1. Introduction
Antibiotic resistance is a global health crisis with far-reaching implications. The overuse and misuse of antibiotics have accelerated the emergence of resistant bacterial strains, making it increasingly difficult to treat infections that are easily managed [
1,
2]. This challenge is particularly acute in the case of pneumonia, where rapid and accurate detection of antibiotic resistance is crucial for effective treatment. The rise of multidrug-resistant pathogens has led to longer hospital stays, increased medical costs, and higher mortality rates, underscoring the urgent need for early and precise diagnostic methods to identify resistant strains and guide appropriate therapies [
3].
Pneumonia remains one of the leading causes of morbidity and mortality worldwide, affecting millions of people annually. In Jordan, the burden of pneumonia is significant, particularly among vulnerable populations, such as the elderly and those with underlying health conditions. Timely and effective treatment is essential for preventing complications and reducing mortality. However, the increasing prevalence of antibiotic-resistant bacteria complicates treatment efforts, making it challenging to achieve positive patient outcomes [
4].
Various diagnostic techniques have been employed to identify the causative pathogens of pneumonia, ranging from traditional microbiological culture to advanced molecular methods [
5,
6]. Although culture methods are considered the gold standard, they are time-consuming and can delay the initiation of appropriate therapy [
7]. In contrast, molecular techniques such as polymerase chain reaction (PCR) offer rapid and highly sensitive detection of pathogens and antibiotic-resistance genes, enabling quicker clinical decision-making. PCR has emerged as a powerful tool for the diagnosis of pneumonia, especially in cases where rapid identification of resistant strains is crucial [
8].
The growing threat of antibiotic resistance in pneumonia-causing pathogens presents a significant public health challenge. With the rise of multidrug-resistant strains, there is a pressing need for diagnostic methods that can quickly and accurately identify resistant pathogens [
9].
The primary objective of this study was to detect antibiotic resistance genes in sputum and bronchoalveolar lavage (BAL) samples obtained from patients with pneumonia using PCR. In addition, this study aimed to evaluate the prevalence of specific resistance genes and assess the clinical and demographic factors associated with antibiotic resistance in patients with pneumonia. These findings are expected to provide valuable insights into the prevalence of antibiotic resistance in Jordan and help refine diagnostic practices to enhance patient outcomes in the treatment of pneumonia.
This study aimed to address this need by developing and validating PCR-based diagnostic techniques that can be integrated into routine clinical practice. By focusing on the detection of antibiotic resistance genes in sputum and BAL samples from patients with pneumonia, this study seeks to improve the management of pneumonia in Jordan and contribute to the global effort to combat antibiotic resistance.
3. Results
The findings of this study, including demographic and clinical features, hospitalization characteristics, clinical outcomes, microbial pathogenesis, clinical features, risk factors, and pneumonia severity, are summarized in
Table 10. A comprehensive overview of these results is provided in
Table 11, which consolidates the key data points for clarity and ease of referencing.
3.1. Demographic and Clinical Features
In total, 114 patients with community-acquired pneumonia (CAP) were included in this study. The study had a median age of 73 years (IQR: 47–79 years) and a mean age of 64.114 years (±20.331), reflecting a predominantly elderly population. The youngest patient was 16 years old, whereas the oldest was 95 years old. Male patients constituted 55.263% (n = 63), whereas females accounted for 44.737% (n = 51). This gender distribution aligns with global data on pneumonia prevalence, where males are typically more affected due to higher risk factors, such as smoking and chronic lung diseases.
3.1.1. Length of Stay and Hospitalization Characteristics
The median length of hospital stay (LOS) was 7 days (IQR: 3–10), with a mean of 11.272 days (±15.091). Hospital stays ranged from a minimum of one day to a maximum of 83 days, reflecting significant variability in disease severity. Among patients requiring ICU admission, the median ICU length of stay was 1 d (IQR: 0–5), with a mean of 4.763 days (±11.882). The median number of hospitalizations was 2 (IQR: 1–3) with a mean of 2.772 (±3.005), and the median number of pneumonia episodes was 0.5 (IQR: 0–1) with a mean of 0.895 (±1.366).
3.1.2. Clinical Outcomes
Of the 114 patients, 23.684% (n = 27) succumbed to their illness, indicating a high mortality rate consistent with severe pneumonia in older populations. Conversely, 76.316% (n = 87) of patients showed clinical improvement and were successfully discharged. This mortality rate highlights the burden of CAP in vulnerable populations, particularly in the presence of comorbidities or antibiotic resistance.
3.1.3. Microbial Pathogenesis
Microbiological analysis has revealed diverse etiologies of CAP, with bacterial pathogens being the most frequently identified. Bacterial infections accounted for 28.947% (n = 33) of the cases, followed by bacterial-viral co-infections (24.561%, n = 28). Viral infections alone constituted 20.175% (n = 23) of the cases, and polymicrobial infections were common, including polybacterial (10.526%, n = 12) and polymicrobial viral infections (13.158%, n = 15). Fungal-bacterial co-infections were the least frequent, comprising 1.754% (n = 2). These findings emphasize the complexity of CAP pathogenesis, with a significant proportion of cases involving mixed infections, which may complicate the treatment strategies. The data were obtained from another study in which PCR tests were performed on these samples.
