Prevalence and Associated Mortality of Infections by Multidrug-Resistant Organisms in Pediatric Intensive Care Units in Argentina (PREV-AR-P)
Abstract
1. Introduction
2. Results
2.1. Characteristics of Participating Centers
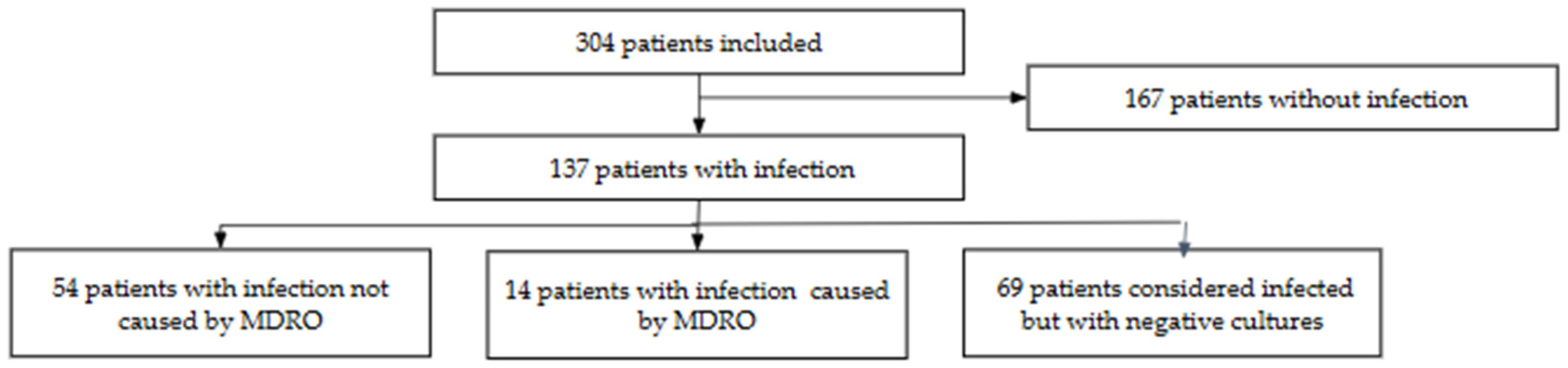
2.2. Characteristics of the Entire Population
2.3. Prevalence and Characteristics of Patients with MDRO Infections
2.4. Prevalence of CPE Colonization
2.5. Sites and Microorganisms Isolated
2.6. ICU Outcomes
3. Discussion
4. Patients and Methods
4.1. Outcomes
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Antimicrobial Resistance. U.S. Actions & Events to Combat. 2024. Available online: https://www.cdc.gov/antimicrobial-resistance/programs/ar-actions-events.html?CDC_AAref_Val=https://www.cdc.gov/drugresistance/us-activities.html (accessed on 4 February 2025).
- World Health Organization (WHO). Yearbook of the United Nations 2005; UN: New York, NY, USA, 2005; pp. 1572–1573.
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Collaborators. Correction to The burden of antimicrobial resistance in the Americas in 2019: A cross-country systematic analysis. Lancet Reg. Health Am. 2023, 28, 100632. [Google Scholar]
- Vanegas, J.M.; Parra, O.L.; Jiménez, J.N. Molecular epidemiology of carbapenem resistant gram-negative bacilli from infected pediatric population in tertiary–care hospitals in Medellín, Colombia: An increasing problem. BMC Infect. Dis. 2016, 16, 463. [Google Scholar] [CrossRef]
- Chiotos, K.; Tamma, P.D.; Flett, K.B.; Naumann, M.; Karandikar, M.V.; Bilker, W.B.; Zaoutis, T.; Han, J. Multicenter Study of the Risk Factors for Colonization or Infection with Carbapenem-Resistant Enterobacteriaceae in Children. Antimicrob. Agents Chemother. 2017, 61, e01440-17. [Google Scholar] [CrossRef] [PubMed]
- Ara-Montojo, M.F.; Escosa-García, L.; Alguacil-Guillén, M.; Seara, N.; Zozaya, C.; Plaza, D.; Schuffelmann-Gutiérrez, C.; de la Vega, A.; Fernández-Cambor, A.; Ramos-Boluda, E.; et al. Predictors of mortality and clinical characteristics among carbapenem-resistant or carbapenemase-producing Enterobacteriaceae bloodstream infections in Spanish children. J. Antimicrob. Chemother. 2021, 76, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Yang, J.; Niu, M.; Zhu, H.; Zhou, Y.; Lu, J. Risk factors and clinical outcomes of carbapenem-resistant Klebsiella pneumoniae bacteraemia in children: A retrospective study. Int. J. Antimicrob. Agents 2023, 62, 106933. [Google Scholar] [CrossRef]
- López-Cubillos, J.F.; Díaz, A.; Cárdenas, V.C.; Camacho-Moreno, G.; Cantor, E.; Arcila, E.M.; Hurtado, I.C.; Correa, A.M.; Tierradentro, T.; Ramírez, O.; et al. Carbapenem resistance in Enterobacterales bloodstream infections among children with cancer or post-haematopoietic stem cell transplant: A retrospective cohort study. J. Antimicrob. Chemother. 2023, 78, 2462–2470. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lu, C.Y.; Yen, T.Y.; Chang, L.Y.; Chen, J.M.; Lee, P.I.; Huang, L.M. Clinical characteristics and outcomes of carbapenem-resistant Enterobacterales bacteremia in pediatric patients. J. Microbiol. Immunol. Infect. 2023, 56, 84–92. [Google Scholar] [CrossRef]
