.4. Anti-Inflammatory and Host-Modulatory Effects
EOs modulate host immunity and inflammation. T. vulgaris EO nanoemulsion reduces inflammation by 80% and NF-κB levels in a murine acne model, outperforming clindamycin in efficacy [103]. Zanthoxylum rhoifolium EO reduces gingival bleeding via cyclooxygenase inhibition [183]. TTEO reduces matrix metalloproteinase-8 levels in gingival crevicular fluid by 82.8%, correlating with suppressed pro-inflammatory cytokines (TNF-α, IL-1β, and IL-8) [184].
7.5.5. Synergistic and Multifaceted Actions
EOs exhibit synergistic effects against multispecies biofilms. Linalool reduces S. pyogenes biofilm hydrophobicity (48% vs. 92% control), upregulates protease SpeB, and downregulates virulence genes (mga and hasA) [129]. Garlic EO alters multispecies biofilm composition, suppressing L. monocytogenes while favoring S. Typhimurium and P. aeruginosa [172]. C. limon EO combined with Lactobacillus pentosus metabolites upregulates biofilm repressor sinR (2.099-fold) and downregulates matrix promoters spo0A and calY (0.314–0.238-fold) in B. cereus [185].
8. Synergistic Effects of EOs in Biofilm Inhibition
8.1. Synergy with Enzymes
The combination of EOs with enzymes enhances biofilm disruption. For example, Pinus sylvestris and C. limon EOs co-administered with DNase I synergistically degraded N. gonorrhoeae biofilms, achieving >95% biomass dispersal compared with ≤60% with individual agents. This synergy stems from DNase I degrading eDNA, which otherwise impedes EO penetration. Notably, no such effect was observed when these EOs were paired with O. vulgare or C. cassia [114].
8.2. Synergy with Antimicrobial and Antifungal Agents
EOs enhance the efficacy of antibiotics, antifungals, and antimicrobial agents by reducing resistance and improving penetration. Cinnamon EO lowered colistin’s MIC from 16 µg/mL to 2 µg/mL against S. aureus, increasing inhibition zone diameters from 6.0 mm to 38.0 mm (Fractional Inhibitory Concentration Index (FICI) = 0.4) [92]. T. vulgaris EO synergized with chlorhexidine gluconate against S. mutans (FICI = 0.25) [115]. Nanoformulated C. zeylanicum EO and its cinnamaldehyde-rich fraction reduced fluconazole’s FICI to 0.26 against resistant C. auris strains, improving survival in Galleria mellonella models [105]. Citral combined with fluconazole reduced azole-resistant C. albicans biofilm metabolic activity (FICI = 0.5) [186]. T. vulgaris EO and thymol further synergized with fluconazole and amphotericin B, reducing MICs 32-fold against resistant Candida spp. (FICI ≤ 0.156) [109]. M. alternifolia and E. globulus EOs restored vancomycin sensitivity in Enterococcus faecium, enabling efficacy at 500-fold lower concentrations [121].
8.3. Advanced Delivery Systems and Mechanisms of Synergy
Nanoparticles enhance EO delivery and biofilm penetration. Oregano derivatives (thymol and carvacrol) combined with bioAgNPs reduced E. coli and EAEC biofilm biomass by 99% [93]. Nano-gold conjugated with L. angustifolia EO disrupted P. mirabilis biofilms at 16 µg/mL via structural collapse [98]. Composite materials like PLA/Fe₃O4@EG coatings reduced E. coli and S. aureus biofilm CFUs 2.5–10-fold [94]. Cellulose-based materials loaded with cinnamaldehyde delayed S. mutant’s growth by 35 h [187].
Surfactants improve EO bioavailability. Dioctyl sodium sulfosuccinate (DSS) combined with Pelargonium graveolens or Mentha piperita EOs reduced Candida tropicalis biofilm inhibition concentrations 2.5–20-fold (FICI = 0.23–0.45) [188]. Niosome-encapsulated oregano EO reduced biofilm formation 2–4-fold more effectively than free oil [181], while nanoencapsulated thyme and oregano EO inhibited S. aureus, E. coli, and C. albicans biofilms at 0.03–0.25 mg/mL [145]. Synergy often involves membrane disruption and enhanced penetration. Thymol with bioAgNPs suppressed C. violaceum QS by 95% [93]. Nanoencapsulated cinnamon oil and colistin-lysed S. aureus biofilms form “ghost cells” [92].
8.4. Synergistic Combinations and Whole-EO Efficacy
Whole EOs often outperform isolated components. Myrtus communis EO (containing myrtenyl acetate, 1,8-cineole, α-pinene, and linalool) inhibited E. coli at 6 mg/mL, outperforming individual constituents [189]. Similarly, O. vulgare and Illicium verum EOs exhibited broader activity than their isolated terpenes [130,155].
Clove and cinnamon EOs reduced MIC values in S. aureus (0.0156/0.0078 mg/mL) and S. epidermidis (0.0625/0.0312 mg/mL) via interactions between eugenol and cinnamaldehyde [190]. O. vulgare paired with C. ladaniferus, C. aurantium var. amara, or Juniperus communis showed synergy (FICI = 0.312–0.5) against S. aureus [174].
9. Application of Drug Nano-Delivery Technology in EOs
9.1. Nanoemulsions for Enhanced Solubility and Stability
Nanoemulsions, submicron oil-in-water emulsions stabilized by surfactants, are widely employed to overcome the hydrophobicity and volatility of EOs. By reducing the droplet size to 50–200 nm, nanoemulsions increase the surface area of EOs, enhancing their dispersion, dissolution rate, and bioavailability [191]. This nanostructuring protects EOs from oxidation, evaporation, and degradation, prolonging shelf life while improving penetration through biological membranes for topical and transdermal delivery [192]. For instance, thyme, lavender, and TTEO nanoemulsions exhibit superior antimicrobial activity due to enhanced interaction with microbial cell membranes, effectively inhibiting biofilm-forming pathogens. Similarly, Eucalyptus and peppermint oil nanoemulsions enable sustained anti-inflammatory effects in conditions like arthritis by facilitating deeper tissue penetration and controlled release [193,194,195]. A notable example is the clove oil nanoemulsion (eugenol ≥ 50%), which reduced S. aureus biofilm biomass by 52% at sub-MICs, outperforming free EO by improving eugenol’s bioavailability and prolonging antibacterial action [196].
9.2. Lipid-Based Nanocarriers: Liposomes, SLNs, and NLCs
Lipid-based systems, including liposomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs), address the instability and poor solubility of EOs [197]. Liposomes, composed of phospholipid bilayers, encapsulate both hydrophilic and hydrophobic compounds, protecting EOs like thyme and lavender from oxidative degradation while enhancing skin penetration [198]. SLNs and NLCs, with solid lipid cores, provide controlled release and long-term stability. For example, SLNs loaded with Nigella sativa or clove oil improve antibacterial efficacy through sustained release. NLCs co-encapsulating EOs with flavonoids (e.g., curcumin) achieve synergistic antimicrobial effects against MDR pathogens. These systems also enhance food preservation and cosmetic applications by reducing EO volatility and toxicity [194,199,200]. One study demonstrated that cinnamon oil nanoliposomes and colistin eradicated S. aureus biofilms through enhanced cellular uptake and synergistic interactions [92]. Similarly, lavender essential oil conjugated with gold nanoparticles eliminated P. mirabilis biofilms at 16 µg/mL, leveraging nanoparticle-enhanced cellular uptake while reducing cytotoxicity [98].
9.3. Polymeric Nanoparticles for Targeted Delivery
Biopolymeric nanoparticles, such as chitosan, poly(lactic-co-glycolic acid) (PLGA), and cellulose derivatives, offer biodegradability and biocompatibility for EO delivery [201]. Chitosan nanoparticles crosslinked with tripolyphosphate stabilize EOs like clove and peppermint, enhancing antimicrobial activity against S. aureus and E. coli via electrostatic interactions with bacterial membranes [196]. PLGA nanoparticles enable sustained release of curcumin and resveratrol, reducing oxidative stress in arthritis models [202]. Cellulose nanocrystals (CNCs) improve thyme oil stability and prolong antimicrobial action [203]. For instance, the encapsulation of lemongrass EO (citral-rich) into chitosan microparticles enhanced antifungal efficacy against C. albicans biofilms, achieving 84% biomass reduction at 8 × MIC due to improved mucoadhesion and controlled release [204].
9.4. Antimicrobial Applications Against MDR Pathogens
Nano-delivery systems enhance EO efficacy against MDR bacteria and fungi [205]. Nanoemulsions of C. zeylanicum and T. vulgaris oils disrupt fungal cell walls [99], while liposomal Eucalyptus camaldulensis EO combats C. albicans [92]. Synergistic combinations of EOs (e.g., carvacrol and thymol) with antibiotics restore sensitivity in resistant strains by inhibiting efflux pumps and biofilm formation [93]. Ethosomes and silver nanoparticles (AgNPs) conjugated with EOs such as clove and cinnamon further enhance penetration and biofilm disruption [92,95]. A study combining cinnamon essential oil with biogenic silver nanoparticles reduced MBIC50 values against MDR S. agalactiae 4–8-fold, disrupting EPSs and enhancing EO diffusion [95]. Similarly, oregano EO-loaded niosomes increased antibiofilm activity 2–4-fold against Vibrio vulnificus through improved stability under gastrointestinal conditions and controlled release [181].
10. Limitations and Challenges
EOs’ use as biofilm inhibitors has several limitations, including methodological constraints, biological variability, formulation issues, and gaps in mechanistic understanding. Recent studies have extensively reported these challenges, highlighting the complexities of translating EO-based antibiofilm strategies into clinical and industrial applications.
10.1. Methodological Limitations and In Vitro Model Constraints
A significant concern in EO research is the reliance on in vitro models, which often fail to replicate the complexity of in vivo environments or polymicrobial interactions. Many studies depend on monoculture biofilms formed by standard ATCC bacterial strains, neglecting the heterogeneity and dynamics observed in natural biofilms. Additionally, biofilm assessment methods, such as tetrazolium salt assays and microscopy, often lack critical parameters like MBIC and microbial biofilm eradication concentrations (MBECs), reducing the accuracy and reproducibility of results. For instance, using C. zeylanicum EO against mature Staphylococcus schleiferi biofilms revealed reduced efficacy in static in vitro models, demonstrating the difficulty in targeting established biofilms [206]. Similarly, many studies overlook long-term exposure and in vivo validation, limiting the clinical relevance of EO findings.
10.2. Chemical Variability and Standardization Issues
The chemical composition of EOs is inherently variable, influenced by factors such as plant chemotype, geographic origin, and extraction methods. This variability introduces significant unpredictability in EO activity. For example, O. vulgare EO showed strain-specific activity against L. monocytogenes, with efficacy fluctuating based on the levels of thymol and carvacrol. This variation complicates the standardization of EO formulations for consistent antibiofilm efficacy. Additionally, many studies fail to report the complete chemical profiles of EOs.