3.1.4. Clinical Features and Risk Factors
The most common clinical features in this study included shortness of breath (SOB), which was observed in 54.369% (n = 56) of the patients, and cough, which occurred in 13.158% (n = 15). Fever was noted in 13.592% (n = 14) of cases, while sputum production was recorded in 6.140% (n = 7). Less frequent symptoms included pleuritic chest pain (8.738%; n = 9). Hemoptysis or rust-colored sputum was absent in 103 patients (100%) due to 11 missing values, suggesting that none of the patients with available data exhibited these symptoms.
Regarding comorbidities, COPD was present in 3.509% (n = 4) of the patients, and asthma was documented in 5.263% (n = 6). A significant majority (91.228%, n = 104) of the patients had not received immunosuppressive treatment, indicating that most individuals in this study did not have this specific risk factor.
In terms of respiratory distress, 7.018% (n = 8) of the patients demonstrated signs of respiratory distress, while 84.211% (n = 96) did not exhibit these signs. The prevalence of aspiration pneumonia was 15.358% (n = 16), and 5.769% (n = 6) of the patients required intubation with mechanical ventilation, reflecting the severity of pneumonia in these cases.
Regarding prior antibiotic use, 91.228% (n = 104) of patients did not have any antibiotic exposure within 30 days preceding their current illness. This high proportion suggests that recent antibiotic use may not be a significant factor in the selection of antibiotic-resistant strains in this population.
3.1.5. Severity and Guideline Adherence
The CURB-65 score, used to assess pneumonia severity, showed that 24.561% (n = 28) of patients had a score of 0 and 57.895% (n = 66) had a score of 1, indicating low-risk CAP. A detailed summary of pneumonia severity is provided in
Table 11 for a comprehensive overview.
The PSI (pneumonia severity index), also known as the PORT score, was used to assess the severity of pneumonia and to predict patient outcomes. Based on PSI scores, patients were categorized into four risk classes, with each class corresponding to specific mortality rates and clinical management strategies.
A total of 59.649% (n = 68) of patients had a PSI score ≤50, indicating a low mortality risk (0.1%) and were typically managed with outpatient care. This class represented the largest proportion of the studies.
A total of 24.561% (n = 28) of the patients had PSI scores between 51 and 70, placing them at low to moderate risk, with a mortality rate of 0.6%. These patients may require either outpatient care or observation admission, depending on their clinical judgment.
A total of 8.772% (n = 10) of the patients had PSI scores between 71 and 90, indicating moderate risk with a mortality rate of 0.9%. Inpatient admission is generally recommended for these patients because of the potential for complications and the need for closer monitoring.
A total of 7.018% (n = 8) of patients had PSI scores > 130, classifying them as high-risk with a mortality rate of 9.3%. These patients typically require inpatient admission and intensive management because of their significantly elevated risk of adverse outcomes.
Guideline-concordant antibiotic use was recorded in only 35.106% (n = 33) of the cases, underscoring suboptimal adherence to treatment protocols. Completion of the prescribed antibiotic therapy was achieved in 63.158% (n = 72) of patients, while 36.842% (n = 42) either discontinued treatment prematurely or received incomplete regimens.
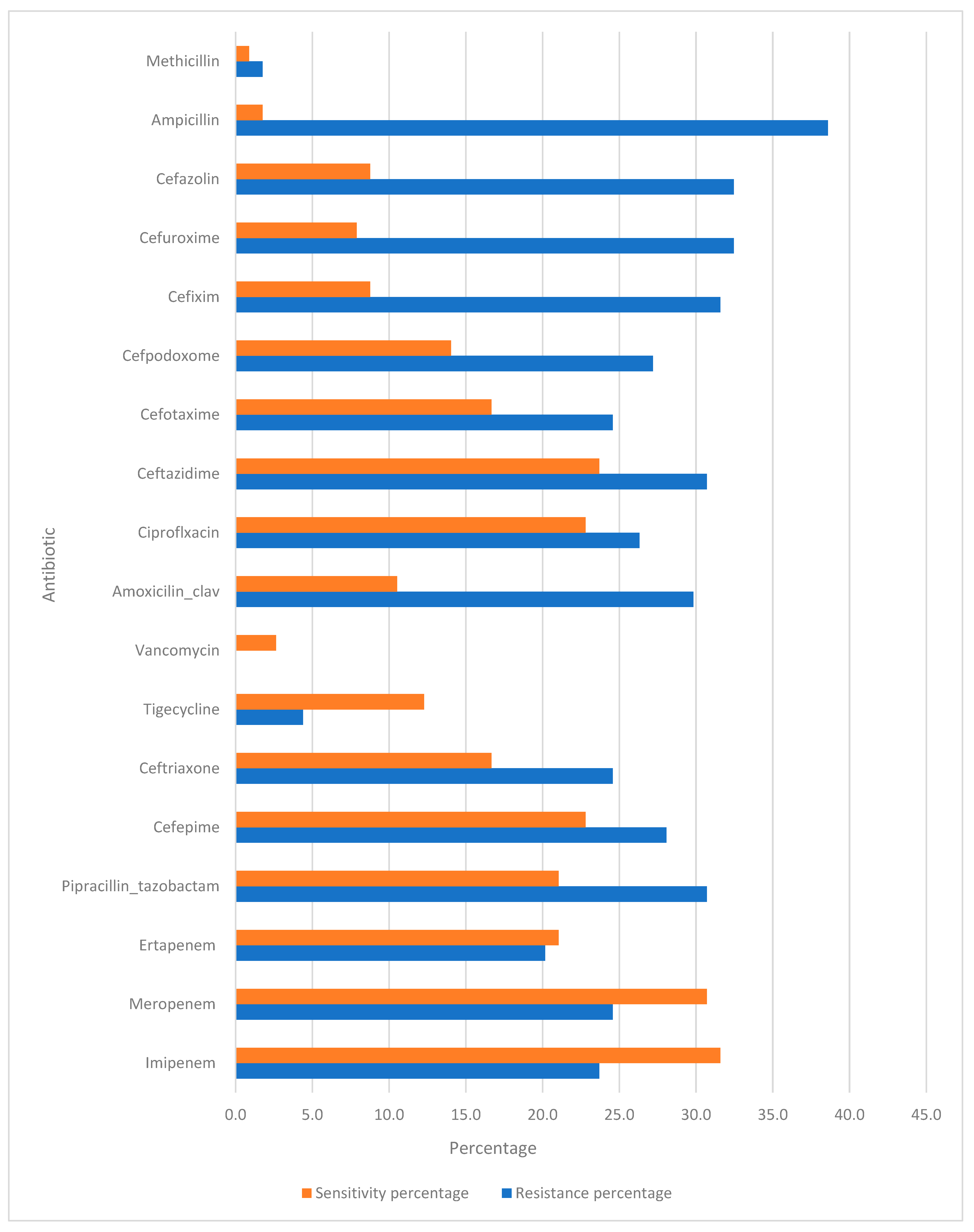
