Abstract
Background: Nosocomial lower respiratory tract infections (nLRTIs) are associated with unfavorable clinical outcomes and significant healthcare costs. nLRTIs include hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), and other ICU-acquired pneumonia phenotypes. While risk factors for mortality in these infections are critical to guide preventive strategies, it remains unclear whether they vary based on their requirement of invasive mechanical ventilation (IMV) at any point during the hospitalization. Objectives: This study aims to identify risk factors associated with short- and long-term mortality in patients with nLRTIs, considering differences between those requiring IMV and those who do not. Methods: This multinational prospective cohort study included ICU-admitted patients diagnosed with nLRTI from 28 hospitals across 13 countries in Europe and South America between May 2016 and August 2019. Patients were selected based on predefined inclusion and exclusion criteria, and clinical data were collected from medical records. A random forest classifier determined the most optimal clustering strategy when comparing pneumonia site acquisition [ward or intensive care unit (ICU)] versus intensive mechanical ventilation (IMV) necessity at any point during hospitalization to enhance the accuracy and generalizability of the regression models. Results: A total of 1060 patients were included. The random forest classifier identified that the most efficient clustering strategy was based on ventilation necessity. In total, 76.4% of patients [810/1060] received IMV at some point during the hospitalization. Diabetes mellitus was identified to be associated with 28-day mortality in the non-IMV group (OR [IQR]: 2.96 [1.28–6.80], p = 0.01). The 90-day mortality-associated factor was MDRP infection (1.98 [1.13–3.44], p = 0.01). For ventilated patients, chronic liver disease was associated with 28-day mortality (2.38 [1.06–5.31] p = 0.03), with no variable showing statistical and clinical significance at 90 days. Conclusions: The risk factors associated with 28-day mortality differ from those linked to 90-day mortality. Additionally, these factors vary between patients receiving invasive mechanical ventilation and those in the non-invasive ventilation group. This underscores the necessity of tailoring therapeutic objectives and preventive strategies with a personalized approach.
1. Introduction
Nosocomial lower respiratory tract infections (nLRTI), encompasses hospital-acquired pneumonia (HAP), hospital-acquired pneumonia that requires ventilation (VHAP), ventilator-associated pneumonia (VAP), ventilator-associated tracheitis (VAT), and intensive care unit-acquired pneumonia that does not require ventilation (ICU-AP) [1]. HAP and VAP are two conditions in the latest international clinical guidelines [2,3]. For instance, HAP is often a less severe disease; however, up to 50% of patients can develop serious complications such as acute respiratory failure or even sepsis and septic shock that require management at the intensive care unit (ICU) [2,4]. On the other hand, up to 90% of pneumonia episodes in the ICU occur in patients undergoing (IMV), making this complication of ventilation a significant concern for clinicians [5,6]. Overall, patients who develop nosocomial pneumonia and require treatment in the ICU are at increased risk of mortality and additional burden on hospital stays and healthcare costs per patient, ranging from 10,000 to 40,000 USD [7].
Identifying risk factors to enhance patients’ clinical outcomes has become a priority [8]. Treatment approaches for nLRTIs typically involve broad-spectrum antibiotics targeting common pathogens such as Acinetobacter baumannii, Klebsiella spp., Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), and resistant strains of Pseudomonas aeruginosa as described in the first analysis of this ENIRRI cohort [1]. Therapeutic empirical approaches include broad-spectrum antibiotics targeting prevalent pathogens depending on the local epidemiology; however, the increasing prevalence of multidrug-resistant pathogens (MDRP) poses significant challenges for effective therapy. Resistance mechanisms, including β-lactamase production, efflux pumps, and porin channel mutations, contribute to treatment failure and necessitate ongoing evaluation of antimicrobial efficacy [2,8,9,10]. This underscores the need for targeted empirical therapy based on local resistance patterns and pathogen sensitivity profiles. Novel antibiotics, such as β-lactam/β-lactamase inhibitor combinations and advanced carbapenems, show promise in overcoming resistance. However, it remains uncertain whether mortality risk factors differ between different phenotypes or subtypes of nLRTI such as those who are receiving IMV and those who are not.
Given the lack of sufficient evidence regarding the optimal method for grouping patients with nLRTIs and identifying factors associated with worse clinical outcomes, we proposed this large multinational study, the ENIRRI. Our study aims to determine the most effective way to categorize patients with nLRTIs and to evaluate the risk factors associated with mortality among these groups.