10.3. Efficacy Limitations and Cytotoxicity Concerns
EOs often require high concentrations to inhibit biofilm formation, raising concerns regarding their cytotoxicity. For instance, S. aromaticum EO exhibited moderate cytotoxicity to human cells at doses higher than 0.5% (v/v), limiting its therapeutic potential [207]. Some EOs, such as M. piperita EO, were found to disrupt beneficial microbiota, raising ecological safety concerns [208]. Furthermore, when used at higher concentrations, certain EOs, like TTEO, exhibited adverse effects such as skin irritation [209]. These cytotoxicity and safety issues necessitate careful dose optimization and rigorous toxicological assessments.
10.4. Strain-Specific Activity and Biofilm Stage Dependency
EO efficacy is highly strain-dependent and often varies across different biofilm stages. For example, C. zeylanicum EO showed limited effectiveness against some E. coli isolates, while T. vulgaris EO demonstrated strain-specific activity against P. aeruginosa biofilms but was less effective against mature biofilms. Some EOs, such as O. vulgare and T. vulgaris, have been reported to enhance biofilm formation at sub-inhibitory concentrations, complicating their therapeutic application. The diminished activity of EOs against mature biofilms, such as the reduced effectiveness of geranium EO against C. albicans after 72–96 h, highlights the challenges of targeting well-established biofilms.
10.5. Formulation and Delivery Challenges
Formulation issues, including the volatility and poor solubility of EOs, complicate their application in real-world settings. Encapsulation strategies, such as nanoemulsions, have been explored to enhance EO stability and solubility, yet some formulations, like those for T. zygis EO, showed only moderate efficiency. Moreover, matrix effects, such as the presence of organic matter in food matrices, can reduce EO efficacy. For example, O. vulgare EO exhibited weaker activity against Salmonella biofilms in chicken meat models compared with laboratory media. Additionally, the sensory properties of EOs, such as the pungent aroma of A. sativum EO, may limit their industrial applications, especially in food-related products.
10.6. Gaps in Mechanistic and Compositional Understanding
Despite promising results, the mechanistic understanding of how EOs inhibit biofilm formation remains incomplete. Studies have highlighted the disruption of extracellular matrix components and QS pathways as potential mechanisms. For instance, eugenol and carvacrol have been shown to disrupt biofilms of S. mutans, but their interactions with eDNA or matrix proteins remain poorly defined. Additionally, the synergistic effects of EOs with conventional antibiotics have been sparsely explored. However, some studies suggest enhanced antibiofilm activity when EOs are combined with nanoparticles or encapsulated in specific formulations. The absence of detailed compositional profiles in many studies, such as the lack of complete chemical analysis for L. angustifolia EO, further complicates understanding their antibiofilm actions.
10.7. Clinical and Industrial Translation Barriers
Translating EO-based antibiofilm therapies into clinical and industrial settings faces numerous barriers. The lack of in vivo validation for most EOs, such as P. graveolens EO against Candida spp., limits their clinical applicability. Additionally, challenges related to formulation instability, as seen with Matricaria chamomilla EO in creams, and sensory incompatibility, such as the alteration in taste in food products by A. sativum EO, further hinder the widespread use of EOs in medical and industrial contexts. Moreover, the inconsistent biofilm inhibitory effects of EOs and the need for high concentrations raise questions about their feasibility as standalone therapeutic agents.
11. Future Perspectives on the Use of EOs for Biofilm Inhibition
The escalating challenge of antimicrobial resistance has sparked renewed interest in EOs as promising natural alternatives for inhibiting biofilms. Their broad-spectrum antimicrobial activity, biocompatibility, and multi-target mechanisms position them as viable candidates across various sectors, including clinical, industrial, and environmental applications.
11.1. Clinical and Therapeutic Applications
EOs demonstrate significant potential in managing infections associated with MDR pathogens and biofilm-related diseases. O. vulgare and C. cassia EOs have effectively disrupted P. aeruginosa biofilms via QS inhibition and matrix degradation. T. vulgaris EO also shows promise in combating MRSA biofilms, with potential applications in wound dressings and catheter coatings to prevent device-associated infections.
In oral care, M. piperita and S. aromaticum EOs have demonstrated efficacy against cariogenic biofilms, particularly S. mutans. Formulations of EO-based mouthwashes and nanoparticle-based delivery systems could improve their retention and biofilm inhibition in oral environments. Furthermore, TTEO, used in topical formulations, offers low-toxicity solutions for chronic wound infections.
11.2. Food Safety and Agricultural Uses
EOs like thymol and carvacrol, derived from T. vulgaris and Origanum spp., have demonstrated robust activity against foodborne pathogens, including L. monocytogenes and S. enterica, through QS inhibition. These EOs offer eco-friendly alternatives for antimicrobial packaging, surface sanitization, and agricultural biocontrol. For instance, C. verum EO has been effective against biofilms from spoilage organisms on meat-processing equipment.
Developing EO-infused food packaging and spray sanitizers could enhance food preservation and improve food safety protocols. However, real-world testing in food systems and validation in industrial settings, especially regarding scalability and sensory impacts, are critical.
11.3. Formulation and Delivery Innovations
One of the significant challenges with EO applications is their stability and bioavailability. Nanoencapsulation techniques, such as liposomal or chitosan-based delivery systems, have shown promise in enhancing the stability and penetration of EOs into biofilms. For instance, nanoemulsified O. vulgare EO has proven effective against oral biofilms while reducing cytotoxicity. Additionally, EO-based nanoencapsulated formulations for medical devices have demonstrated improved biofilm inhibition, enhancing the potential for clinical applications.
Combining EOs with conventional antibiotics, such as ciprofloxacin and C. cassia EO, is a promising strategy to combat MDR pathogens. These synergistic formulations could reduce the effective antibiotic doses and mitigate the evolution of resistance.
11.4. Challenges and Future Research Directions
Despite their potential, several challenges remain in the clinical and industrial application of EOs for biofilm inhibition. The pharmacokinetics, in vivo toxicity, and biocompatibility of EOs need to be rigorously tested to ensure their safety in human applications. For example, Rosmarinus officinalis EO, although effective against acne-causing C. acnes, requires standardization to avoid skin irritation.
Future studies should focus on the mechanistic elucidation of biofilm formation through advanced techniques, such as proteomics and transcriptomics, to identify specific molecular targets and pathways. Moreover, research into synergistic combinations, formulation innovations such as thermoresponsive systems, and the scalability of EO applications will be critical to their widespread adoption.
12. Conclusions
Biofilms represent a formidable challenge in clinical and industrial settings, serving as a cornerstone of bacterial persistence and antimicrobial resistance. Their complex architecture, reinforced by extracellular matrices and adaptive mechanisms such as metabolic dormancy, efflux pump activation, and QS-mediated communication, renders them highly resistant to conventional therapies. These microbial communities are implicated in chronic and recurrent infections, particularly those associated with medical devices, where they evade immune responses and subvert antibiotic efficacy. The resilience of biofilms is further exacerbated by their capacity to harbor MDR pathogens, underscoring the urgent need for innovative strategies to disrupt their formation and persistence.
EOs have emerged as a promising alternative in this context due to their multifaceted antibiofilm and antimicrobial properties. These natural compounds exhibit broad-spectrum activity, targeting critical biofilm processes such as initial bacterial adhesion, matrix destabilization, and QS interference. Their ability to disrupt membrane integrity and impair virulence pathways, even at sub-inhibitory concentrations, positions them as effective agents against Gram-positive and Gram-negative pathogens. Notably, EOs demonstrate synergistic potential when combined with conventional antibiotics, enhancing therapeutic outcomes while mitigating the risk of resistance development—a significant advantage over single-target antimicrobials.
Despite their potential, the clinical translation of EOs faces notable limitations. Variability in chemical composition, influenced by botanical sources and extraction methods, compromises reproducibility and standardization. Furthermore, insufficient in vivo validation and stability, bioavailability, and potential cytotoxicity challenges hinder their widespread application. Current research gaps, including the lack of pharmacokinetic data and optimized delivery systems, must be addressed to ensure safety and efficacy in real-world settings.
Future advancements should prioritize the integration of EOs into multifunctional strategies. Innovations in formulation technologies—such as encapsulation, nanocarriers, and biofilm-resistant coatings—could enhance their stability and targeted delivery. Combining EOs with emerging approaches, including phage therapy, metabolic inhibitors, or immune-modulating agents, may further amplify their antibiofilm efficacy. Additionally, rigorous clinical trials and standardized protocols are essential to validating their therapeutic potential and establishing safety profiles.
In conclusion, EOs represent a valuable, naturally derived arsenal in the fight against biofilm-associated infections and antimicrobial resistance. Their multi-targeted mechanisms, interdisciplinary research, and formulation science advancements offer a sustainable pathway to revitalizing antimicrobial stewardship. By addressing existing limitations and fostering innovative synergies, EOs may ultimately redefine therapeutic paradigms, providing practical solutions for persistent infections in an era increasingly threatened by MDR pathogens.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14050503/s1, Table S1. Anti-biofilm Activities of Essential Oils and Their Combinations Against Different Microorganisms. Table S2. Chemical Composition and Biofilm Inhibition Efficacy of Essential Oils from Various Medicinal Plant Sources. References [210,211,212,213,214,215,216,217,218,219,220,221] are cited in the supplementary materials.