3.2. Antibiotic Sensitivity Test
The management of community-acquired pneumonia (CAP) is increasingly challenged by the rising incidence of antibiotic resistance. The data provided in
Figure 1 highlight the resistance and sensitivity patterns of various antibiotics used. Understanding these patterns is crucial for developing effective treatment strategies.
Imipenem and Meropenem: Both carbapenems, Imipenem and Meropenem, exhibit resistance rates of 23.7% and 24.6%, respectively. The corresponding sensitivity percentages were 31.6% and 30.7%.
Ertapenem: This carbapenem showed a slightly lower resistance rate (20.2%), with a sensitivity of 21.1%.
Piperacillin–Tazobactam: With a resistance rate of 30.7% and sensitivity of 21.1%, this combination antibiotic is frequently used for its synergistic effect against a wide range of pathogens.
Cefepime, Ceftriaxone, and Ceftazidime: These cephalosporins showed varying resistance rates (28.1%, 24.6%, and 30.7%, respectively) and sensitivities (22.8%, 16.7%, and 23.7%, respectively). Ceftriaxone and Ceftazidime are often used as first-line agents for CAP because of their efficacy against Streptococcus pneumoniae and Haemophilus influenzae. Cefepime is typically reserved for more severe cases or suspected Gram-negative infections owing to its extended spectrum.
Tigecycline: This antibiotic has a notably low resistance rate of 4.4% and sensitivity of 12.3%.
Amoxicillin–Clavulanate and Ciprofloxacin: Amoxicillin–Clavulanate showed a resistance rate of 29.8% and a sensitivity of 10.5%, whereas ciprofloxacin had a resistance rate of 26.3% and a sensitivity of 22.8%. Both antibiotics are commonly used for outpatient treatment of CAP.
Cefotaxime, Cefpodoxime, Cefixime, Cefuroxime, and Cefazolin: These cephalosporins exhibited significant resistance rates ranging from 24.6% to 32.5%, with sensitivities between 7.9% and 16.7%. Their use in CAP treatment is often guided by the susceptibility results.
Ampicillin and Methicillin: Ampicillin showed a high resistance rate of 38.6% with a minimal sensitivity of 1.8%, reflecting limited efficacy against common CAP pathogens. The exceptionally low resistance (1.8%) and sensitivity (0.9%) confirmed its limited role, which is primarily of historical interest.
3.3. Antibiotic Utilization Patterns
The antibiotic utilization patterns in this study reflect the use of empirical and subsequent therapies. The findings are summarized in
Table 12 which provides a detailed breakdown of empiric therapy, next-line therapy, and initial continuation therapy.
3.4. Empiric and Subsequent Antibiotic Use
3.4.1. Empiric Therapy
Empiric therapy was prescribed to most patients (n = 114), with meropenem being the most frequently used antibiotic, accounting for 31.60% (n = 36) of cases. Piperacillin–tazobactam was the second most common empiric antibiotic used in 26.30% (n = 30) of cases. Other antibiotics included amoxicillin–clavulanate (7.90%, n = 9), which was favored for moderate infections, and ceftriaxone (6.10%, n = 7), which is often prescribed for community-acquired infections. The less frequently used antibiotics included levofloxacin (3.50%, n = 4) for respiratory pathogens, azithromycin (4.40%, n = 5) for atypical coverage, and ampicillin (3.50%, n = 4) for Gram-positive infections. Vancomycin was prescribed in 23.70% (n = 27) of cases, reflecting its role in managing suspected MRSA infections. Targeted therapies such as doxycycline (0.90%, n = 1) and amikacin (1.80%, n = 2) were sparingly used for atypical and Gram-negative pathogens, respectively.