- Almasian Tehrani, N.; Azimi, L.; Armin, S.; Soleimani, N.; Fallah, F.; Karimi, A.; Shamsian, B.; Nazari, S.; Alebouyeh, M. Endogenous Bacteremia Caused by Intestinal Colonization of Carbapenem-Resistant Enterobacteriaceae (CRE) in Immunocompromised Children. Trop. Med. Infect. Dis. 2023, 8, 402. [Google Scholar] [CrossRef]
- Ruvinsky, S.; Voto, C.; Roel, M.; Portillo, V.; Naranjo Zuñiga, G.; Ulloa-Gutierrez, R.; Comandé, D.; Ciapponi, A.; Aboud, G.; Brizuela, M.; et al. Carbapenem-resistant Enterobacteriaceae bacteremia in pediatric patients in Latin America and the Caribbean: A systematic review and meta-analysis. Antibiotics 2024, 13, 1117. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Gossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [PubMed]
- Fink, G.; D’Acremont, V.; Leslie, H.H.; Cohen, J. Antibiotic exposure among children younger than 5 years in low-income and middle-income countries: A cross-sectional study of nationally representative facility-based and household-based surveys. Lancet Infect. Dis. 2020, 20, 179–187. [Google Scholar] [CrossRef]
- Cornistein, W.; Balasini, C.; Nuccetelli, Y.; Rodriguez, V.M.; Cudmani, N.; Roca, M.V.; Sadino, G.; Brizuela, M.; Fernández, A.; González, S.; et al. Prevalence and mortality associated with multidrug-resistant infections in adult intensive care units in Argentina (PREV-AR). Antimicrob. Agents Chemother. 2025, 69, e0142624. [Google Scholar] [CrossRef] [PubMed]
- Saleem, Z.; Godman, B.; Hassali, M.A.; Hashmi, F.K.; Azhar, F.; Rehman, I.U. Point prevalence surveys of health-care-associated infections: A systematic review. Pathog. Glob. Health 2019, 113, 191–205. [Google Scholar] [CrossRef]
- Magill, S.S.; Edwards, J.R.; Bamberg, W. Multistate point-prevalence survey of healthcare-associated infections. N. Engl. J. Med. 2014, 386, 1198–1208. [Google Scholar] [CrossRef]
- Kepenekli, E.; Soysal, A.; Yalindag-Ozturk, N.; Ozgur, O.; Ozcan, I.; Devrim, I.; Akar, S.; Bakir, M.; Turkish PICU-HCAI Study Group. Healthcare-associated infections in pediatric intensive care units in Turkey: A national point-prevalence survey. Jpn. J. Infect. Dis. 2015, 68, 381–386. [Google Scholar] [CrossRef]
- Le, N.K.; Hf, W.; Vu, P.D.; Khu, D.T.K.; Le, H.T.; Hoang, B.T.N.; Than Vo, V.; Minh Lam, Y.; Tien Viet Vu, D.; Hoai Nguyen, T.; et al. High prevalence of hospital-acquired infections caused by gram-negative carbapenem resistant strains in Vietnamese pediatric ICUs: A multi-centre point prevalence survey. Medicine 2016, 95, e4099. [Google Scholar] [CrossRef]
- Zingg, W.; Hopkins, S.; Gayet-Ageron, A.; Holmes, A.; Sharland, M.; Suetens, C.; ECDC PPS Study Group. Health-care-associated infections in neonates, children, and adolescents: An analysis of paediatric data from the European Centre for Disease Prevention and Control point-prevalence survey. Lancet Infect. Dis. 2017, 17, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Alemu, A.Y.; Endalamaw, A.; Belay, D.M.; Mekonen, D.K.; Birhan, B.M.; Bayih, W.A. Healthcare-associated infection and its determinants in Ethiopia: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241073. [Google Scholar] [CrossRef] [PubMed]
- Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals—2022–2023. 6 May 2024. Available online: https://www.ecdc.europa.eu/en/publications-data/PPS-HAI-AMR-acute-care-europe-2022-2023 (accessed on 19 January 2025).