2. Results
A total of 1060 patients admitted to the ICU were included in the study, with the majority receiving IMV (76.4% [810/1060]) (Figure 1). Most of the patients enrolled were male, 72.5% (769/1060), with a mean age of 64 years across the entire cohort. The Random Forest classifier model found that the most optimal way of categorizing patients was into non-IMV and IMV groups, achieving an AUC ROC of 0.70 ± 0.02. The AUC graph and variables included alongside their feature importance can be found in the Supplementary Materials (Figure S2). In contrast, classifying patients based on whether their condition was acquired in the ward or the ICU resulted in a lower performance, with an AUC ROC of 0.68 ± 0.03 (Figure S3).
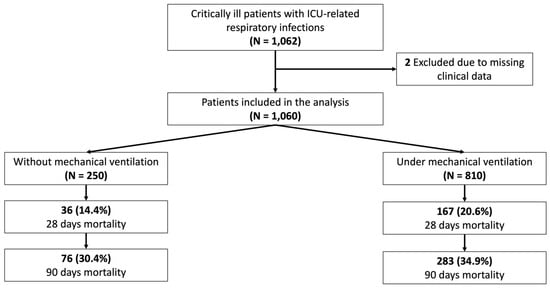
Figure 1.
Study flowchart of included patients diagnosed with nLRTIs and clinical outcomes.
The cohort was then divided into those not under invasive mechanical ventilation (non-IMV) (250/1060) and those under invasive mechanical ventilation (IMV) (810/1060). Non-IMV patients received various forms of respiratory support, distributed as follows: 72.4% (181/250) received supplementary oxygen, 13.6% (34/250) were managed with non-invasive positive pressure ventilation (NIPPV), and 14% (35/250) were treated with a high-flow nasal cannula (HFNC). Additionally, non-IMV patients also had a higher median [IQR] age (non-IMV: 66 [57–75] vs. IMV: 63 [49–73], p = 0.003) and had more comorbid conditions. The most frequent past medical condition for both non-IMV and IMV patients was a history of immunocompromise (non-IMV: 37.6% [94/250] vs. IMV: 21.0% [170/810], p < 0.001), chronic renal failure (non-IMV: 14.8% [37/250] vs. IMV: 10.2% [83/810], p = 0.05), and chronic heart disease (non-IMV: 30.8% [77/250] vs. IMV: 25.8% [209/810], p = 0.12). Furthermore, IMV patients presented more severe disease at nLRTI diagnosis based on median [IQR] SAPS II (non-IMV: 41 [30–54] vs. IMV: 48 [38–59], p < 0.001) and SOFA score (non-IMV: 6 [4–9] vs. IMV: 8 [5–10], p < 0.001). All the demographic characteristics are shown in Table 1.

Table 1.
Demographic and Clinical Characteristics of Patients with nLRTI stratified by those requiring IMV.
Regarding nLRTI etiology, non-IMV patients developed MRSA infections more frequently (non-IMV: 8.4% [21/250] vs. IMV: 4.2% [34/810], p < 0.001), while IMV patients were more frequently infected by Pseudomonas aeruginosa (non-IMV: 6.4% [16/250] vs. IMV: 17.0% [138/810], p = 0.01). Also, IMV patients were infected in a higher percentage by MDR pathogens (non-IMV: 18.0% [45/250] vs. IMV: 26.8% [217/810], p = 0.01). Finally, ventilated patients had worse clinical outcomes based on the median [IQR] ICU length of stay (LOS) (non-IMV: 14 [7–25] vs. IMV: 22 [13–37], p < 0.001) and hospital LOS (non-IMV: 36 [23–61] vs. IMV: 39 [22–65], p = 0.38) as well as mortality at 28 days (non-IMV: 14.4% [36/250] vs. IMV: 20.6% [167/810], p = 0.03), and mortality at 90 days (non-IMV: 30.4% [76/250] vs. IMV: 34.9% [283/810], p = 0.19) (Table 1).
2.1. Mortality Analysis in the Whole Cohort
Logistic regression was performed to identify the risk factors associated with 28 d and 90 d mortality within the whole cohort presented in Table S2 and Table S3, respectively. For both time points, a higher SAPS II score was an independent risk factor (28 d: 1.04 [1.03–1.05], p < 0.001; 90 d: 1.03 [1.02–1.04], p < 0.001). Notably, the need for IMV was only related to mortality at 28 days (28 d: 1.53 [1.01–2.32], p = 0.04; 90 d: 1.26 [0.91–1.75], p < 0.001). Finally, age was analyzed as a continuous variable, exposing a statistically significant relationship with mortality at both time points (28 d: 1.02 [1.00–1.03], p = 0.01; 90 d: 1.02 [1.01–1.03], p < 0.001). The model used had an appropriate fitness determined by the Hosmer–Lemeshow test of 0.15 for 28-day mortality and 0.58 for 90-day mortality.