3.4.2. Next-Line Therapy
Next-line therapies were initiated in patients who did not respond to empirical treatment or required escalation due to multidrug-resistant organisms. Amoxicillin–clavulanate was the most commonly used next-line antibiotic, prescribed in 14.04% (n = 16) of cases to narrow down therapy when pathogens were susceptible. Azithromycin and colistin were each used in 9.64% (n = 11) of the cases, with colistin targeting multidrug-resistant Gram-negative pathogens and azithromycin providing coverage for respiratory and atypical pathogens.
3.4.3. Initial Continuation Therapy
Empiric antibiotics were continued as the initial therapy in some cases when clinical progress or culture results supported their use. Colistin was the most frequently continued antibiotic, used in 11.40% (n = 13) of the cases, primarily for resistant Gram-negative organisms. Doxycycline was continued in 7.02% (n = 8) of cases of atypical pathogens, whereas azithromycin was continued in 6.14% (n = 7) of patients with respiratory or atypical infections. Other antibiotics included amikacin (4.39%, n = 5), metronidazole (1.75%, n = 2) for anaerobic infections, levofloxacin (2.63%, n = 3), and amoxicillin–clavulanate (0.88%, n = 1). Ceftriaxone was continued in 0.88% (n = 1) of the cases when susceptibility testing confirmed its efficacy.
The hospital opted for carbapenems as empiric therapy for patients with CAP due to their broad-spectrum activity against a wide range of pathogens, including resistant strains. Carbapenems, particularly meropenem, are effective against Gram-negative bacteria that are commonly involved in severe CAP, such as those caused by multidrug-resistant organisms. Their strong effectiveness against organisms, such as Escherichia coli and Klebsiella pneumoniae, which frequently exhibit resistance to other antibiotic classes, makes them a reliable choice in critical settings.
Additionally, the rapid deterioration of patients with CAP and the potential for severe complications necessitate the use of powerful antibiotics that can cover a variety of possible pathogens during the initial treatment phase. The increasing prevalence of resistant bacteria calls for aggressive empirical therapy, and carbapenems serve to bridge this gap until susceptibility patterns can be confirmed through microbiological testing. This approach aims not only to ensure immediate treatment efficacy but also to reduce the risk of treatment failure and the associated morbidity and mortality in these patients.
3.5. Antibiotic Resistance Gene Detection
This study investigated the prevalence of antibiotic resistance genes in patients with pneumonia, focusing on several classes of antibiotics. The data revealed variable levels of resistance and sensitivity to different antibiotics, as well as significant variation in the detection of specific resistance genes. A detailed overview of all detected resistance genes and their associations is provided in
Table 13.
3.5.1. Imipenem Resistance
Oxa-40-like gene was detected in 7% of samples from patients resistant to imipenem, and 93% of samples did not exhibit this gene. The p-value for this association was 0.915, indicating no significant correlation between Oxa-40-like genes and imipenem resistance.
Oxa-48-like gene was found in 13.2% of imipenem-resistant patients, with 86.8% of samples undetected. A p-value of 0.200 suggests that there is no statistically significant relationship between the presence of this gene and imipenem resistance.
Oxa-51-like gene detection was higher, with 74% of the imipenem-resistant samples showing the gene and 64.9% undetected. The p-value for this gene was 0.062, which was statistically significant, but the association was not strong enough to conclude a definitive relationship.
Imp gene had a detection rate of 4.4% in imipenem-resistant samples, with a p-value of 0.209, indicating no significant association.
Kpc was detected in only 1.8% of resistant samples, with a p-value of 0.535, indicating that Kpc does not significantly contribute to imipenem resistance in the study population.
Ndm was present in 33.3% of imipenem-resistant samples, and the p-value of 0.601 suggests no significant impact on resistance patterns.
Vim gene was detected in 8.8% of resistant samples, with a p-value of 0.074, indicating a potential but non-significant association.
3.5.2. Meropenem Resistance
Similar to Imipenem, meropenem resistance showed no significant association with the tested genes (p-values for Oxa-40-like, Oxa-48-like, Imp, and Kpc ranged from 0.200 to 0.930). The Oxa-51-like gene exhibited a trend towards significance, with a p-value of 0.093, but the association was not strong enough to conclude a definitive relationship. Vim was found in 8.8% of meropenem-resistant samples, with 91.2% of samples undetected. The p-value of 0.08 shows a potential, but non-significant, association with meropenem resistance.
3.5.3. Ertapenem Resistance
The Oxa-51-like gene showed the only significant association with etapenem resistance, with a p-value of 0.046, indicating that it may contribute to resistance in a portion of the population. Other genes tested, including Oxa40-like, Oxa-48-like, and Imp, showed no significant association with ertapenem resistance, indicating that they may not play a major role in resistance in this population.
3.5.4. Methicillin Resistance
The Mec A gene was detected in 76.3% of samples resistant to methicillin, with a highly significant p-value of 0.000, indicating a strong association between Mec A and methicillin resistance.
3.5.5. Resistance to Other Antibiotics
Pipracillin-tazobactam resistance was linked to the Tem gene (70.2% of resistant samples), but no statistically significant association was found (p = 0.904).
Cefepime resistance also showed a strong association with Tem (70.2% resistance), but this was not statistically significant (p = 0.848). The Ctx-M-1 and shv genes were detected at varying frequencies in different antibiotic resistance profiles, although the p-values were consistently higher than 0.05, indicating no significant correlation.