- Martins, C.; Lima, D.; Cortez Ferreira, M.; Verdelho Andrade, J.; Dias, A. Healthcare-associated infections in pediatric patients: A decade of experience in an intensive care unit. Acta Med. Port. 2025, 38, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Folgori, L.; Bernaschi, P.; Piga, S.; Carletti, M.; Cunha, F.P.; Lara, P.H.R.; Cafieiro de Castro Peixoto, N.; Gomes Alvez Guimaraes, B.; Sharland, M.; Araujo da Silva, A.R.; et al. Healthcare-associated infections in pediatric and neonatal intensive care units: Impact of underlying risk factors and antimicrobial resistance on 30-day case-fatality in Italy and Brazil. Infect. Control Hosp. Epidemiol. 2016, 37, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- El-Nawawy, A.; Ashraf, G.A.; Antonios, M.A.M.; Meheissen, M.A.; El-Alfy, M.M.R. Incidence of multidrug-resistant organisms among children admitted to pediatric intensive care units in a developing country. Microb. Drug Resist. 2018, 24, 1198–1206. [Google Scholar] [CrossRef]
- de Souza, D.C.; Gonçalves Martin, J.; Soares Lanziotti, V.; de Oliveira, C.F.; Tonial, C.; de Carvalho, W.B.; Fioretto, J.R.; Piva, J.P.; Troster, E.J.; Siqueira Bossa, A.; et al. The epidemiology of sepsis in paediatric intensive care units in Brazil (the Sepsis PREvalence Assessment Database in Pediatric population, SPREAD PED): An observational study. Lancet Child. Adolesc. Health 2021, 5, 873–881. [Google Scholar] [CrossRef]
- Fresán-Ruiz, E.; Pons-Tomás, G.; de Carlos-Vicente, J.C.; Bustinza-Arriortua, A.; Slocker-Barrio, M.; Belda-Hofheinz, S.; Nieto-Moro, M.; Uriona-Tuma, S.N.; Pinós-Tella, L.; Morteruel-Arizcuren, E.; et al. Device exposure and patient risk factors’ impact on the healthcare-associated infection rates in PICUs. Children 2022, 9, 1669. [Google Scholar] [CrossRef]
- Aguilera-Alonso, D.; Escosa-García, L.; Epalza, C.; Bravo-Queipo-de-Llano, B.; Camil Olteanu, F.; Cendejas-Bueno, E.; Orellana, M.A.; Cercenado, E.; Saavedra-Lozano, J. Antibiotic resistance in bloodstream isolates from high-complexity paediatric units in Madrid, Spain: 2013–2021. J. Hosp. Infect. 2023, 139, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Xu, H.; Jing, C.; Deng, J.; Wang, H.; Hua, C.; Chen, Y.; Zhang, T.; Zhang, H.; Chen, Y.; et al. Bacterial epidemiology and antimicrobial resistance profiles in children reported by the ISPED program in China, 2016 to 2020. Microbiol. Spectr. 2021, 9, e0028321. [Google Scholar] [CrossRef]
- Saeedi, F.A.; Hegazi, M.A.; Alsaedi, H.; Alganmi, A.H.; Mokhtar, J.A.; Metwalli, E.M.; Hamadallah, H.; Siam, G.S.; Alaqla, A.; Alsharabi, A.; et al. Multidrug-resistant bacterial infections in pediatric patients hospitalized at King Abdulaziz University Hospital, Jeddah, western Saudi Arabia. Children 2024, 11, 444. [Google Scholar] [CrossRef]
- González-Anleo, C.; Girona-Alarcón, M.; Casaldàliga, A.; Bobillo-Perez, S.; Fresán, E.; Solé-Ribalta, A.; Velasco-Arnaiz, E.; Monsonís, M.; Urrea, M.; Jordan, I. Risk factors for multidrug-resistant bacteria in critically ill children and MDR score development. Eur. J. Pediatr. 2024, 183, 5255–5265. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, Z. What we can do? The risk factors for multi-drug-resistant infection in pediatric intensive care unit (PICU): A case-control study. Ital. J. Pediatr. 2020, 46, 17. [Google Scholar] [CrossRef]