Author Contributions
Conceptualization, A.T. and A.M.; methodology, A.T., A.M. and T.I.; validation, A.T., A.M. and N.A.I.; investigation, A.T. and A.M.; writing—original draft preparation, T.I. and A.M.; writing—review and editing, A.T. and A.M.; supervision, A.T. and A.M.; funding, N.A.I. and T.I. project administration, A.T. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the Structure and Contribution towards Bacterial Resistance in Antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding Bacterial Biofilms: From Definition to Treatment Strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Trubenová, B.; Roizman, D.; Moter, A.; Rolff, J.; Regoes, R.R. Population Genetics, Biofilm Recalcitrance, and Antibiotic Resistance Evolution. Trends Microbiol. 2022, 30, 841–852. [Google Scholar] [CrossRef]
- Gilbert, P.; Maira-Litran, T.; McBain, A.J.; Rickard, A.H.; Whyte, F.W. The Physiology and Collective Recalcitrance of Microbial Biofilm Communities. Adv. Microb. Physiol. 2002, 46, 202–256. [Google Scholar]
- Yan, J.; Bassler, B.L. Surviving as a Community: Antibiotic Tolerance and Persistence in Bacterial Biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Jiang, Y.; Geng, M.; Bai, L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms 2020, 8, 1222. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Maggio, F.; Rossi, C.; Serio, A.; Chaves-Lopez, C.; Casaccia, M.; Paparella, A. Anti-Biofilm Mechanisms of Action of Essential Oils by Targeting Genes Involved in Quorum Sensing, Motility, Adhesion, and Virulence: A Review. Int. J. Food Microbiol. 2025, 426, 110874. [Google Scholar] [CrossRef]
- Lu, L.; Hu, W.; Tian, Z.; Yuan, D.; Yi, G.; Zhou, Y.; Cheng, Q.; Zhu, J.; Li, M. Developing Natural Products as Potential Anti-Biofilm Agents. Chin. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Soulaimani, B. Comprehensive Review of the Combined Antimicrobial Activity of Essential Oil Mixtures and Synergism with Conventional Antimicrobials. Nat. Prod. Commun. 2025, 20, 1934578X251328241. [Google Scholar] [CrossRef]
- Moghaddam, M.; Mehdizadeh, L. Chapter 13—Chemistry of Essential Oils and Factors Influencing Their Constituents. In Soft Chemistry and Food Fermentation; Grumezescu, A.M., Holban, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2017; pp. 379–419. ISBN 978-0-12-811412-4. [Google Scholar]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Prakash, B. Effect of Environmental Factors on Essential Oil Biosynthesis, Chemical Stability, and Yields. In Plant Essential Oils: From Traditional to Modern-day Application; Prakash, B., Dubey, N.K., Freitas Brilhante de São José, J., Eds.; Springer Nature: Singapore, 2024; pp. 225–247. ISBN 978-981-9943-70-8. [Google Scholar]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Zouari-Bouassida, K.; Trigui, M.; Makni, S.; Jlaiel, L.; Tounsi, S. Seasonal Variation in Essential Oils Composition and the Biological and Pharmaceutical Protective Effects of Mentha Longifolia Leaves Grown in Tunisia. BioMed Res. Int. 2018, 2018, 7856517. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The Biofilm Life Cycle: Expanding the Conceptual Model of Biofilm Formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral Microbial Biofilms: An Update. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Dreifus, J.E.; O’Neal, L.; Jacobs, H.M.; Subramanian, A.S.; Howell, P.L.; Wozniak, D.J.; Parsek, M.R. The Sia System and C-Di-GMP Play a Crucial Role in Controlling Cell-Association of Psl in Planktonic P. aeruginosa. J. Bacteriol. 2022, 204, e00335-22. [Google Scholar] [CrossRef]
- Sundstrom, P. Adhesins in Candida albicans. Curr. Opin. Microbiol. 1999, 2, 353–357. [Google Scholar] [CrossRef]
- Boisvert, A.-A.; Cheng, M.P.; Sheppard, D.C.; Nguyen, D. Microbial Biofilms in Pulmonary and Critical Care Diseases. Ann. Am. Thorac. Soc. 2016, 13, 1615–1623. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. The MSCRAMM Family of Cell-Wall-Anchored Surface Proteins of Gram-Positive Cocci. Trends Microbiol. 2019, 27, 927–941. [Google Scholar] [CrossRef]
- Schilcher, K.; Horswill, A.R. Staphylococcal Biofilm Development: Structure, Regulation, and Treatment Strategies. Microbiol. Mol. Biol. Rev. MMBR 2020, 84, e00026-19. [Google Scholar] [CrossRef] [PubMed]
- Briard, B.; Muszkieta, L.; Latgé, J.-P.; Fontaine, T. Galactosaminogalactan of Aspergillus Fumigatus, a Bioactive Fungal Polymer. Mycologia 2016, 108, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Nero, T.; Weerasekera, R.; Matej, K.H.; Hinbest, A.; Jiang, Z.; Lee, R.F.; Wu, L.; Chak, C.; Nijjer, J.; et al. Vibrio Cholerae Biofilms Use Modular Adhesins with Glycan-Targeting and Nonspecific Surface Binding Domains for Colonization. Nat. Commun. 2023, 14, 2104. [Google Scholar] [CrossRef]
- Moreau, A.; Nguyen, D.T.; Hinbest, A.J.; Zamora, A.; Weerasekera, R.; Matej, K.; Zhou, X.; Sanchez, S.; Rodriguez Brenes, I.; Tai, J.-S.B.; et al. Surface Remodeling and Inversion of Cell-Matrix Interactions Underlie Community Recognition and Dispersal in Vibrio Cholerae Biofilms. Nat. Commun. 2025, 16, 327. [Google Scholar] [CrossRef]
- Temme, J.S.; Tan, Z.; Li, M.; Yang, M.; Wlodawer, A.; Huang, X.; Schneekloth, J.S.; Gildersleeve, J.C. Insights into Biofilm Architecture and Maturation Enable Improved Clinical Strategies for Exopolysaccharide-Targeting Therapeutics. Cell Chem. Biol. 2024, 31, 2096–2111.e7. [Google Scholar] [CrossRef]
- Quan, K.; Hou, J.; Zhang, Z.; Ren, Y.; Peterson, B.W.; Flemming, H.-C.; Mayer, C.; Busscher, H.J.; van der Mei, H.C. Water in Bacterial Biofilms: Pores and Channels, Storage and Transport Functions. Crit. Rev. Microbiol. 2022, 48, 283–302. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans Biofilms: Development, Regulation, and Molecular Mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Cooney, C.; Sommer, B.; Marzinelli, E.M.; Figueira, W.F. The Role of Microbial Biofilms in Range Shifts of Marine Habitat-Forming Organisms. Trends Microbiol. 2024, 32, 190–199. [Google Scholar] [CrossRef]
- Deo, R.; Lakra, U.; Ojha, M.; Nigam, V.K.; Sharma, S.R. Exopolysaccharides in Microbial Interactions: Signalling, Quorum Sensing, and Community Dynamics. Nat. Prod. Res. 2024, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Guilhen, C.; Forestier, C.; Balestrino, D. Biofilm Dispersal: Multiple Elaborate Strategies for Dissemination of Bacteria with Unique Properties. Mol. Microbiol. 2017, 105, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas Aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Perchikov, R.; Cheliukanov, M.; Plekhanova, Y.; Tarasov, S.; Kharkova, A.; Butusov, D.; Arlyapov, V.; Nakamura, H.; Reshetilov, A. Microbial Biofilms: Features of Formation and Potential for Use in Bioelectrochemical Devices. Biosensors 2024, 14, 302. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Stoodley, P. Developmental Regulation of Microbial Biofilms. Curr. Opin. Biotechnol. 2002, 13, 228–233. [Google Scholar] [CrossRef]
- Sahreen, S.; Mukhtar, H.; Imre, K.; Morar, A.; Herman, V.; Sharif, S. Exploring the Function of Quorum Sensing Regulated Biofilms in Biological Wastewater Treatment: A Review. Int. J. Mol. Sci. 2022, 23, 9751. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, P.; Sarmah, B.K.; Nandi, S.P. Quorum Sensing: Its Role in Microbial Social Networking. Res. Microbiol. 2020, 171, 159–164. [Google Scholar] [CrossRef]
- Li, Q.; Mao, S.; Wang, H.; Ye, X. The Molecular Architecture of Pseudomonas Aeruginosa Quorum-Sensing Inhibitors. Mar. Drugs 2022, 20, 488. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharma, P.; Capalash, N. Quorum Sensing in Acinetobacter Baumannii. In Quorum Sensing vs Quorum Quenching: A Battle with No End in Sight; Kalia, V.C., Ed.; Springer: New Delhi, India, 2015; pp. 101–113. ISBN 978-81-322-1982-8. [Google Scholar]
- Li, H.; He, B.; Ma, N.; Liu, C.; Cai, K.; Zhang, X.; Ma, X. Quorum Sensing of Bifidobacteria: Research and Progress. Microbiol. Res. 2025, 294, 128102. [Google Scholar] [CrossRef]
- Jung, S.A.; Hawver, L.A.; Ng, W.-L. Parallel Quorum Sensing Signaling Pathways in Vibrio Cholerae. Curr. Genet. 2016, 62, 255–260. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Bai, G.; Zhou, X.; Wu, H. Cyclic Di-AMP Mediates Biofilm Formation. Mol. Microbiol. 2016, 99, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Castro, M.; Copaja, S.; Guiliani, N. Biofilm Formation by the Acidophile Bacterium Acidithiobacillus Thiooxidans Involves C-Di-GMP Pathway and Pel Exopolysaccharide. Genes 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, C.; Raheem, D.; Ramos, F.; Saraiva, A.; Raposo, A. Microbial Biofilms in the Food Industry-A Comprehensive Review. Int. J. Environ. Res. Public Health 2021, 18, 2014. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Waters, C.M. A Tangled Web: Regulatory Connections between Quorum Sensing and Cyclic Di-GMP. J. Bacteriol. 2012, 194, 4485–4493. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Liu, J.; Chen, Y.; Zhou, X.; Ru, Y.; Zhu, J.; Liu, W. cAMP and C-Di-GMP Synergistically Support Biofilm Maintenance through the Direct Interaction of Their Effectors. Nat. Commun. 2022, 13, 1493. [Google Scholar] [CrossRef]