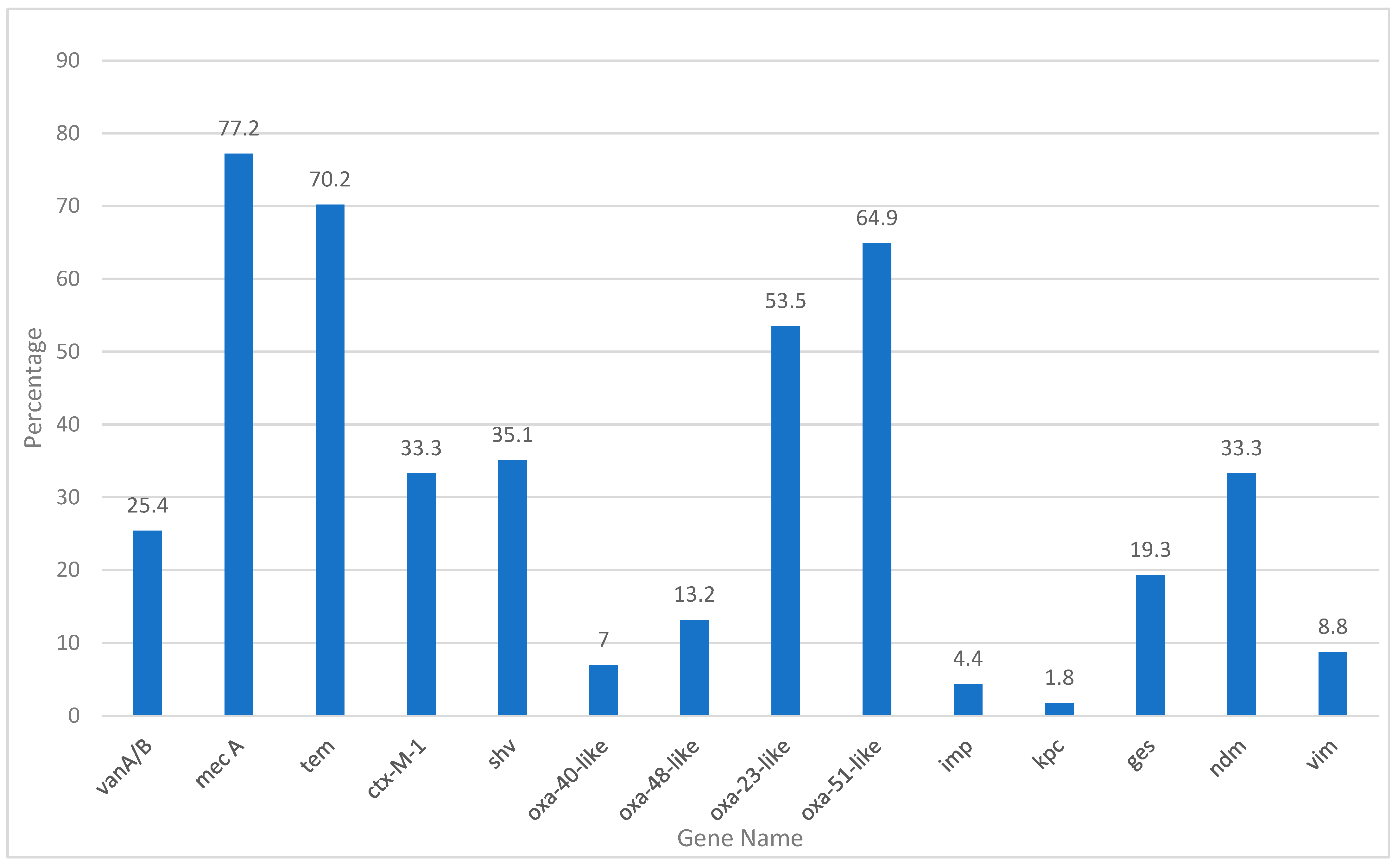
3.6. Antibiotic Resistance Gene Prevalence
In this study, the prevalence of 14 antibiotic resistance genes was evaluated in the tested samples. Mec A was the most commonly detected gene, identified in 87 samples, corresponding to 77.2% of the total samples analyzed (
Table 14 and
Figure 2). This gene, known to confer resistance to methicillin and other beta-lactam antibiotics, reflects the significant presence of methicillin-resistant
Staphylococcus aureus (MRSA) or other resistant strains. Similarly, the
Tem gene, which is associated with extended-spectrum beta-lactamases (ESBLs), was detected in 80 samples (70.2%), indicating beta-lactam resistance (
Table 14 and
Figure 2).
Other notable findings include the Oxa-51-like and Oxa-23-like genes detected in 71 (64.9%) and 61 (53.5%) samples, respectively. These genes are typically linked to carbapenem resistance in Acinetobacter baumannii and other Gram-negative pathogens. Their high prevalence signals a significant threat posed by carbapenem-resistant organisms in the tested population. Furthermore, the Ctx-M-1 gene, another key marker for ESBL production, was identified in 38 samples (33.3%), underscoring the continued prevalence of β-lactamase-producing organisms.
Genes associated with other forms of resistance, such as
shv and
Ndm, were also frequently detected. The
shv gene, which codes for beta-lactamases, was identified in 40 samples (35.1%), whereas the
Ndm gene, responsible for carbapenemase production, was present in 38 samples (33.3%) (
Table 14 and
Figure 2). These findings highlight the growing challenge of multidrug-resistant pathogens, particularly those that produce carbapenemases and ESBLs.