- Macesic, N.; Uhlemann, A.C.; Peleg, A.Y. Multidrug-resistant Gram-negative bacterial infections. Lancet 2025, 405, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Tfifha, M.; Ferjani, A.; Mallouli, M.; Mlika, N.; Abroug, S.; Boukadida, J. Carriage of multidrug-resistant bacteria among pediatric patients before and during their hospitalization in a tertiary pediatric unit in Tunisia. Libyan J. Med. 2018, 13, 1419047. [Google Scholar] [CrossRef]
- Najem, S.; Eick, D.; Boettcher, J.; Aigner, A.; Aboutara, M.; Fenner, I.; Reinshagen, K.; Koenigs, I. High prevalence of multidrug-resistant Gram-negative bacteria carriage in children screened prospectively for multidrug resistant organisms at admission to a paediatric hospital, Hamburg, Germany, September 2018 to May 2019. Euro Surveill 2022, 27, 2001567. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Nguyen, D.C.; Scaggs Huang, F.A.; Qureshi, N.K.; Charnot-Katsikas, A.; Bartlett, A.H.; Zheng, X.; Hujer, A.M.; Domitrovic, T.N.; Marshall, S.H.; et al. A Multi-Centered Case-Case-Control Study of Factors Associated With Klebsiella pneumoniae Carbapenemase-Producing Enterobacteriaceae Infections in Children and Young Adults. Pediatr. Infect. Dis. J. 2019, 38, 490–495. [Google Scholar] [CrossRef]
- Avcu, G.; Erci, E.; Bilen, N.M.; Ersayoglu, I.; Ozek, G.; Celtik, U.; Terek, D.; Cilli, F.; Bal, Z.S. Clinical outcomes and the impact of treatment modalities in children with carbapenem-resistant Enterobacteriaceae bloodstream infections: A retrospective cohort study from a tertiary university hospital. J. Antimicrob. Chemother. 2025, 80, 147–153. [Google Scholar] [CrossRef]
- Slocker-Barrio, M.; López-Herce-Cid, J.; Bustinza-Arriortúa, A.; Fresán-Ruiz, E.; Jordán-García, I.; de Carlos-Vicente, J.C.; Morteruel-Arizcuren, E.; García-Soler, P.; Nieto-Moro, M.; Schüffelmann, C.; et al. Increase in incidence rates and risk factors for multidrug resistant bacteria in septic children: A nationwide Spanish cohort study (2013–2019). Antibiotics 2023, 12, 1626. [Google Scholar] [CrossRef]
- Liu, R.; Yu, Z.; Xiao, C.; Xu, F.; Xiao, S.; He, J.; Shi, Y.; Hua, Y.; Zhou, J.; Zhang, G.; et al. Epidemiology and clinical characteristics of pediatric sepsis in PICUs in Southwest China: A prospective multicenter study. Pediatr. Crit. Care Med. 2024, 25, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Aldarhami, A.; Bokhary, N.A.; Bazaid, M.B.; Qusty, M.F.; AlGhamdi, T.H.; Almarashi, A.A. Prevalence and risk factors associated with drug resistant bacteria in neonatal and pediatric intensive care units: A retrospective study in Saudi Arabia. Medicine 2023, 102, e35638. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, S.J.; Jhang, W.K. Comparison of the clinical characteristics and clinical outcomes of culture-positive septic shock and culture-negative septic shock among pediatric patients. PLoS ONE 2023, 18, e0288615. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Chakraborty, M.; Sardar, S.; De, R.; Biswas, M.; Mascellino, M.T.; Miele, M.C.; Biswas, S.; Nandi Mitra, A. Current trends in antimicrobial resistance patterns in bacterial pathogens among adult and pediatric patients in the intensive care unit in a tertiary care hospital in Kolkata, India. Antibiotics 2023, 12, 459. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.M.N.D.; Buonora, S.N.; Souza, C.L.P.; Simões Júnior, R.; Silva, T.C.D.; Bom, G.J.T.; da Silva Teixeira, C.H.; Araujo da Silva, A.R. Surveillance of multidrug-resistant bacteria in pediatric and neonatal intensive care units in Rio de Janeiro State, Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190205. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Gracida-Osorno, C.; Luna-Chi, I.G.; Jiménez-Guillermo, J.G.; Molina-Salinas, G.M. High prevalence of antimicrobial resistance among gram-negative isolated bacilli in intensive care units at a tertiary-care hospital in Yucatán Mexico. Medicina 2019, 55, 588. [Google Scholar] [CrossRef]
- Chu, V.T.; Tsitsiklis, A.; Mick, E.; Ambroggio, L.; Kalantar, K.L.; Glascock, A.; Osborne, C.M.; Wagner, B.D.; Matthay, M.A.; DeRisi, J.L.; et al. The antibiotic resistance reservoir of the lung microbiome expands with age in a population of critically ill patients. Nat. Commun. 2024, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Waterlow, N.R.; Cooper, B.S.; Robotham, J.V.; Knight, G.M. Antimicrobial resistance prevalence in bloodstream infection in 29 European countries by age and sex: An observational study. PLoS Med. 2024, 21, e1004301. [Google Scholar] [CrossRef] [PubMed]
- Adam, H.J.; Baxter, M.R.; Davidson, R.J.; Rubinstein, E.; Fanella, S.; Karlowsky, J.A.; Lagacé-Wiens, P.R.S.; Hoban, D.J.; Zhanel, G.G.; Canadian Antimicrobial Resistance Alliance (CARA). Comparison of pathogens and their antimicrobial resistance patterns in paediatric, adult and elderly patients in Canadian hospitals. J. Antimicrob. Chemother. 2013, 68, i31–i37. [Google Scholar] [CrossRef]
- Balamuth, F.; Scott, H.F.; Weiss, S.L.; Webb, M.; Chamberlain, J.M.; Bajaj, L.; Depinet, H.; Grundmeier, R.; Campos, D.; Deakyne Davies, S.; et al. Validation of the pediatric Sequential Organ Failure Assessment score and evaluation of third international consensus definitions for sepsis and septic shock definitions in the pediatric emergency department. JAMA Pediatr. 2022, 176, 672–678. [Google Scholar] [CrossRef]
- Goldstein, B.; Giroir, B.; Randolph, A.; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr. Crit. Care Med. 2005, 6, 2–8. [Google Scholar] [CrossRef]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schalapbach, L.; Tasker, R.; Argent, A.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46, 10–67. [Google Scholar] [CrossRef]
- Calandra, T.; Cohen, J.; International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit. Care Med. 2005, 33, 1538–1548. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.; Akova, M.; Harbath, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef] [PubMed]
- AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use. 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 26 December 2024).

| Characteristics | Centers (n = 50) |
|---|---|
| Hospital category | |
| Public | 37 (73.6%) |
| Private * | 13 (26.4%) |
| Type of hospital | |
| General (admission of adult and pediatric patients) | 33 (66%) |
| Specialized (admission of pediatric patients only) | 17 (34%) |
| Number of hospital beds | 153 [90–130] |
| Number of ICU beds | 8 [6–15] |
| Number of patients included per hospital | 3 [2–8] |
| Other resources | |
| Hospital-acquired infection control committee | 48 (96.3%) |
| Antimicrobial stewardship program | 24 (47.2%) |