2.2. Mortality Stratified by Ventilation Status
An adjusted logistic regression model was fitted for 28 d and 90 d mortality between non-IMV and IMV patients (Table 2, Table 3, Table 4 and Table 5). The risk factor that showed the strongest association with mortality in non-IMV patients at 28 days was diabetes mellitus (OR [95% CI]) (2.96 [1.28–6.80], p = 0.01) and the SAPS II score (1.05 [1.02–1.07], p < 0.01). At 90 days, they were the SAPS II score (1.04 [1.02–1.06], p < 0.01) and an MDRP agent causing pneumonia (1.98 [1.13–3.44] p = 0.01). The model used had an appropriate fitness determined by the Hosmer–Lemeshow test of 0.87 for 28 d mortality and 0.30 for 90 d mortality (Table 2 and Table 3).

Table 2.
Bivariate and multivariate analysis for 28 days mortality among non-IMV patients.

Table 3.
Bivariate and multivariate analysis for 90-day mortality among non-IMV patients.

Table 4.
Bivariate and multivariate analysis for 28 days mortality among patients under IMV.

Table 5.
Bivariate and multivariate analysis for 90 days mortality among patients under IMV.
Regarding the patients who required IMV (Table 4 and Table 5), a higher SAPS II score at nLRTI diagnosis (28 d: 1.04 [1.02–1.05], p < 0.01; 90 d: 1.03 [1.02–1.05], p < 0.01) and older age (28 d: 1.02 [1.01–1.04], p < 0.01; 90 d: 1.03 [1.01–1.04], p < 0.01) showed significant association with 28 d and 90 d mortality. Furthermore, chronic liver disease showed an association with 28-day mortality (2.38 [1.06–5.31], p = 0.03). Further information regarding the 90-day mortality model can be found in Table 5. Although included in this model, MRSA infections did not show a significant association at any point. The model used had an appropriate fitness determined by the Hosmer–Lemeshow test of 0.28 for 28-day mortality and 0.06 for 90-day mortality.
3. Discussion
This multicenter and multinational prospective cohort study is focused on patients with nLRTI admitted to the ICU. After a non-supervised statistical analysis, we found that the best way to cluster patients into comparable groups with nLRTI was by the requirement of invasive mechanical ventilation at any time point during their hospitalization. Notably, over two-thirds of patients required mechanical ventilation and had an increased 28-day mortality risk. Consequently, the study identified the risk factors associated with 28-day and 90-day mortality in non-IMV and IMV patients. Among non-IMV patients, diabetes mellitus and MDRP infection were found to be independent factors associated with 28 d and 90 d mortality when the model was adjusted by older age and severity at diagnosis using the SAPS II score. For IVM patients, chronic liver disease was found to be strongly associated with 28 d mortality when the model was adjusted by severity according to the SAPS II score and age.
nLRTI is commonly regarded as the most frequently acquired infection in the ICU [11,12]. It has been estimated that approximately 65% of nosocomial infections originate from respiratory sources [13]. These infections occur at a rate of 5 to 10 cases per 1000 hospital admissions in Europe and the United States [14,15,16]. In ventilated patients, it is of particular concern, as VAP accounts for 10 to 40% of ICU pneumonia cases. In some countries, it can reach over 90% of patients who are intubated and mechanically ventilated [14,15,16,17]. In our cohort, which included patients from Europe and South America, more than two-thirds of nLRTI cases were reported in ventilated patients, aligning with previous research findings. While Shah et al. have reported a 5% decrease in the incidence of VAP in the United States over the last decade [18], the overall prevalence has largely remained stable. Among patients with nLRTI, those who received IMV faced a significantly increased 28-day mortality risk compared to those without mechanical ventilation. The worldwide all-cause mortality rate associated with VAP falls within the range of 20% to 50% [14], which is consistent with our findings. Notably, our study is a pioneer in prospectively assessing the issue of nosocomial pneumonia in several countries in Europe and South America. By including a larger, more diverse patient population, we have reduced random error and addressed the demographic heterogeneity, enhancing our results’ robustness and generalizability. It remains crucial to identify risk predictors promptly to identify at-risk patients effectively.