The detection of VanA/B (29 samples, 25.4%), ges (22 samples, 19.3%), and Oxa-48-like bacteria (15 samples, 13.2%) further emphasized the presence of resistance mechanisms in the population. The ges gene, which is associated with resistance to aminoglycosides, and Oxa-48-like, a carbapenemase gene, further highlight the diverse range of resistance mechanisms present in the tested samples.
Lower frequencies of detection were observed for
Oxa-40-like (7%),
Vim (8.8%), and
Imp (4.4%) genes.
Oxa-40-like and
Vim genes, both related to carbapenem resistance, were detected in a smaller proportion of samples; however, their presence indicates potential concerns in the resistance landscape. The
imp gene, found in only five samples (4.4%), also contributes to carbapenem resistance, although it appears to be less prevalent in this study. The
Kpc gene, which is associated with carbapenemase production, was the least commonly detected, appearing in only two samples (1.8%) (
Table 14 and
Figure 2).
The distribution of these resistance genes demonstrated a complex and multifaceted resistance profile within the population. The high prevalence of Mec A, Tem, and shv suggests a substantial burden of β-lactam and methicillin resistance. Meanwhile, the presence of carbapenemase genes, such as Oxa-51-like, Oxa-23-like, Ndm, and Kpc, emphasizes the growing threat of multidrug-resistant, Gram-negative bacteria. Additionally, the detection of VanA/B and ges highlights the ongoing issue of glycopeptide and aminoglycoside resistance.
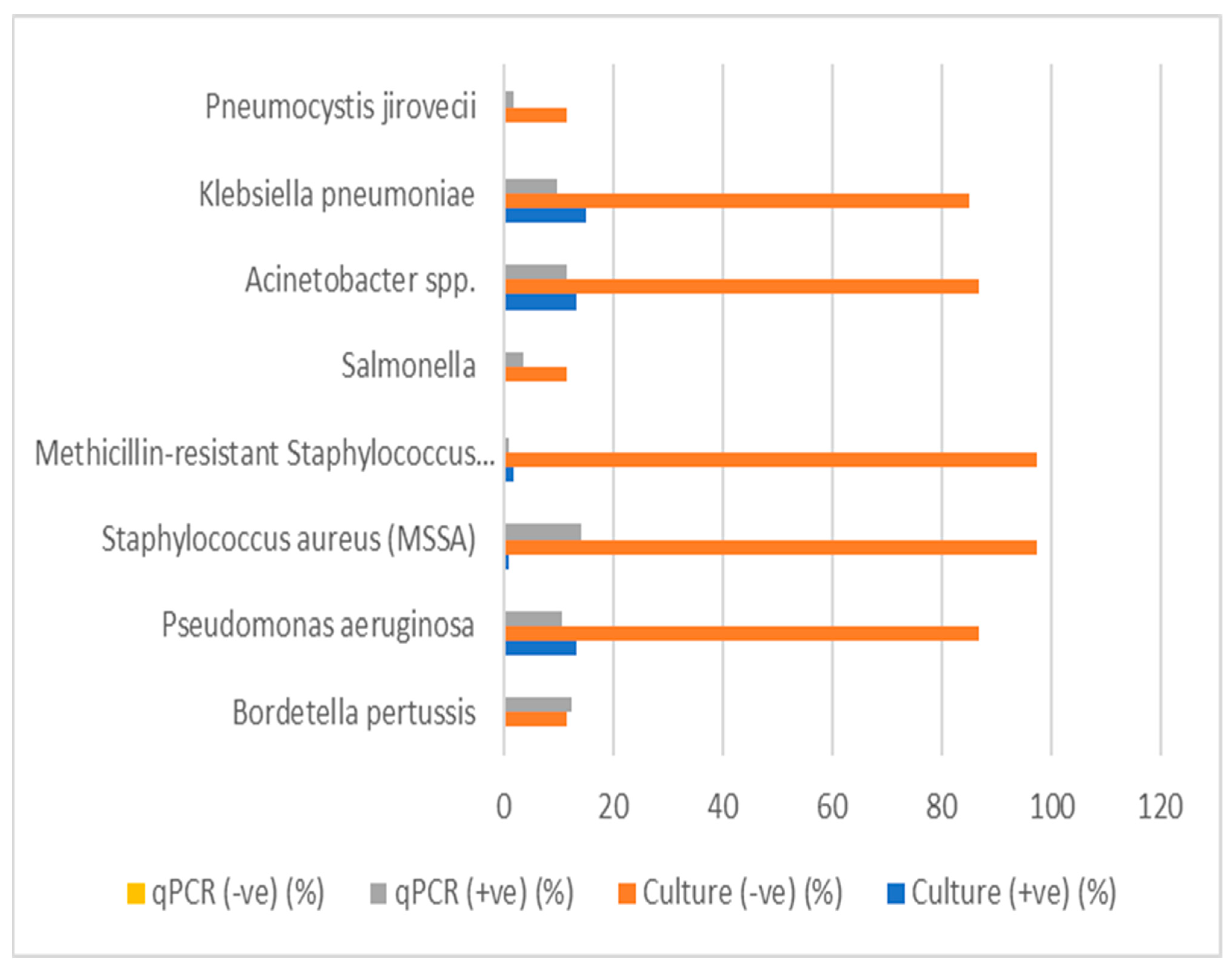
3.7. Comparison Between Culture and qPCR
A comparison of culture and qPCR detection of various respiratory pathogens was conducted to assess the diagnostic performance of both methods. The results for each pathogen are summarized in
Table 15 and
Figure 3. The table includes valid percentages for positive and negative culture results, qPCR results, and
p-values for statistical significance.