| Frequency of CPE colonization screening | |
| Weekly | 40 (79%) |
| At ICU admission | 30 (60%) |
| Prior to surgery | 12 (23%) |
| Methods used to detect mechanisms of bacterial resistance | |
| Phenotypic | 45 (89%) |
| Molecular | 20 (40%) |
| Immunochromatographic | 34 (68%) |
| Variable | All Patients n = 304 | Patients Without Infection n = 167 | Patients with Infection n = 137 | p Value |
|---|---|---|---|---|
| Age (months) | 27 [5–88] | 25 [5–85] | 34 [6–88] | 0.44 |
| Gender (male) | 168 (55.2) | 90 (53.9) | 78 (56.9) | 0.60 |
| Comorbidity | 99 (32.5) | 45 (26.9) | 54 (39.4) | 0.021 |
| Respiratory disease | 62 (20.4) | 34 (20.3) | 28 (20.4) | 0.73 |
| Prematurity | 52 (17.1) | 33 (19.8) | 19 (13.9) | 0.04 |
| Bronchopulmonary dysplasia | 39 (12.8) | 29 (17.4) | 10 (7.3) | 0.009 |
| Congenital heart disease | 32 (10.5) | 23 (13.8) | 9 (6.6) | 0.04 |
| Immunosuppression | 18 (5.9) | 10 (6.0) | 8 (5.8) | 1.00 |
| Oncologic/Hematologic disease | 18 (5.9) | 8 (4.8) | 10 (7.3) | 0.36 |
| Chemotherapy in the previous 6 months | 10 (3.3) | 3 (1.8) | 7 (5.1) | 0.12 |
| Obesity | 14 (4.6) | 7 (4.2) | 7 (5.1) | 0.71 |
| Chronic liver disease | 6 (1.9) | 3 (1.8) | 3 (2.2) | 1.00 |
| Chronic renal disease | 4 (1.3) | 3 (1.8) | 1 (0.7) | 0.63 |
| Solid organ transplantation | 3 (1.0) | 2 (1.2) | 1 (0.7) | 1.00 |
| Cystic fibrosis | 3 (1.0) | 1 (0.6) | 2 (1.5) | 0.59 |
| Diabetes | 2 (0.7) | 1 (0.6) | 1 (0.7) | 1.00 |
| Bone marrow transplantation | 1 (0.3) | 1 (0.6) | 0 (0.0) | 1.00 |
| Predisposing factors for MDRO infections | ||||
| Hospital admission in the previous 6 months | 141 (46.3) | 85 (51.0) | 56 (40.9) | 0.08 |
| Use of antibiotics in the previous 6 months | 149 (49.0) | 92 (56.0) | 57 (41.6) | 0.02 |
| Colonization with MDRO in the previous 6 months | 35 (11.5) | 21 (12.6) | 14 (10.2) | 0.52 |
| Time from ICU admission to the study day (days) | 7 [2–34] | 10 [2–45] | 6 [3–17] | 0.07 |
| Time from hospital admission to the study day (days) | 11 [3–29] | 15 [4–50] | 7 [3–18] | 0.02 |
| Colonization with CPE * | 40 (13.2) | 21 (12.6) | 19 (13.9) | 0.87 |
| Time from diagnosis of colonization to the study day (days) | 21 [8–77] | 24 [14–102] | 16 [1–64] | 0.30 |
| Mode of infection acquisition: | - | |||
| Community | 66 (21.7) | 66 (48.2) | ||
| ICU | 52 (17.1) | 52 (38.0) | ||
| Hospital non-ICU | 10 (3.2) | 10 (5.2) | ||
| Nursing home | 9 (2.9) | 9 (6.7) | ||
| Healthcare-associated infections ** | 69 (22.6) | 69 (50.4) | - | |
| Status at admission | ||||
| SOFA at admission | 3 [2–6] | 3 [1–6] | 4 [2–7] | 0.03 |
| SOFA on the study day | 2 [1–4] | 1 [0.3] [8–77] | 3 [1.5] | 0.0000 |
| Type of admission | 0.021 | |||
| medical | 232 (76.6) | 118 (71.1) | 114 (83.2) | |
| elective surgery | 56 (18.5) | 40 (24) | 16 (11.7) | |
| emergency surgery | 15 (5) | 8 (4.8) | 7 (5.1) | |
| Trauma | 2 (3.0) | 3 (1.8) | 6 (4.4) | 0.19 |
| Clinical status on the study day | ||||
| Septic shock | 12 (3.9) | 12 (8.8) | - | |
| Sepsis | 45 (14.8) | 45 (32.9) | ||
| Infection status category | ||||
| Isolation of non-MDRO | 54 (17.7) | 54 (39.4) | - | |
| Isolation of MDRO | 14 (4.6) | 14 (10.2) | - | |
| No bacteriologic confirmation (probable or possible infection) | 69 (22.6) | 69 (50.4) | - | |
| Outcomes | ||||
| Mortality *** | 16 (5.3 [3.0–8.4]) | 7 (4.2 [1.7–8.5]) | 9 (6.6 [3.1–12.1]) | 0.36 |