- Wall, G.; Montelongo-Jauregui, D.; Vidal Bonifacio, B.; Lopez-Ribot, J.L.; Uppuluri, P. Candida albicans Biofilm Growth and Dispersal: Contributions to Pathogenesis. Curr. Opin. Microbiol. 2019, 52, 1–6. [Google Scholar] [CrossRef]
- Pierce, C.G.; Vila, T.; Romo, J.A.; Montelongo-Jauregui, D.; Wall, G.; Ramasubramanian, A.; Lopez-Ribot, J.L. The Candida albicans Biofilm Matrix: Composition, Structure and Function. J. Fungi 2017, 3, 14. [Google Scholar] [CrossRef]
- Dhaked, H.P.S.; Cao, L.; Biswas, I. Redox Sensing Modulates the Activity of the ComE Response Regulator of Streptococcus Mutans. J. Bacteriol. 2021, 203, e0033021. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, X.; Wang, C.; Song, J.; Xu, J.; Liu, X.; Qian, Y.; Suo, H. New Strategies and Mechanisms for Targeting Streptococcus Mutans Biofilm Formation to Prevent Dental Caries: A Review. Microbiol. Res. 2024, 278, 127526. [Google Scholar] [CrossRef]
- Crabbé, A.; Jensen, P.Ø.; Bjarnsholt, T.; Coenye, T. Antimicrobial Tolerance and Metabolic Adaptations in Microbial Biofilms. Trends Microbiol. 2019, 27, 850–863. [Google Scholar] [CrossRef]
- Walters, M.C.; Roe, F.; Bugnicourt, A.; Franklin, M.J.; Stewart, P.S. Contributions of Antibiotic Penetration, Oxygen Limitation, and Low Metabolic Activity to Tolerance of Pseudomonas Aeruginosa Biofilms to Ciprofloxacin and Tobramycin. Antimicrob. Agents Chemother. 2003, 47, 317–323. [Google Scholar] [CrossRef]
- Gloag, E.S.; Fabbri, S.; Wozniak, D.J.; Stoodley, P. Biofilm Mechanics: Implications in Infection and Survival. Biofilm 2020, 2, 100017. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Biofilm Resilience: Molecular Mechanisms Driving Antibiotic Resistance in Clinical Contexts. Biology 2025, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, J.L. Biofilm-Related Disease. Expert Rev. Anti Infect. Ther. 2018, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, H.; Xiong, J.; Yang, G.-X.; Hu, J.-F.; Zhu, Q.; Chen, Z. Staphylococcus aureus Biofilm: Formulation, Regulatory, and Emerging Natural Products-Derived Therapeutics. Biofilm 2024, 7, 100175. [Google Scholar] [CrossRef]
- Kulshrestha, A.; Gupta, P. Polymicrobial Interaction in Biofilm: Mechanistic Insights. Pathog. Dis. 2022, 80, ftac010. [Google Scholar] [CrossRef]
- Penesyan, A.; Paulsen, I.T.; Kjelleberg, S.; Gillings, M.R. Three Faces of Biofilms: A Microbial Lifestyle, a Nascent Multicellular Organism, and an Incubator for Diversity. Npj Biofilms Microbiomes 2021, 7, 80. [Google Scholar] [CrossRef]
- Saharan, B.S.; Beniwal, N.; Duhan, J.S. From Formulation to Function: A Detailed Review of Microbial Biofilms and Their Polymer-Based Extracellular Substances. The Microbe 2024, 5, 100194. [Google Scholar] [CrossRef]
- Hussaini, I.M.; Oyewole, O.A.; Sulaiman, M.A.; Dabban, A.I.; Sulaiman, A.N.; Tarek, R. Microbial Anti-Biofilms: Types and Mechanism of Action. Res. Microbiol. 2024, 175, 104111. [Google Scholar] [CrossRef]
- Zhang, R.; Neu, T.R.; Blanchard, V.; Vera, M.; Sand, W. Biofilm Dynamics and EPS Production of a Thermoacidophilic Bioleaching Archaeon. New Biotechnol. 2019, 51, 21–30. [Google Scholar] [CrossRef]
- Liu, J.; Wu, S.; Feng, L.; Wu, Y.; Zhu, J. Extracellular Matrix Affects Mature Biofilm and Stress Resistance of Psychrotrophic Spoilage Pseudomonas at Cold Temperature. Food Microbiol. 2023, 112, 104214. [Google Scholar] [CrossRef] [PubMed]
- Mahto, K.U.; Vandana; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial Biofilm and Extracellular Polymeric Substances in the Treatment of Environmental Pollutants: Beyond the Protective Role in Survivability. J. Clean. Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Lee, Y. Biofilm Formation and Antimicrobial Resistance in Enterococcus. Infect. Chemother. 2017, 49, 236–237. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Malviya, J.; Alameri, A.A.; Al-Janabi, S.S.; Fawzi, O.F.; Azzawi, A.L.; Obaid, R.F.; Alsudani, A.A.; Alkhayyat, A.S.; Gupta, J.; Mustafa, Y.F.; et al. Metabolomic Profiling of Bacterial Biofilm: Trends, Challenges, and an Emerging Antibiofilm Target. World J. Microbiol. Biotechnol. 2023, 39, 212. [Google Scholar] [CrossRef]
- Hajiagha, M.N.; Kafil, H.S. Efflux Pumps and Microbial Biofilm Formation. Infect. Genet. Evol. 2023, 112, 105459. [Google Scholar] [CrossRef]
- Lewis, K. Persister Cells and the Riddle of Biofilm Survival. Biochem. Biokhimiia 2005, 70, 267–274. [Google Scholar] [CrossRef]
- Ranieri, M.R.; Whitchurch, C.B.; Burrows, L.L. Mechanisms of Biofilm Stimulation by Subinhibitory Concentrations of Antimicrobials. Curr. Opin. Microbiol. 2018, 45, 164–169. [Google Scholar] [CrossRef]
- Whelan, S.; O’Grady, M.C.; Corcoran, G.D.; Finn, K.; Lucey, B. Effect of Sub-Inhibitory Concentrations of Nitrofurantoin, Ciprofloxacin, and Trimethoprim on In Vitro Biofilm Formation in Uropathogenic Escherichia coli (UPEC). Med. Sci. 2023, 11, 1. [Google Scholar] [CrossRef]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Salinas-Delgado, G.A.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Battle Royale: Immune Response on Biofilms—Host-Pathogen Interactions. Curr. Res. Immunol. 2023, 4, 100057. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. The Role of Alginate in Bacterial Biofilm Formation. In Extracellular Sugar-Based Biopolymers Matrices; Cohen, E., Merzendorfer, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 517–537. ISBN 978-3-030-12919-4. [Google Scholar]
- Le, K.Y.; Park, M.D.; Otto, M. Immune Evasion Mechanisms of Staphylococcus Epidermidis Biofilm Infection. Front. Microbiol. 2018, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Bera, J.H.; Raj A, L.S.; Gang, S.; Patel, D.N. Chapter 22—Biofilm: A Threat to Medical Devices. In Microbial Biofilms; Sarma, H., Joshi, S., Lahiri, D., Ray, R.R., Davoodbasha, M., Eds.; Advances in Biotechnology and Bioengineering; Academic Press: Cambridge, MA, USA, 2023; pp. 369–390. ISBN 978-0-323-95715-1. [Google Scholar]
- Michaelis, C.; Grohmann, E. Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef]
- Vishakha, K.; Das, S.; Das, S.K.; Banerjee, S.; Ganguli, A. Antibacterial, Anti-Biofilm, and Anti-Virulence Potential of Tea Tree Oil against Leaf Blight Pathogen Xanthomonas Oryzae Pv. Oryzae Instigates Disease Suppression. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2022, 53, 19–32. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.; Junka, A.F.; Migdał, P.; Korzeniowska-Kowal, A.; Wzorek, A.; Bartoszewicz, M. The Antibiofilm Activity of Selected Substances Used in Oral Health Prophylaxis. BMC Oral Health 2022, 22, 509. [Google Scholar] [CrossRef]
- Ersanli, C.; Tzora, A.; Skoufos, I.; Fotou, K.; Maloupa, E.; Grigoriadou, K.; Voidarou, C.C.; Zeugolis, D.I. The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains. Antibiotics 2023, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Pourkhosravani, E.; Dehghan Nayeri, F.; Mohammadi Bazargani, M. Decoding Antibacterial and Antibiofilm Properties of Cinnamon and Cardamom Essential Oils: A Combined Molecular Docking and Experimental Study. AMB Express 2021, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-Review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: https://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf (accessed on 1 February 2025).
- Kamimura, R.; Kanematsu, H.; Ogawa, A.; Kogo, T.; Miura, H.; Kawai, R.; Hirai, N.; Kato, T.; Yoshitake, M.; Barry, D.M. Quantitative Analyses of Biofilm by Using Crystal Violet Staining and Optical Reflection. Materials 2022, 15, 6727. [Google Scholar] [CrossRef]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Vallé, Q.; Hancock, R.E.W. Critical Assessment of Methods to Quantify Biofilm Growth and Evaluate Antibiofilm Activity of Host Defence Peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef]
- Beyenal, H.; Donovan, C.; Lewandowski, Z.; Harkin, G. Three-Dimensional Biofilm Structure Quantification. J. Microbiol. Methods 2004, 59, 395–413. [Google Scholar] [CrossRef]
- Schlafer, S.; Meyer, R.L. Confocal Microscopy Imaging of the Biofilm Matrix. J. Microbiol. Methods 2017, 138, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Sedano, A.J.; Zapana-García, J.; Águila, E.V.-D.; Padilla-Huamantinco, P.G.; Guerra, D.G. Quantification of Early Biofilm Growth in Microtiter Plates through a Novel Image Analysis Software. J. Microbiol. Methods 2024, 223, 106979. [Google Scholar] [CrossRef] [PubMed]
- Reichling, J. Anti-Biofilm and Virulence Factor-Reducing Activities of Essential Oils and Oil Components as a Possible Option for Bacterial Infection Control. Planta Med. 2020, 86, 520–537. [Google Scholar] [CrossRef] [PubMed]
- Vidaković Knežević, S.; Knežević, S.; Vranešević, J.; Milanov, D.; Ružić, Z.; Karabasil, N.; Kocić-Tanackov, S. Using Essential Oils to Reduce Yersinia Enterocolitica in Minced Meat and in Biofilms. Foods 2024, 13, 806. [Google Scholar] [CrossRef]
- Zhan, X.; Tan, Y.; Lv, Y.; Fang, J.; Zhou, Y.; Gao, X.; Zhu, H.; Shi, C. The Antimicrobial and Antibiofilm Activity of Oregano Essential Oil against Enterococcus Faecalis and Its Application in Chicken Breast. Foods 2022, 11, 2296. [Google Scholar] [CrossRef]