Our study introduces a different perspective by comparing the risk factors between non-IMV and IMV patients. Among non-IMV patients, past medical conditions, microbial etiology, and systemic corticoids us in previous studies led to increase in short- and mid-term mortality. In 2023, E. Bouza et al. conducted a comparative study on the etiology of nosocomial bacteremic pneumonia in ventilated and non-ventilated patients over the past decade, revealing a higher prevalence of S. aureus and P. aeruginosa in HAP, with an increased mortality rate of more than 40% [19], a trend that is further confirmed by our findings, which present MDRP infections associated with higher mortality rates in non-IMV patients; this association is further supported by an observational study conducted by de Oliveira et al. [20] They found that MDRP infections were linked to higher mortality rates, longer hospital stays, and an elevated risk of requiring mechanical ventilation. These findings align with our observation that MDRP infections emerged as a significant risk factor at the 90-day mark. However, further research is needed to evaluate the impact of inappropriate empiric antibiotic therapy at admission, as well as antibiotic misuse, on the development of nLRTI caused by MDRPs [20].
Regarding comorbidities, diabetes mellitus (DM) has been identified as an independent factor associated with mortality in non-IMV patients. The observation that DM is associated with mortality at 28 days but not at 90 days can be explained by the impact of DM on exacerbating the acute phase of the infection. This chronic condition may not influence long-term mortality, as those who survive the initial phase are less likely to be affected by diabetes during the resolution phase of the illness. This aligns and is further supported by the findings from Equils et al. in a randomized controlled trial on methicillin-resistant Staphylococcus aureus (MRSA) nosocomial pneumonia, where they noted that diabetic patients had higher overall 28-day mortality rates compared to non-diabetic patients (23.5% vs. 14.7%; RD = 8.8%, 95% CI [1.4, 16.3]) but this trend did not persist in long term mortality [21]. Furthermore, Yakoub et al. described this in a 2023 longitudinal cohort study, that DM was a significant risk factor for mortality in nosocomial pneumonia (OR: 2.98, p = 0.004) [22]. Although both studies had a smaller sample size than our cohort, their findings align with our results.
In IVM patients, chronic hepatic disease was associated with 28-day mortality but not with 90-day mortality. Similarly to DM, this may be due to the significant impact of these chronic conditions during the acute phase of infection. Pasquale et al. in 2013 found that patients with liver disease had significantly higher 28-day and 90-day mortality rates (63% vs. 28%, p < 0.001; 72% vs. 38%, p < 0.001, respectively) compared to non-chronic liver disease patients. Although their results seem contradictory in terms of temporality to ours, their cohort only included patients who acquired pneumonia in the ICU. In contrast, our ventilated group included patients who were ventilated before developing an nLRTI and those who developed HAP after required ventilation. These differences in patient populations likely contributed to the variations in long-term outcomes between the two studies [23]. In the same vein, our results are further supported by Maruyama et al. [24] who found that chronic liver disease was a significant factor associated with 30-day mortality due to pneumonia from any cause, not specifically nosocomial acquired, with an (OR: 3.029, 95% CI [1.126–8.149], p = 0.028) in a sample size similar to ours. However, our study specifically found an association of chronic liver disease with mortality in IMV patients [24].
For IVM and non-IMV patients, the SAPS II score showed a statistically significant association with the evaluated outcome. SAPS II is a score that provides an estimate of the risk of death within the first 24 h from calculation without having to specify a primary diagnosis [16]; showing a discriminative power for ICU 24 h mortality in nosocomial pneumonia with an AUC [95% CI] 0.752 [0.656–0.848] and a specificity of 83.93 [25] when calculated on the day of nLRTI diagnosis, demonstrating that this score is rather valuable for the acute phase of the illness. While the SAPS II score was included in our regression models to determine associated factors at 28 and 90 days, it cannot be interpreted as a risk factor despite its statistical significance. The very low hazard ratio indicates that this variable primarily serves to adjust the model rather than to predict long-term mortality independently. Its discriminative power for mortality over extended periods is minimal, as supported by studies like Iwashyna et al., which showed that the predictive ability of acute illness severity scores, such as SAPS II, diminishes over time. Specifically, it found that while such scores accurately predict outcomes during ICU admission, their predictive power wanes after approximately 10 days in the ICU, giving way to other factors like age, sex, and chronic health status [26].