Bordetella pertussis: A low percentage (12.3%) of positive results were detected using qPCR, with no culture-positive samples. The p-value (0.152) indicated no statistically significant difference between the culture and qPCR results for this pathogen.
A number of pathogens, including Streptococcus pneumoniae, Mycoplasma pneumoniae, non-typeable Haemophilus influenzae, Moraxella catarrhalis, Chlamydia pneumoniae, and Legionella pneumophila, showed a 100% negative culture rate; however, no positive qPCR results were found for most pathogens. The p-values were marked as “a”, indicating that no statistical test was conducted or that these pathogens did not meet the testing criteria.
Pseudomonas aeruginosa: This pathogen showed 13.2% positive culture results and 10.5% positive qPCR results, with a
p-value of <0.001 (
Table 15), suggesting a highly significant difference between the culture and qPCR detection methods.
Methicillin-sensitive Staphylococcus aureus (MSSA) and MRSA: While MSSA and MRSA showed a low percentage of positive culture results (0.9% and 1.8%, respectively), both showed significantly higher qPCR positivity (14% for MSSA and 0.9% for MRSA). The p-values were 0.778 for MSSA and <0.001 for MRSA, indicating no significant difference for MSSA, but a significant difference for MRSA.
Salmonella, Acinetobacter spp., Klebsiella pneumoniae: Salmonella had a culture positivity of 0%; however, qPCR detected 3.5% positive samples, with a p-value of 0.465, suggesting no significant difference between the two methods. Acinetobacter spp. and Klebsiella pneumoniae showed similar findings, with culture positivity percentages of 13.2% and 14.9%, respectively, and qPCR positivity of 11.4% and 9.6%, respectively. Both had p-values of <0.001, indicating no significant differences.
Legionella longbeachae: This pathogen showed no percentage of positive culture (0%) and no qPCR positivity (0%), with all results marked as “not available” for qPCR.
Pneumocystis jirovecii: This pathogen showed only qPCR positivity of 1.8%.
4. Discussion
This study used PCR to detect antibiotic resistance genes in pneumonia pathogens, providing rapid and precise identification of genetic resistance mechanisms. These findings shed light on the genetic basis of antibiotic resistance in patients with pneumonia, highlighting current management challenges. Empirical antibiotic treatment poses significant challenges, particularly in low- and middle-income countries with widespread multidrug-resistant bacteria [
14]. Rapid molecular PCR-based diagnostics are urgently needed to guide targeted antibiotic therapy and mitigate delayed treatment, mortality, and antimicrobial resistance [
15,
16].
This study used a multiplex PCR assay for the rapid detection of 14 antibiotic resistance genes directly from respiratory samples to facilitate timely diagnosis and targeted therapy. Our study identified the
Mec A gene in 77.2% of the samples, making it the most prevalent resistance gene detected. This finding corroborates the global evidence underscoring
Mec A’s critical role in methicillin resistance, particularly among
Staphylococcus aureus isolates. The strong association between
Mec A and methicillin resistance (
p < 0.001) emphasizes its clinical significance in managing
S. aureus-related infections, including pneumonia [
17].
Carbapenem resistance genes (
Oxa-48-like,
Ndm,
Kpc) exhibited varying prevalence rates, with
Oxa-48-like detected in 13.1% of samples. Although statistical significance was not achieved (
p > 0.05), these genes highlight the emerging threat of carbapenemase-producing pathogens in multidrug-resistant pneumonia [
18].
ESBL genes, specifically
Ctx-M-1 and
Shv, have confirmed the prevalence of beta-lactam resistance. Notably,
Ctx-M-1 (33.3%) contributes significantly to global cephalosporin resistance, emphasizing the importance of considering ESBL-producing bacteria in empirical pneumonia treatment [
19]
The
Oxa-51-like gene demonstrated a borderline significant association with ertapenem resistance (
p = 0.046), suggesting its potential role in conferring resistance. Typically intrinsic to
Acinetobacter baumannii [
18], this finding underscores the need for targeted surveillance of
A. baumannii infections, particularly in mechanically ventilated patients.
The hospital selected antibiotics, such as meropenem and piperacillin–tazobactam, primarily for their broad-spectrum efficacy against various pathogens, particularly those causing severe infections. Meropenem has emerged as the most frequently used empirical antibiotic because of its effectiveness against resistant Gram-negative bacteria. In contrast, piperacillin–tazobactam was chosen because of its synergistic effects and ability to cover both Gram-positive and Gram-negative organisms.
However, the widespread use of these antibiotics poses the risk of developing resistance. Notably, the prevalence of resistance genes, such as
Oxa-51-like and
Oxa-23-like, signifies a considerable threat to carbapenem-resistant organisms. Resistance rates for carbapenems were concerning, with 23.7% for imipenem and 24.6% for meropenem; similar resistance was observed with piperacillin–tazobactam at 30.7% [
20].