- Kačániová, M.; Čmiková, N.; Vukovic, N.L.; Verešová, A.; Bianchi, A.; Garzoli, S.; Ben Saad, R.; Ben Hsouna, A.; Ban, Z.; Vukic, M.D. Citrus Limon Essential Oil: Chemical Composition and Selected Biological Properties Focusing on the Antimicrobial (In Vitro, In Situ), Antibiofilm, Insecticidal Activity and Preservative Effect against Salmonella Enterica Inoculated in Carrot. Plants 2024, 13, 524. [Google Scholar] [CrossRef]
- Ellboudy, N.M.; Elwakil, B.H.; Shaaban, M.M.; Olama, Z.A. Cinnamon Oil-Loaded Nanoliposomes with Potent Antibacterial and Antibiofilm Activities. Molecules 2023, 28, 4492. [Google Scholar] [CrossRef]
- Scandorieiro, S.; Teixeira, F.M.M.B.; Nogueira, M.C.L.; Panagio, L.A.; de Oliveira, A.G.; Durán, N.; Nakazato, G.; Kobayashi, R.K.T. Antibiofilm Effect of Biogenic Silver Nanoparticles Combined with Oregano Derivatives against Carbapenem-Resistant Klebsiella Pneumoniae. Antibiotics 2023, 12, 756. [Google Scholar] [CrossRef]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogoșanu, G.D.; Mogoantă, L.; Iordache, F.; Holban, A.M.; Vasile, B.Ș.; Bîrcă, A.C.; Oprea, O.-C.; et al. MAPLE Coatings Embedded with Essential Oil-Conjugated Magnetite for Anti-Biofilm Applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef]
- Abd El-Aziz, N.K.; Ammar, A.M.; El-Naenaeey, E.Y.M.; El Damaty, H.M.; Elazazy, A.A.; Hefny, A.A.; Shaker, A.; Eldesoukey, I.E. Antimicrobial and Antibiofilm Potentials of Cinnamon Oil and Silver Nanoparticles against Streptococcus Agalactiae Isolated from Bovine Mastitis: New Avenues for Countering Resistance. BMC Vet. Res. 2021, 17, 136. [Google Scholar] [CrossRef]
- Alibi, S.; Ben Selma, W.; Ramos-Vivas, J.; Smach, M.A.; Touati, R.; Boukadida, J.; Navas, J.; Ben Mansour, H. Anti-Oxidant, Antibacterial, Anti-Biofilm, and Anti-Quorum Sensing Activities of Four Essential Oils against Multidrug-Resistant Bacterial Clinical Isolates. Curr. Res. Transl. Med. 2020, 68, 59–66. [Google Scholar] [CrossRef]
- Oz, Y.; Nabawy, A.; Fedeli, S.; Gupta, A.; Huang, R.; Sanyal, A.; Rotello, V.M. Biodegradable Poly(lactic acid) Stabilized Nanoemulsions for the Treatment of Multidrug-Resistant Bacterial Biofilms. ACS Appl. Mater. Interfaces 2021, 13, 40325–40331. [Google Scholar] [CrossRef] [PubMed]
- Fadel, B.A.; Elwakil, B.H.; Fawzy, E.E.; Shaaban, M.M.; Olama, Z.A. Nanoemulsion of Lavandula Angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria. Molecules 2023, 28, 6988. [Google Scholar] [CrossRef] [PubMed]
- Rosato, R.; Napoli, E.; Granata, G.; Di Vito, M.; Garzoli, S.; Geraci, C.; Rizzo, S.; Torelli, R.; Sanguinetti, M.; Bugli, F. Study of the Chemical Profile and Anti-Fungal Activity against Candida auris of Cinnamomum Cassia Essential Oil and of Its Nano-Formulations Based on Polycaprolactone. Plants 2023, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Maniki, E.; Kostoglou, D.; Paterakis, N.; Nikolaou, A.; Kourkoutas, Y.; Papachristoforou, A.; Giaouris, E. Chemical Composition, Antioxidant, and Antibiofilm Properties of Essential Oil from Thymus Capitatus Plants Organically Cultured on the Greek Island of Lemnos. Molecules 2023, 28, 1154. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, B.; Biernasiuk, A.; Skiba, A.; Skalicka-Woźniak, K.; Ludwiczuk, A. Composition, Anti-MRSA Activity and Toxicity of Essential Oils from Cymbopogon Species. Molecules 2021, 26, 7542. [Google Scholar] [CrossRef]
- Somrani, M.; Debbabi, H.; Palop, A. Antibacterial and Antibiofilm Activity of Essential Oil of Clove against Listeria Monocytogenes and Salmonella Enteritidis. Food Sci. Technol. Int. Cienc. Tecnol. Los Aliment. Int. 2022, 28, 331–339. [Google Scholar] [CrossRef]
- Abdelhamed, F.M.; Abdeltawab, N.F.; ElRakaiby, M.T.; Shamma, R.N.; Moneib, N.A. Antibacterial and Anti-Inflammatory Activities of Thymus vulgaris Essential Oil Nanoemulsion on Acne Vulgaris. Microorganisms 2022, 10, 1874. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Ożarowski, M.; Seremak-Mrozikiewicz, A.; Wolski, H. Anti-Candida and Antibiofilm Activity of Selected Lamiaceae Essential Oils. Front. Biosci. Landmark Ed. 2023, 28, 28. [Google Scholar] [CrossRef]
- Di Vito, M.; Garzoli, S.; Rosato, R.; Mariotti, M.; Gervasoni, J.; Santucci, L.; Ovidi, E.; Cacaci, M.; Lombarini, G.; Torelli, R.; et al. A New Potential Resource in the Fight against Candida auris: The Cinnamomum Zeylanicum Essential Oil in Synergy with Antifungal Drug. Microbiol. Spectr. 2023, 11, e04385-22. [Google Scholar] [CrossRef]
- Peng, J.; Chen, G.; Guo, S.; Lin, Z.; Zeng, Y.; Ren, J.; Wang, Q.; Yang, W.; Liang, Y.; Li, J. Anti-Bacterial and Anti-Biofilm Activities of Essential Oil from Citrus Reticulata Blanco Cv. Tankan Peel Against Listeria Monocytogenes. Foods 2024, 13, 3841. [Google Scholar] [CrossRef] [PubMed]
- Pejčić, M.; Stojanović-Radić, Z.; Genčić, M.; Dimitrijević, M.; Radulović, N. Anti-Virulence Potential of Basil and Sage Essential Oils: Inhibition of Biofilm Formation, Motility and Pyocyanin Production of Pseudomonas Aeruginosa Isolates. Food Chem. Toxicol. 2020, 141, 111431. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Garzoli, S.; Ben Hsouna, A.; Bianchi, A.; Kluz, M.I.; Elizondo-Luevano, J.H.; Ban, Z.; Ben Saad, R.; Mnif, W.; Haščík, P. The Potential of Thymus Serpyllum Essential Oil as an Antibacterial Agent against Pseudomonas Aeruginosa in the Preservation of Sous Vide Red Deer Meat. Foods 2024, 13, 3107. [Google Scholar] [CrossRef] [PubMed]
- Jafri, H.; Ahmad, I. Thymus vulgaris Essential Oil and Thymol Inhibit Biofilms and Interact Synergistically with Antifungal Drugs against Drug Resistant Strains of Candida albicans and Candida tropicalis. J. Mycol. Médicale 2020, 30, 100911. [Google Scholar] [CrossRef]
- Park, S.-Y.; Raka, R.N.; Hui, X.-L.; Song, Y.; Sun, J.-L.; Xiang, J.; Wang, J.; Jin, J.-M.; Li, X.-K.; Xiao, J.-S.; et al. Six Spain Thymus Essential Oils Composition Analysis and Their in Vitro and in Silico Study against Streptococcus Mutans. BMC Complement. Med. Ther. 2023, 23, 106. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Gaspar, C.; Rolo, J.; Palmeira-de-Oliveira, R.; Teixeira, J.P.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Comparative Efficacy of Essential Oils against Cutibacterium Acnes: Effect upon Strains from Phylotypes with Different Virulence Patterns. Microb. Pathog. 2025, 199, 107159. [Google Scholar] [CrossRef]
- Liaqat, I.; Ibtisam, R.; Hussain, M.I.; Muhammad, N.; Andleeb, S.; Naseem, S.; Ali, A.; Latif, A.A.; Ali, S.; Aftab, M.N.; et al. Medicinal Plants Exhibited Promising Potential to Inhibit Biofilm Formation by Catheter-Associated Bacteria in UTI Patients from Lahore, Pakistan. J. Oleo Sci. 2025, 74, 221–232. [Google Scholar] [CrossRef]
- Badekova, K.Z.; Atazhanova, G.A.; Kacergius, T.; Akhmetova, S.B.; Smagulov, M.K. Formulation of an Origanum Vulgare Based Dental Gel with Antimicrobial Activity. J. Taibah Univ. Med. Sci. 2021, 16, 712–718. [Google Scholar] [CrossRef]
- Jurado, P.; Uruén, C.; Martínez, S.; Lain, E.; Sánchez, S.; Rezusta, A.; López, V.; Arenas, J. Essential Oils of Pinus Sylvestris, Citrus Limon and Origanum Vulgare Exhibit High Bactericidal and Anti-Biofilm Activities against Neisseria Gonorrhoeae and Streptococcus Suis. Biomed. Pharmacother. Biomedecine Pharmacother. 2023, 168, 115703. [Google Scholar] [CrossRef]
- de Oliveira Carvalho, I.; Purgato, G.A.; Píccolo, M.S.; Pizziolo, V.R.; Coelho, R.R.; Diaz-Muñoz, G.; Alves Nogueira Diaz, M. In Vitro Anticariogenic and Antibiofilm Activities of Toothpastes Formulated with Essential Oils. Arch. Oral Biol. 2020, 117, 104834. [Google Scholar] [CrossRef]
- Wijesinghe, G.K.; de Oliveira, T.R.; Maia, F.C.; de Feiria, S.B.; Barbosa, J.P.; Joia, F.; Boni, G.C.; Höfling, J.F. Efficacy of True Cinnamon (Cinnamomum verum) Leaf Essential Oil as a Therapeutic Alternative for Candida Biofilm Infections. Iran. J. Basic Med. Sci. 2021, 24, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Somrani, M.; Inglés, M.-C.; Debbabi, H.; Abidi, F.; Palop, A. Garlic, Onion, and Cinnamon Essential Oil Anti-Biofilms’ Effect against Listeria Monocytogenes. Foods 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Kosari, F.; Taheri, M.; Moradi, A.; Hakimi Alni, R.; Alikhani, M.Y. Evaluation of Cinnamon Extract Effects on clbB Gene Expression and Biofilm Formation in Escherichia coli Strains Isolated from Colon Cancer Patients. BMC Cancer 2020, 20, 267. [Google Scholar] [CrossRef]
- Song, Y.-M.; Zhou, H.-Y.; Wu, Y.; Wang, J.; Liu, Q.; Mei, Y.-F. In Vitro Evaluation of the Antibacterial Properties of Tea Tree Oil on Planktonic and Biofilm-Forming Streptococcus Mutans. AAPS Pharmscitech 2020, 21, 227. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-Ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef]
- Iseppi, R.; Mariani, M.; Benvenuti, S.; Truzzi, E.; Messi, P. Effects of Melaleuca Alternifolia Chell (Tea Tree) and Eucalyptus Globulus Labill. Essential Oils on Antibiotic-Resistant Bacterial Biofilms. Molecules 2023, 28, 1671. [Google Scholar] [CrossRef]
- Cai, K.; Liu, Y.; Yue, Y.; Liu, Y.; Guo, F. Essential Oil Nanoemulsion Hydrogel with Anti-Biofilm Activity for the Treatment of Infected Wounds. Polymers 2023, 15, 1376. [Google Scholar] [CrossRef]