| Length of ICU stay (days) | 22 [8–67] | 27 [7–81] | 20 [9–64] | 0.05 |
| Length of hospital stay (days) | 30 [14–87] | 32 [11–93] | 30 [17–73] | 0.02 |
| Variable | Patients with Non-MDRO Infections n = 54 | Patients with MDRO Infections n = 14 | p Value |
|---|---|---|---|
| Age (months) | 26 [4–93] | 43 [9,10] | 0.40 |
| Gender (male) | 35 (64.8) | 9 (64.3) | 0.97 |
| Comorbid condition | |||
| Respiratory disease | 8 (14.8) | 2 (14.3) | 0.96 |
| Obesity | 2 (3.7) | 1 (7.1) | 0.51 |
| Diabetes | 0 (0.0) | 0 (0.0) | 1.00 |
| Chronic liver disease | 1 (1.9) | 1 (7.1) | 0.37 |
| Chronic renal disease | 0 (0.0) | 1 (7.1) | 0.21 |
| Immunosuppression | 3 (5.6) | 2 (14.3) | 0.27 |
| Bone marrow transplantation | 1 (1.9) | 0 (0.0) | 1.00 |
| Solid organ transplantation | 0 (0.0) | 1 (7.1) | 0.21 |
| Oncologic/Hematologic disease | 5 (9.3) | 1 (7.1) | 0.64 |
| Chemotherapy in the previous 6 months | 2 (3.7) | 1 (7.1) | 0.68 |
| Human immunodeficiency virus infection | 0 (0.0) | 0 (0.0) | 1.00 |
| Bronchopulmonary dysplasia | 3 (5.6) | 2 (14.3) | 0.27 |
| Cystic fibrosis | 1 (1.9) | 0 (0.0) | 0.79 |
| Congenital heart disease | 6 (11.1) | 1 (7.1) | 1.0 |
| Prematurity | 12 (22.2) | 1 (7.1) | 0.28 |
| Predisposing factors for MDRO infections | |||
| Hospital admission in the previous 6 months | 29 (53.7) | 5 (31.7) | 0.37 |
| Use of antibiotics in the previous 6 months | 29 (53.7) | 8 (57.1) | 0.81 |
| Colonization with MDRO in the previous 6 months | 7 (13.0) | 5 (35.7) | 0.047 |
| Colonization with carbapenemase-producing Enterobacterales during ICU stay * | 9 (16.7) | 8 (66.7) | 0.002 |
| Time from colonization to the study day (days) | 45 [13–111] | 16 [1–42] | 0.23 |
| Time from ICU admission to the study day (days) | 5 [3–14] | 35 [10–78] | 0.06 |
| Time from hospital admission to the study day (days) | 12 [5–46] | 54 [15–107] | 0.008 |
| Time from hospital admission to diagnosis of infection (days) | 8 [0–30] | 73 [14–99] | 0.003 |
| Time from diagnosis of infection to the study day (days) | 5 [3–12] | 8 [2–12] | 0.77 |
| Mode of acquisition of infection: Community/ICU/hospital non-ICU/nursing home | 17/26/4/4 (33.3/51.0/7.8/7.8) | 2/11/0/1 (14.3/78.6/0/7.1) | 0.268 |
| Healthcare-associated infection ** | 34 (63) | 12 (85.7) | 0.165 |
| Status at admission | |||
| SOFA at admission | 4 [2–8] | 5 [3–9] | 0.42 |
| Type of admission (medical/elective surgery/emergency surgery) | 43/8/3 (80.0/15.0/5.0) | 10/3/1 (71.4/21.4/7.1) | 0.80 |
| Trauma | 3 (5.6) | 1 (7.1) | 0.82 |
| Clinical status on the study day | |||
| Septic shock | 6 (11.1) | 2 (14.3) | 0.67 |
| Sepsis | 15 (27.8) | 7 (50.0) | 0.36 |
| SOFA at the study day | 3 [1–5] | 3 [3–8] | 0.20 |
| Outcomes | |||
| Mortality *** | 4 (7.4 [2.0–17.9]) | 1 (7.1 [0.3–33.9]) | 0.97 |
| Length of ICU stay (days) | 25 [12–27] | 81 [22–150] | 0.0007 |
| Length of Hospital stay (days) | 33 [19–83] | 116 [46–168] | 0.0001 |
| Antimicrobial use | |||
| By therapeutic approach | 0.874 | ||
| Empiric | 16 (30.0) | 4 (28.6) | |
| Targeted | 36 (66.7) | 10 (71.4) | |
| By concordance with antimicrobial susceptibility | 0.916 | ||
| Adequate | 46 (85.2) | 13 (92.9) | |
| Inadequate | 4 (7.5) | 1 (7.1) | |
| By type of antimicrobial | 0.007 | ||
| New | 1 (1.85) | 3 (21.4) | |
| Traditional | 50 (92.6) | 11 (78.6) | |