Discrepancies arise when sensitivity tests are compared with antibiotic utilization. For example, although the sensitivity to meropenem is 30.7%, it is used in 31.6% of cases, indicating that prescriptions often occur despite a significant portion of pathogens exhibiting resistance. Similarly, although piperacillin–tazobactam has a low sensitivity (21.1%), it is frequently used. This gap stresses the importance of aligning empirical treatment decisions with actual resistance patterns and highlights the necessity for ongoing surveillance and antibiotic stewardship programs to effectively combat resistance development. Given the rising failure rates of conventional antibiotics, there is an urgent need to explore alternative therapeutic strategies. Natural products, with their diverse bioactive compounds, and bacteriophage therapy, which utilizes viruses that specifically target bacteria, represent promising options to combat resistant pathogens [
21,
22].
The results of this study highlight the significant diagnostic advantages of qPCR over traditional culture-based methods for detecting various respiratory pathogens. For example, qPCR was identified in 12.3% of cases, while no positive results were obtained through culture, although the
p-value (0.152) was not statistically significant [
23].
Pseudomonas aeruginosa displayed a significant difference between culture (13.2%) and qPCR (10.5%), with a highly significant
p-value (<0.001). This emphasizes the ability of qPCR to complement or even surpass culture methods for detecting certain pathogens [
24]. Similarly, both MSSA and MRSA showed low culture positivity (0.9% and 1.8%, respectively), but much higher qPCR detection rates (14% for MSSA and 0.9% for MRSA). The
p-value for MSSA (0.778) indicated no significant difference, while MRSA (
p < 0.001) highlighted qPCR’s superiority of qPCR in detecting resistant strains. Other pathogens, such as
Salmonella,
Acinetobacter spp., and
Klebsiella pneumoniae, presented with low culture positivity but comparable qPCR detection, with
p-values suggesting no significant difference for
Salmonella (0.465) but highly significant differences for
Acinetobacter spp. and
Klebsiella pneumoniae (both <0.001). The results of
Pneumocystis jirovecii indicated the importance of including this fungus in clinical practice [
25,
26]. Overall, the findings of this study strongly support the integration of qPCR into routine clinical diagnostics [
27].
A comparison of culture and PCR methods for detecting bacteria revealed that, in some cases, culture proved more effective than PCR, which is typically expected to be more sensitive. However, errors in performing PCR, such as sample handling, DNA extraction, personnel errors, and contamination, can lead to false-negative results. For example, Pseudomonas aeruginosa showed 13.2% culture positivity compared to 10.5% using qPCR. Similarly, for Staphylococcus aureus (MRSA), culture positivity was 0.9%, whereas qPCR positivity was notably higher at 14%. In the case of Acinetobacter spp., 13.2% were culture-positive and 11.4% were qPCR-positive, while Klebsiella pneumoniae showed 14.9% culture positivity and 9.6% qPCR positivity.
PCR offers several advantages over traditional culture-based methods, making it a superior diagnostic tool for many clinical settings. One of the key benefits of PCR is its high sensitivity, which allows the detection of low levels of pathogens that may go undetected with culture methods. Additionally, PCR has high specificity, enabling the precise identification of particular DNA sequences from complex mixtures [
8,
16,
28]. This method is also extremely fast compared with traditional techniques, providing rapid results that can significantly expedite clinical decision-making and patient management [
29]. Another advantage is that PCR can be performed easily and can use various fluid samples, including blood, without being affected by prior antimicrobial therapy. This minimizes the risk of the emergence of multidrug-resistant pathogens and reduces the need for unnecessarily administered drugs [
29]. Furthermore, PCR minimizes the risk of cross-contamination and allows for the simultaneous identification of multiple microorganisms and resistance genes, thus enhancing diagnostic throughput and accuracy. However, it is important to note that PCR also has some limitations, such as higher costs compared to traditional methods, inability to assess susceptibility to all antibiotics, and challenges in diagnosing infections due to non-viable organism detection [
16,
25]. Despite these drawbacks, the significant advantages of PCR make it an invaluable tool in modern diagnostics, especially in the fight against antibiotic resistance.
Despite the valuable insights provided by this study, several limitations should be considered when interpreting the results. First, the sample size, although adequate for a preliminary analysis, may not fully represent the broader population of patients with pneumonia. A larger cohort across multiple centers is needed to confirm the generalizability of these findings, particularly given the variation in resistance patterns that may exist across different geographical regions.
Second, the study focused primarily on a targeted set of resistance genes, which may not capture the full spectrum of resistance mechanisms present in pneumonia. It is possible that other uncharacterized genes or mechanisms, such as efflux pump systems or alterations in antibiotic targets, contribute to resistance but were not included in the analysis. Additionally, this study did not assess the phenotypic resistance of the pathogens, which would have provided complementary data on the clinical significance of the detected genes.
Furthermore, although PCR is a powerful tool for identifying resistance genes, it does not provide information about the functional expression of these genes in bacterial populations. In some cases, resistance genes may be present but do not actively contribute to the resistance phenotype. Future studies using whole-genome sequencing or transcriptomic approaches could provide a more comprehensive understanding of the genetic and phenotypic relationships between resistance genes and phenotypes.
Another limitation is the use of a single diagnostic kit (Bacresista GLA kit), which may not have detected all possible resistance genes, particularly those that are rare or specific to certain pathogens. Future work could involve using broader or more customized panels for PCR detection to ensure the inclusion of a wider variety of resistance determinants.