- Gupta, P.; Pruthi, V.; Poluri, K.M. Mechanistic Insights into Candida Biofilm Eradication Potential of Eucalyptol. J. Appl. Microbiol. 2021, 131, 105–123. [Google Scholar] [CrossRef]
- Khedhri, S.; Polito, F.; Caputo, L.; Manna, F.; Khammassi, M.; Hamrouni, L.; Amri, I.; Nazzaro, F.; De Feo, V.; Fratianni, F. Chemical Composition, Phytotoxic and Antibiofilm Activity of Seven Eucalyptus Species from Tunisia. Molecules 2022, 27, 8227. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Pathrose, B.; Olatunji, O.J.; Al-Ansari, A.; Alfarhan, A.; Ramesh, V. Chemical Composition, Antioxidant, Anti-Bacterial, and Anti-Cancer Activities of Essential Oils Extracted from Citrus limetta Risso Peel Waste Remains after Commercial Use. Molecules 2022, 27, 8329. [Google Scholar] [CrossRef]
- Marinković, J.; Ćulafić, D.M.; Nikolić, B.; Đukanović, S.; Marković, T.; Tasić, G.; Ćirić, A.; Marković, D. Antimicrobial Potential of Irrigants Based on Essential Oils of Cymbopogon martinii and Thymus Zygis towards in Vitro Multispecies Biofilm Cultured in Ex Vivo Root Canals. Arch. Oral Biol. 2020, 117, 104842. [Google Scholar] [CrossRef]
- Ngo-Mback, M.N.L.; Babii, C.; Jazet Dongmo, P.M.; Kouipou Toghueo, M.R.; Stefan, M.; Fekam Boyom, F. AntiCandidal and Synergistic Effect of Essential Oil Fractions from Three Aromatic Plants Used in Cameroon. J. Mycol. Médicale 2020, 30, 100940. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Karuppaiah, A.; Nithyanth, M.; Baberoselin, R.; Ramesh, S.; Geetha, N.; Veintramuthu, S. Formulation Development of Cream with Mupirocin and Essential Oils for Eradication of Biofilm Mediated Antimicrobial Resistance. Arch. Microbiol. 2021, 203, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Praseetha, S.; Sukumaran, S.T.; Dan, M.; Augustus, A.R.; Pandian, S.K.; Sugathan, S. The Anti-Biofilm Potential of Linalool, a Major Compound from Hedychium larsenii, against Streptococcus Pyogenes and Its Toxicity Assessment in Danio Rerio. Antibiotics 2023, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- Noumi, E.; Ahmad, I.; Adnan, M.; Patel, H.; Merghni, A.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Kadri, A.; Caputo, L.; et al. Illicium verum L. (Star anise) Essential Oil: GC/MS Profile, Molecular Docking Study, In Silico ADME Profiling, Quorum Sensing, and Biofilm-Inhibiting Effect on Foodborne Bacteria. Molecules 2023, 28, 7691. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, G.; Li, J.; Chen, J.; Li, L.; Li, Z.; Zhang, X.; Zhang, S.; Thorne, R.F.; Zhang, S. Antimicrobial Activity of Lemongrass Essential Oil (Cymbopogon flexuosus) and Its Active Component Citral Against Dual-Species Biofilms of Staphylococcus aureus and Candida Species. Front. Cell. Infect. Microbiol. 2020, 10, 603858. [Google Scholar] [CrossRef]
- Pedonese, F.; Longo, E.; Torracca, B.; Najar, B.; Fratini, F.; Nuvoloni, R. Antimicrobial and Anti-Biofilm Activity of Manuka Essential Oil against Listeria Monocytogenes and Staphylococcus aureus of Food Origin. Ital. J. Food Saf. 2022, 11, 10039. [Google Scholar] [CrossRef]
- Kabotso, D.E.K.; Neglo, D.; Gaba, S.E.; Danyo, E.K.; Dayie, A.D.; Asantewaa, A.A.; Kotey, F.C.N.; Dayie, N.T.K.D. In Vitro Evaluation of Rosemary Essential Oil: GC-MS Profiling, Antibacterial Synergy, and Biofilm Inhibition. Pharmaceuticals 2024, 17, 1653. [Google Scholar] [CrossRef]
- Kim, E.S.; Park, B.-I.; Kim, Y.-H.; Kang, J.; You, Y.-O. The Inhibitory Effect of Agastache Rugosa Essential Oil on the Dental Biofilm. Molecules 2024, 29, 4907. [Google Scholar] [CrossRef]
- Ghannay, S.; Aouadi, K.; Kadri, A.; Snoussi, M. In Vitro and In Silico Screening of Anti-Vibrio Spp., Antibiofilm, Antioxidant and Anti-Quorum Sensing Activities of Cuminum cyminum L. Volatile Oil. Pharmaceuticals 2022, 11, 2236. [Google Scholar] [CrossRef]
- Kačániová, M.; Vukovic, N.L.; Čmiková, N.; Galovičová, L.; Schwarzová, M.; Šimora, V.; Kowalczewski, P.Ł.; Kluz, M.I.; Puchalski, C.; Bakay, L.; et al. Salvia sclarea Essential Oil Chemical Composition and Biological Activities. Int. J. Mol. Sci. 2023, 24, 5179. [Google Scholar] [CrossRef] [PubMed]
- Ramić, D.; Bucar, F.; Kunej, U.; Dogša, I.; Klančnik, A.; Smole Možina, S. Antibiofilm Potential of Lavandula Preparations against Campylobacter Jejuni. Appl. Environ. Microbiol. 2021, 87, e0109921. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, O.O. Chapter 19—Toxicity and Safety of Essential Oil. In Applications of Essential Oils in the Food Industry; Adetunji, C.O., Sharifi-Rad, J., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 235–241. ISBN 978-0-323-98340-2. [Google Scholar]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2021, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Elbestawy, M.K.M.; El-Sherbiny, G.M.; Moghannem, S.A. Antibacterial, Antibiofilm and Anti-Inflammatory Activities of Eugenol Clove Essential Oil against Resistant Helicobacter Pylori. Molecules 2023, 28, 2448. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Kim, H.-E.; Han, S.-J.; Choi, J.-S. Antibacterial and Antibiofilm Activities of Cinnamon Essential Oil Nanoemulsion against Multi-Species Oral Biofilms. Sci. Rep. 2021, 11, 5911. [Google Scholar] [CrossRef]
- Brożyna, M.; Paleczny, J.; Kozłowska, W.; Chodaczek, G.; Dudek-Wicher, R.; Felińczak, A.; Gołębiewska, J.; Górniak, A.; Junka, A. The Antimicrobial and Antibiofilm In Vitro Activity of Liquid and Vapour Phases of Selected Essential Oils against Staphylococcus aureus. Pathogens 2021, 10, 1207. [Google Scholar] [CrossRef]
- Fekry, M.; Yahya, G.; Osman, A.; Al-Rabia, M.W.; Mostafa, I.; Abbas, H.A. GC-MS Analysis and Microbiological Evaluation of Caraway Essential Oil as a Virulence Attenuating Agent against Pseudomonas Aeruginosa. Molecules 2022, 27, 8532. [Google Scholar] [CrossRef]
- Vasconcelos, P.G.S.; Abuna, G.F.; e Silva, J.P.R.; Tavares, J.F.; Costa, E.M.M.d.B.; Murata, R.M. Syzygium Aromaticum Essential Oil and Its Major Constituents: Assessment of Activity against Candida spp. and Toxicity. PLoS ONE 2024, 19, e0305405. [Google Scholar] [CrossRef]
- Kapustová, M.; Puškárová, A.; Bučková, M.; Granata, G.; Napoli, E.; Annušová, A.; Mesárošová, M.; Kozics, K.; Pangallo, D.; Geraci, C. Biofilm Inhibition by Biocompatible Poly(ε-Caprolactone) Nanocapsules Loaded with Essential Oils and Their Cyto/Genotoxicity to Human Keratinocyte Cell Line. Int. J. Pharm. 2021, 606, 120846. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Sienkiewicz, M.; Pruss, A.; Łopusiewicz, Ł.; Arszyńska, N.; Wojciechowska-Koszko, I.; Kilanowicz, A.; Kot, B.; Dołęgowska, B. Antibacterial and Anti-Biofilm Activities of Essential Oil Compounds against New Delhi Metallo-β-Lactamase-1-Producing Uropathogenic Klebsiella Pneumoniae Strains. Antibiotics 2022, 11, 147. [Google Scholar] [CrossRef]
- Purkait, S.; Bhattacharya, A.; Bag, A.; Chattopadhyay, R.R. Evaluation of Antibiofilm Efficacy of Essential Oil Components β-Caryophyllene, Cinnamaldehyde and Eugenol Alone and in Combination against Biofilm Formation and Preformed Biofilms of Listeria Monocytogenes and Salmonella Typhimurium. Lett. Appl. Microbiol. 2020, 71, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Pelarti, S.; Karimi Zarehshuran, L.; Babaeekhou, L.; Ghane, M. Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities of Artemisia dracunculus Essential Oil (EO): A Study against Salmonella Enterica Serovar Typhimurium and Staphylococcus aureus. Arch. Microbiol. 2021, 203, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to Nature: Combating Candida albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Gamal El-Din, M.I.; Youssef, F.S.; Altyar, A.E.; Ashour, M.L. GC/MS Analyses of the Essential Oils Obtained from Different Jatropha Species, Their Discrimination Using Chemometric Analysis and Assessment of Their Antibacterial and Anti-Biofilm Activities. Plants 2022, 11, 1268. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, J.; Zou, Y.; Li, S.; Zhao, D.; Wang, H.; Xia, X. Anti-Biofilm Activity of Laurel Essential Oil against Vibrio parahaemolyticus. Foods 2023, 12, 3658. [Google Scholar] [CrossRef]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Antibacterial and Antibiofilm Efficacy of Thyme (Thymus vulgaris L.) Essential Oil against Foodborne Illness Pathogens, Salmonella Enterica Subsp. Enterica Serovar Typhimurium and Bacillus Cereus. Antibiotics 2023, 12, 485. [Google Scholar] [CrossRef]
- Bakó, C.; Balázs, V.L.; Kerekes, E.; Kocsis, B.; Nagy, D.U.; Szabó, P.; Micalizzi, G.; Mondello, L.; Krisch, J.; Pethő, D.; et al. Flowering Phenophases Influence the Antibacterial and Anti-Biofilm Effects of Thymus vulgaris L. Essential Oil. BMC Complement. Med. Ther. 2023, 23, 168. [Google Scholar] [CrossRef]
- Chatterjee, B.; Vittal, R.R. Quorum Sensing Modulatory and Biofilm Inhibitory Activity of Plectranthus barbatus Essential Oil: A Novel Intervention Strategy. Arch. Microbiol. 2021, 203, 1767–1778. [Google Scholar] [CrossRef]
- Merghni, A.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Adnan, M.; Snoussi, M.; Noumi, E. Comparative Study of Antibacterial, Antibiofilm, Antiswarming and Antiquorum Sensing Activities of Origanum Vulgare Essential Oil and Terpinene-4-Ol against Pathogenic Bacteria. Life 2022, 12, 1616. [Google Scholar] [CrossRef]