| By AWARE classification (WHO) | 0.048 | ||
| Access | 26 (48.1) | 5 (35.7) | |
| Watch | 23 (42.6) | 5 (35.7) | |
| Reserve | 3 (5.5) | 4 (28.6) |
| Multidrug-Resistant Microorganisms (n = 14) | Non-Multidrug-Resistant Microorganisms (n = 54) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site of Infection | Total Number | A.∙baumanii | DT-P.∙aeruginosa | CPE | ESBL | VRE | MRSA | Total Number | S.∙pneumoniae | P.∙aeruginosa | S.∙aureus | E.∙coli | S.∙coagulase negative | Proteus sp. | KESC | S.∙pyogenes | Other |
| Community-acquired pneumonia | 2 (14.2%) | 2 | 17 (23.6%) | 4 | 1 | 1 | 3 | 8 | |||||||||
| Hospital-acquired pneumonia | - | 3 (4%) | 1 | 1 | 1 | ||||||||||||
| Ventilator-associated pneumonia | 6 (42.9%) | 1 | 2 | 3 | 17 (23.6%) | 6 | 3 | 1 | 7 | ||||||||
| Catheter-related bloodstream infection | 3 (21.4%) | 3 | 8 (11.1%) | 1 | 2 | 2 | 3 | ||||||||||
| Primary bacteremia | - | 1 (1%) | 1 | ||||||||||||||
| Clostridium difficile diarrhea | - | 3 (4%) | 1 | 2 | |||||||||||||
| Cardiovascular infections | - | - | |||||||||||||||
| Gynecological and obstetric | - | ||||||||||||||||
| UTI-community | - | 2 (3%) | 1 | 1 | |||||||||||||
| UTI-related to catheter | 1 (7.1%) | 1 | 6 (8.3%) | 3 | 2 | 1 | |||||||||||
| Post-surgical meningitis | - | 3 (4%) | 2 | 1 | |||||||||||||
| Surgical site infection | 1 (7.1%) | 1 | 3(4%) | 1 | 2 | ||||||||||||
| Intraabdominal infection | 1 (7.1%) | 1 | 3 (4%) | 1 | 2 | ||||||||||||
| Skin and soft tissue | - | 1 (1%) | 1 | ||||||||||||||
| Osteoarticular infection | - | 1(1%) | 1 | ||||||||||||||
| Other | - | 4 (6%) | 1 | 1 | 1 | 1 | |||||||||||
| Total number | 14 (100%) | 0 | 1 | 4 | 7 | 0 | 2 | 72 (100%) | 10 | 9 | 5 | 6 | 1 | 7 | 5 | 25 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornistein, W.; Balasini, C.; Nuccetelli, Y.; Rodriguez, V.M.; Cudmani, N.; Roca, M.V.; Sadino, G.; Brizuela, M.; Fernández, A.; González, S.; et al. Prevalence and Associated Mortality of Infections by Multidrug-Resistant Organisms in Pediatric Intensive Care Units in Argentina (PREV-AR-P). Antibiotics 2025, 14, 493. https://doi.org/10.3390/antibiotics14050493
Cornistein W, Balasini C, Nuccetelli Y, Rodriguez VM, Cudmani N, Roca MV, Sadino G, Brizuela M, Fernández A, González S, et al. Prevalence and Associated Mortality of Infections by Multidrug-Resistant Organisms in Pediatric Intensive Care Units in Argentina (PREV-AR-P). Antibiotics. 2025; 14(5):493. https://doi.org/10.3390/antibiotics14050493
Chicago/Turabian StyleCornistein, Wanda, Carina Balasini, Yanina Nuccetelli, Viviana M. Rodriguez, Norma Cudmani, Maria Virginia Roca, Graciela Sadino, Martín Brizuela, Analía Fernández, Soledad González, and et al. 2025. "Prevalence and Associated Mortality of Infections by Multidrug-Resistant Organisms in Pediatric Intensive Care Units in Argentina (PREV-AR-P)" Antibiotics 14, no. 5: 493. https://doi.org/10.3390/antibiotics14050493
APA StyleCornistein, W., Balasini, C., Nuccetelli, Y., Rodriguez, V. M., Cudmani, N., Roca, M. V., Sadino, G., Brizuela, M., Fernández, A., González, S., Águila, D., Macchi, A., Staneloni, M. I., & Estenssoro, E. (2025). Prevalence and Associated Mortality of Infections by Multidrug-Resistant Organisms in Pediatric Intensive Care Units in Argentina (PREV-AR-P). Antibiotics, 14(5), 493. https://doi.org/10.3390/antibiotics14050493