- Vrenna, G.; Artini, M.; Ragno, R.; Relucenti, M.; Fiscarelli, E.V.; Tuccio Guarna Assanti, V.; Papa, R.; Selan, L. Anti-Virulence Properties of Coridothymus capitatus Essential Oil against Pseudomonas Aeruginosa Clinical Isolates from Cystic Fibrosis Patients. Microorganisms 2021, 9, 2257. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Gong, X.; Wu, X.; Liu, L.; Chi, F. Rosemary and Tea Tree Essential Oils Exert Antibiofilm Activities In Vitro against Staphylococcus aureus and Escherichia coli. J. Food Prot. 2020, 83, 1261–1267. [Google Scholar] [CrossRef]
- Noumi, E.; Ahmad, I.; Adnan, M.; Merghni, A.; Patel, H.; Haddaji, N.; Bouali, N.; Alabbosh, K.F.; Ghannay, S.; Aouadi, K.; et al. GC/MS Profiling, Antibacterial, Anti-Quorum Sensing, and Antibiofilm Properties of Anethum graveolens L. Essential Oil: Molecular Docking Study and In-Silico ADME Profiling. Plants 2023, 12, 1997. [Google Scholar] [CrossRef] [PubMed]
- Ghannay, S.; Aouadi, K.; Kadri, A.; Snoussi, M. GC-MS Profiling, Vibriocidal, Antioxidant, Antibiofilm, and Anti-Quorum Sensing Properties of Carum carvi L. Essential Oil: In Vitro and In Silico Approaches. Plants 2022, 11, 1072. [Google Scholar] [CrossRef]
- Barak, T.H.; Eryilmaz, M.; Karaca, B.; Servi, H.; Kara Ertekin, S.; Dinc, M.; Ustuner, H. Antimicrobial, Anti-Biofilm, Anti-Quorum Sensing and Cytotoxic Activities of Thymbra spicata L. Subsp. Spicata Essential Oils. Antibiotics 2025, 14, 181. [Google Scholar] [CrossRef]
- Kunicka-Styczyńska, A.; Tyfa, A.; Laskowski, D.; Plucińska, A.; Rajkowska, K.; Kowal, K. Clove Oil (Syzygium aromaticum L.) Activity against Alicyclobacillus acidoterrestris Biofilm on Technical Surfaces. Molecules 2020, 25, 3334. [Google Scholar] [CrossRef] [PubMed]
- Kaskatepe, B.; Aslan Erdem, S.; Ozturk, S.; Safi Oz, Z.; Subasi, E.; Koyuncu, M.; Vlainić, J.; Kosalec, I. Antifungal and Anti-Virulent Activity of Origanum majorana L. Essential Oil on Candida albicans and In Vivo Toxicity in the Galleria Mellonella Larval Model. Molecules 2022, 27, 663. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Galovičová, L.; Valková, V.; Ďuranová, H.; Štefániková, J.; Čmiková, N.; Vukic, M.; Vukovic, N.L.; Kowalczewski, P.Ł. Chemical Composition, Antioxidant, In Vitro and In Situ Antimicrobial, Antibiofilm, and Anti-Insect Activity of Cedar Atlantica Essential Oil. Plants 2022, 11, 358. [Google Scholar] [CrossRef]
- Balázs, V.L.; Filep, R.; Répás, F.; Kerekes, E.; Szabó, P.; Kocsis, B.; Böszörményi, A.; Krisch, J.; Horváth, G. Immortelle (Helichrysum italicum (Roth) G. Don) Essential Oil Showed Antibacterial and Biofilm Inhibitory Activity against Respiratory Tract Pathogens. Molecules 2022, 27, 5518. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Gędas, A.; Simões, M. The Effects of Eugenol, Trans-Cinnamaldehyde, Citronellol, and Terpineol on Escherichia coli Biofilm Control as Assessed by Culture-Dependent and -Independent Methods. Molecules 2020, 25, 2641. [Google Scholar] [CrossRef]
- Guillín, Y.; Cáceres, M.; Stashenko, E.E.; Hidalgo, W.; Ortiz, C. Untargeted Metabolomics for Unraveling the Metabolic Changes in Planktonic and Sessile Cells of Salmonella Enteritidis ATCC 13076 after Treatment with Lippia origanoides Essential Oil. Antibiotics 2023, 12, 899. [Google Scholar] [CrossRef]
- Su, F.; Yang, G.; Hu, D.; Ruan, C.; Wang, J.; Zhang, Y.; Zhu, Q. Chemical Composition, Antibacterial and Antioxidant Activities of Essential Oil from Centipeda minima. Molecules 2023, 28, 824. [Google Scholar] [CrossRef]
- Tartor, Y.H.; Elmowalid, G.A.; Hassan, M.N.; Shaker, A.; Ashour, D.F.; Saber, T. Promising Anti-Biofilm Agents and Phagocytes Enhancers for the Treatment of Candida albicans Biofilm-Associated Infections. Front. Cell. Infect. Microbiol. 2022, 12, 807218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Sun, J.; Song, Y.; Raka, R.N.; Xiang, J.; Wu, H.; Xiao, J.; Jin, J.; Hui, X. Antibacterial Activity of Oregano Essential Oils against Streptococcus Mutans in Vitro and Analysis of Active Components. BMC Complement. Med. Ther. 2023, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Algburi, A.; Asghar, A.; Huang, Q.; Mustfa, W.; Javed, H.U.; Zehm, S.; Chikindas, M.L. Black Cardamom Essential Oil Prevents Escherichia coli O157:H7 and Salmonella Typhimurium JSG 1748 Biofilm Formation through Inhibition of Quorum Sensing. J. Food Sci. Technol. 2021, 58, 3183–3191. [Google Scholar] [CrossRef] [PubMed]
- ALrashidi, A.A.; Noumi, E.; Snoussi, M.; Feo, V.D. Chemical Composition, Antibacterial and Anti-Quorum Sensing Activities of Pimenta dioica L. Essential Oil and Its Major Compound (Eugenol) against Foodborne Pathogenic Bacteria. Plants 2022, 11, 540. [Google Scholar] [CrossRef]
- Dos Santos, E.A.R.; Tadielo, L.E.; Schmiedt, J.A.; Possebon, F.S.; Pereira, M.O.; Pereira, J.G.; Dos Santos Bersot, L. Effect of Ginger Essential Oil and 6-Gingerol on a Multispecies Biofilm of Listeria Monocytogenes, Salmonella Typhimurium, and Pseudomonas Aeruginosa. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2023, 54, 3041–3049. [Google Scholar] [CrossRef]
- Morshdy, A.E.M.A.; El-tahlawy, A.S.; Qari, S.H.; Qumsani, A.T.; Bay, D.H.; Sami, R.; Althubaiti, E.H.; Mansour, A.M.A.; Aljahani, A.H.; Hafez, A.E.-S.E.; et al. Anti-Biofilms’ Activity of Garlic and Thyme Essential Oils against Salmonella Typhimurium. Molecules 2022, 27, 2182. [Google Scholar] [CrossRef]
- Naccari, C.; Ginestra, G.; Micale, N.; Palma, E.; Galletta, B.; Costa, R.; Vadalà, R.; Nostro, A.; Cristani, M. Binary Combinations of Essential Oils: Antibacterial Activity Against Staphylococcus aureus, and Antioxidant and Anti-Inflammatory Properties. Molecules 2025, 30, 438. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, H.; Xiao, X.; Sun, S.; Tong, Y.; Zhuang, S.; Sheng, Z.; Fan, Y.; Ma, W.; Liu, Y.; et al. Shuanghuanglian Volatile Oil Exerts Antipyretic, Anti-Inflammatory, and Antibacterial Synergistic Effects through Multiple Pathways. J. Ethnopharmacol. 2025, 337, 118795. [Google Scholar] [CrossRef]
- Yang, X.; Rajivgandhi, G.N.; Ramachandran, G.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Almanaa, T.N.; Manoharan, N.; Viji, R. Preparative HPLC Fraction of Hibiscus rosa-sinensis Essential Oil against Biofilm Forming Klebsiella pneumoniae. Saudi J. Biol. Sci. 2020, 27, 2853–2862. [Google Scholar] [CrossRef]
- Tritripmongkol, P.; Sangkanu, S.; Boripun, R.; Jeenkeawpieam, J.; Chuprom, J.; Nissapatorn, V.; Pereira, M.d.L.; Paul, A.K.; Mitsuwan, W. Robusta Coffee Extracts Inhibit Quorum Sensing Activity in Chromobacterium Violaceum and Reduce Biofilms against Bacillus Cereus and Staphylococcus aureus. Vet. World 2022, 15, 2391–2398. [Google Scholar] [CrossRef] [PubMed]
- Mathlouthi, A.; Saadaoui, N.; Pennacchietti, E.; De Biase, D.; Ben-Attia, M. Essential Oils from Artemisia Species Inhibit Biofilm Formation and the Virulence of Escherichia coli EPEC 2348/69. Biofouling 2021, 37, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Feng, C.; Zheng, Y.; Sun, Y.; Yan, C.; Zhang, X. Effect of Lemon Essential Oil Microemulsion on the Cariogenic Virulence Factor of Streptococcus Mutans via the Glycolytic Pathway. Oral Health Prev. Dent. 2022, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, J.; Zhang, L.; Zhang, R.; Zhang, S.; Ye, S.; Zhao, Z.; Yang, D. Exploring the Antibacterial Mechanism of Essential Oils by Membrane Permeability, Apoptosis and Biofilm Formation Combination with Proteomics Analysis against Methicillin-Resistant Staphylococcus aureus. Int. J. Med. Microbiol. 2020, 310, 151435. [Google Scholar] [CrossRef]
- Sirati, R.; Khajehrahimi, A.E.; Kazempoor, R.; Kakoolaki, S.; Ghorbanzadeh, A. Development, Physicochemical Characterization, and Antimicrobial Evaluation of Niosome-Loaded Oregano Essential Oil against Fish-Borne Pathogens. Heliyon 2024, 10, e26486. [Google Scholar] [CrossRef]
- Mishra, P.; Gupta, P.; Pruthi, V. Cinnamaldehyde Incorporated Gellan/PVA Electrospun Nanofibers for Eradicating Candida Biofilm. Mater. Sci. Eng. C 2021, 119, 111450. [Google Scholar] [CrossRef]
- Bauer Faria, T.R.; Furletti-Goes, V.F.; Franzini, C.M.; de Aro, A.A.; de Andrade, T.A.M.; Sartoratto, A.; de Menezes, C.C. Anti-Inflammatory and Antimicrobial Effects of Zingiber officinale Mouthwash on Patients with Fixed Orthodontic Appliances. Am. J. Orthod. Dentofacial Orthop. 2021, 159, 21–29. [Google Scholar] [CrossRef]
- Taalab, M.; Mahmoud, S.; Elmoslemany, R.; Abdelaziz, D. Intrapocket Application of Tea Tree Oil Gel in the Treatment of Stage 2 Periodontitis. BMC Oral Health 2021, 21, 239. [Google Scholar] [CrossRef]
- Ammar, H.A.; Samy, R.; Reda, F.M.; Hassanein, W.A. Essential Oils and Lactobacillus Metabolites as Alternative Antibiofilm Agents against Foodborne Bacteria and Molecular Analysis of Biofilm Regulatory Genes. Sci. Rep. 2025, 15, 7576. [Google Scholar] [CrossRef]
- Miranda-Cadena, K.; Marcos-Arias, C.; Perez-Rodriguez, A.; Cabello-Beitia, I.; Mateo, E.; Sevillano, E.; Madariaga, L.; Quindós, G.; Eraso, E. In Vitro and in Vivo Anti- Candida Activity of Citral in Combination with Fluconazole. J. Oral Microbiol. 2022, 14, 2045813. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Göldi, L.; Rohr, N.; Worreth, S.; Dard, E.; Hünerfauth, S.; Töpper, T.; Zurflüh, J.; Braissant, O. Antimicrobial and Mechanical Assessment of Cellulose-Based Thermoformable Material for Invisible Dental Braces with Natural Essential Oils Protecting from Biofilm Formation. Sci. Rep. 2023, 13, 13428. [Google Scholar] [CrossRef]
- Barbarossa, A.; Rosato, A.; Carrieri, A.; Tardugno, R.; Corbo, F.; Clodoveo, M.L.; Fracchiolla, G.; Carocci, A. Antifungal Biofilm Inhibitory Effects of Combinations of Diclofenac and Essential Oils. Antibiotics 2023, 12, 1673. [Google Scholar] [CrossRef]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F. Chemical Composition, Antibiofilm, Cytotoxic, and Anti-Acetylcholinesterase Activities of Myrtus communis L. Leaves Essential Oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Hasnain, S.Z.U.; Baloch, R.; Amin, A.; Nasibova, A.; Selakovic, D.; Rosic, G.L.; Islamov, S.; Naraliyeva, N.; Jaradat, N.; et al. Exploring Essential Oil-Based Bio-Composites: Molecular Docking and in Vitro Analysis for Oral Bacterial Biofilm Inhibition. Front. Chem. 2024, 12, 1383620. [Google Scholar] [CrossRef] [PubMed]
- Begum JP, S.; Sahu, P.; Vinode, R.; Patel, A.; Alomary, M.N.; Begum, M.Y.; Jamous, Y.F.; Siddiqua, A.; Fatease, A.A.; Ansari, M.A. Antimicrobial Nanoemulsion: A Futuristic Approach in Antibacterial Drug Delivery System. J. Saudi Chem. Soc. 2024, 28, 101896. [Google Scholar] [CrossRef]
- Sheikh, A.R.; Wu-Chen, R.A.; Matloob, A.; Mahmood, M.H.; Javed, M.; Sheikh, A.R.; Wu-Chen, R.A.; Matloob, A.; Mahmood, M.H.; Javed, M. Nanoencapsulation of Volatile Plant Essential Oils: A Paradigm Shift in Food Industry Practices. Food Innov. Adv. 2024, 3, 305–319. [Google Scholar] [CrossRef]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly (Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef]
- Gafur, A.; Sukamdani, G.Y.; Kristi, N.; Maruf, A.; Xu, J.; Chen, X.; Wang, G.; Ye, Z. From Bulk to Nano-Delivery of Essential Phytochemicals: Recent Progress and Strategies for Antibacterial Resistance. J. Mater. Chem. B 2020, 8, 9825–9835. [Google Scholar] [CrossRef]
- de Matos, S.P.; Teixeira, H.F.; de Lima, Á.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Doghish, A.S.; El-Dakroury, W.A.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Hashem, A.H. Antimicrobial, Antibiofilm, and Anticancer Activities of Syzygium Aromaticum Essential Oil Nanoemulsion. Molecules 2023, 28, 5812. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Maiti, T.K.; Parvate, S.; Dixit, P.; Singh, J.; Reddy, V.J.; Bhuvanesh, E.; Chattopadhyay, S. Chapter 6—Liposome for Encapsulation of Essential Oil and Fatty Acids. In Liposomal Encapsulation in Food Science and Technology; Anandharamakrishnan, C., Dutta, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 113–124. ISBN 978-0-12-823935-3. [Google Scholar]
- Yadav, R.; Pradhan, M.; Yadav, K.; Mahalvar, A.; Yadav, H. Present Scenarios and Future Prospects of Herbal Nanomedicine for Antifungal Therapy. J. Drug Deliv. Sci. Technol. 2022, 74, 103430. [Google Scholar] [CrossRef] [PubMed]
- Pinilla, C.M.B.; Lopes, N.A.; Brandelli, A. Lipid-Based Nanostructures for the Delivery of Natural Antimicrobials. Molecules 2021, 26, 3587. [Google Scholar] [CrossRef] [PubMed]
- Hossen, M.A.; Shimul, I.M.; Sameen, D.E.; Rasheed, Z.; Dai, J.; Li, S.; Qin, W.; Tang, W.; Chen, M.; Liu, Y. Essential Oil–Loaded Biopolymeric Particles on Food Industry and Packaging: A Review. Int. J. Biol. Macromol. 2024, 265, 130765. [Google Scholar] [CrossRef]
- Alam, J.; Dilnawaz, F.; Sahoo, S.K.; Singh, D.V.; Mukhopadhyay, A.K.; Hussain, T.; Pati, S. Curcumin Encapsulated into Biocompatible Co-Polymer PLGA Nanoparticle Enhanced Anti-Gastric Cancer and Anti-Helicobacter Pylori Effect. Asian Pac. J. Cancer Prev. 2022, 23, 61–70. [Google Scholar] [CrossRef]
- Liu, F.; Jin, P.; Gong, H.; Sun, Z.; Du, L.; Wang, D. Antibacterial and Antibiofilm Activities of Thyme Oil against Foodborne Multiple Antibiotics-Resistant Enterococcus Faecalis. Poult. Sci. 2020, 99, 5127–5136. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; da Rocha, M.G.; Lima, L.R.; Cunha, A.P.; de Oliveira, J.S.; de Andrade, A.R.C.; Ricardo, N.M.P.S.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Rocha, M.F.G.; et al. Essential Oils Encapsulated in Chitosan Microparticles against Candida albicans Biofilms. Int. J. Biol. Macromol. 2021, 166, 621–632. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Ellah, N.H.A.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- Albuquerque, V.d.Q.; Soares, M.J.C.; Matos, M.N.C.; Cavalcante, R.M.B.; Guerrero, J.A.P.; Rodrigues, T.H.S.; Gomes, G.A.; Guedes, R.F.d.M.; Castelo-Branco, D.d.S.C.M.; da Silva, I.N.G.; et al. Anti-Staphylococcal Activity of Cinnamomum Zeylanicum Essential Oil against Planktonic and Biofilm Cells Isolated from Canine Otological Infections. Antibiotics 2021, 11, 4. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of Clove (Syzygium aromaticum) Oil and Its Major Components to Human Skin Cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef]
- Abdelrahman, S.M.; El Samak, M.; El-Baz, L.M.F.; Hanora, A.M.S.; Satyal, P.; Dosoky, N.S. Effects of Mint Oils on the Human Oral Microbiome: A Pilot Study. Microorganisms 2024, 12, 1538. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-J.; Chen, L.-W.; Chen, L.-G.; Chang, T.-L.; Huang, C.-W.; Huang, M.-C.; Wang, C.-C. Correlations of the Components of Tea Tree Oil with Its Antibacterial Effects and Skin Irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Iseppi, R.; Tardugno, R.; Brighenti, V.; Benvenuti, S.; Sabia, C.; Pellati, F.; Messi, P. Phytochemical Composition and in vitro Antimicrobial Activity of Essential Oils from the Lamiaceae Family against Streptococcus agalactiae and Candida albicans Biofilms. Antibiotics 2020, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Brożyna, M.; Paleczny, J.; Kozłowska, W.; Ciecholewska-Juśko, D.; Parfieńczyk, A.; Chodaczek, G.; Junka, A. Chemical Composition and Antibacterial Activity of Liquid and Volatile Phase of Essential Oils against Planktonic and Biofilm-Forming Cells of Pseudomonas Aeruginosa. Molecules 2022, 27, 4096. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Almuhayawi, M.S.; Alqhtani, H.; Al Jaouni, S.K.; Saleh, F.M.; Warrad, M.; Hagagy, N. Anti-Salmonella and Antibiofilm Potency of Salvia officinalis L. Essential Oil against Antibiotic-Resistant Salmonella Enterica. Antibiotics 2022, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, M.; Sahmeddini, S.; Lavaee, F.; Zareshahrabadi, Z.; Zomorodian, K. Antimicrobial and Anti-Biofilm Activities of Thymus fallax Essential Oil against Oral Pathogens. BioMed Res. Int. 2022, 2022, 9744153. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Liu, T.; Liu, Y.; Wu, X.; Liu, L. Mandarin (Citrus reticulata L.) Essential Oil Incorporated into Chitosan Nanoparticles: Characterization, Anti-Biofilm Properties and Application in Pork Preservation. Int. J. Biol. Macromol. 2021, 185, 620–628. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Galovičová, L.; Ivanišová, E.; Štefániková, J.; Valková, V.; Borotová, P.; Kowalczewski, P.Ł.; Kunová, S.; Felšöciová, S.; et al. Biological Activity and Antibiofilm Molecular Profile of Citrus Aurantium Essential Oil and Its Application in a Food Model. Molecules 2020, 25, 3956. [Google Scholar] [CrossRef]
- Garcia, L.G.S.; Rocha, M.G.d.; Freire, R.S.; Nunes, P.I.G.; Nunes, J.V.S.; Fernandes, M.R.; Pereira-Neto, W.A.; Sidrim, J.J.C.; Santos, F.A.; Rocha, M.F.G.; et al. Chitosan Microparticles Loaded with Essential Oils Inhibit Duo-Biofilms of Candida albicans and Streptococcus mutans. J. Appl. Oral Sci. 2023, 31, e20230146. [Google Scholar] [CrossRef]
- Khammassi, M.; Polito, F.; Caputo, L.; Abidi, A.; Mabrouk, Y.; Nazzaro, F.; Fratianni, F.; Anouar, E.H.; Snoussi, M.; Noumi, E.; et al. Antibacterial, Antibiofilm, and Chemical Profiles of Ammi visnaga L. and Foeniculum vulgare Mill. Essential Oils, and ADMET, Molecular Docking Investigation of Essential Oils Major Components. Fitoterapia 2024, 177, 106047. [Google Scholar] [CrossRef]
- Lim, A.C.; Tang, S.G.H.; Zin, N.M.; Maisarah, A.M.; Ariffin, I.A.; Ker, P.J.; Mahlia, T.M.I. Chemical Composition, Antioxidant, Antibacterial, and Antibiofilm Activities of Backhousia citriodora Essential Oil. Molecules 2022, 27, 4895. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Asghar, A.; Algburi, A.; Huang, Q.; Ahmad, T.; Zhong, H.; Javed, H.U.; Ermakov, A.M.; Chikindas, M.L. Anti-Biofilm Potential of Elletaria cardamomum Essential Oil Against Escherichia coli O157:H7 and Salmonella Typhimurium JSG 1748. Front. Microbiol. 2021, 12, 620227. [Google Scholar] [CrossRef]
- Wongsariya, K.; Lapirattanakul, J.; Chewchinda, S.; Kanchanadumkerng, P. Anti-Oral Streptococci and Anti-Biofilm Properties of Etlingera pavieana Essential Oil and Its Bioactive Compounds Proposed for an Alternative Herbal Mouthwash. Heliyon 2024, 10, e31136. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).