It is important to acknowledge the limitations of our study. First, using fixed data represents a significant limitation, particularly in predicting long-term outcomes, as it fails to capture the dynamic changes in patient health over time. Additionally, clustering patients into two groups, including specific subgroups like the IMV group, introduces bias, especially since patients with VHAP, whose nLRTI was not due to ventilation, were included. However, to our knowledge, this is a pioneering study strengthened by its multicentric and multinational scope across Europe and Latin America. Second, the evolving dynamics in pneumonia management, particularly the shift toward non-IMV strategies with high-flow nasal cannulas, further complicates the extrapolation of our results to current practice as the smaller sample was in the non-IMV group. A further limitation of this study is the lack of data on glucose control during hospitalization, as blood glucose measurements were not consistently collected as well as the lack of MELD score or Child-Pugh classification data for chronic liver disease. This prevented us from assessing their potential impact on patient outcomes and mortality in nLRTI.
Nevertheless, it is essential to mention that there is still limited migration to non-IMV strategies, especially in low- and middle-income countries, and the results found are valuable and provide a starting point for future research. Third, although DAGs suggest potential collinearity between variables like SAPS II and IMV, the statistical assessment shows minimal correlation (point-biserial correlation coefficient = −0.0765). Fourth, we could not assess the quality of sputum samples collected for microorganism identification, the techniques used for sample collection, the implementation of preventive strategies against infections caused by microorganisms other than P. aeruginosa and MRSA, or the specific antibiotic treatments administered. This information could have provided additional insights. However, it is essential to mention that even with high-quality sputum, the etiological agent of lower respiratory infections is only identified in 38% of all cases [27]. Fifth, the variability in the time taken to diagnose nosocomial lower respiratory tract infections (nLRTI) and the lack of standardized treatment protocols across different ICUs may have resulted in the omission of specific fungal and viral tests, potentially introducing bias. Although technical and diagnostic capabilities may be limited in some countries, guidelines for managing healthcare-associated respiratory infections are widely disseminated and applied globally, reducing bias. Despite these limitations, our results are valuable because they are consistent with previous studies and provide insights that can help physicians identify specific patient characteristics, ultimately leading to better patient care.
4. Materials and Methods
This multicenter, multinational prospective cohort study represents one of the primary analyses of the European network for ICU-related respiratory infections (ENIRRI) multicenter study across 28 ICUs in 13 countries throughout Europe and Latin America, including Argentina, Belgium, Colombia, Croatia, France, Germany, Ireland, Italy, the Netherlands, Poland, Portugal, Spain, and Turkey. The participating hospitals were selected based on logistical feasibility and their ability to contribute to the study objectives. The study enrolled critically ill patients admitted between 9 May 2016, and 16 August 2019, with each site conducting enrollment over a continuous 12-month period within this timeframe. Consecutive patients aged 18 years or older were included if they developed a lower respiratory tract infection (LRTI) at least 48 h after hospital admission (i.e., nosocomial LRTI), were later admitted to the ICU, and/or developed LRTI during their ICU stay. Follow-up was performed for all enrolled patients until hospital discharge.
The study followed the Code of Ethics of the World Medical Association (Declaration of Helsinki). The study received approval from the institution’s Internal Review Board (Comité Ètic d’Investigació Clínica, registry number HCB/2020/0370) and was registered in ClinicalTrials.gov Identifier (NCT03183921). Additionally, each of the thirteen participating sites obtained approval from its institutional ethics committee to conduct the study. Informed consent from patients was obtained when this was requested per local regulations. All clinical data were anonymized and transferred to the coordinating center for data curation and analysis. Further details are provided in reference [1].
4.1. Definitions
According to the 2016 ATS/IDS guidelines, pneumonia is characterized by new lung infiltrates accompanied by clinical indicators suggestive of an infectious origin, including recent onset of fever, purulent sputum, leukocytosis, and decline in oxygenation. Consequently, nosocomial pneumonia is diagnosed in patients who develop a pulmonary infection after being hospitalized for 48 h or more. HAP is identified explicitly as pneumonia occurring in patients after 48 h of being admitted to the hospital while not receiving invasive mechanical ventilation.
Acute kidney injury (AKI) was defined based on the Kidney Disease Improving Global Outcomes (KDIGO) classification, with a KDIGO Stage ≥ 2 [28]. In contrast, acute respiratory distress syndrome (ARDS) was defined according to the Berlin definition [29]. Multiorgan failure was determined when three or more organ systems failed following the diagnosis of nLRTI, and septic shock was defined as sepsis-induced hypotension with elevated lactate (≥2 mmol/L), persisting despite adequate fluid resuscitation [30,31].
4.2. Data Collection
All data were collected from the medical chart and transferred by the principal investigator to the multinational dataset. Demographics, type of admission, previous treatments, comorbidities, laboratories, Sequential Organ Failure Assessment (SOFA) score [32], new Simplified Acute Physiology Score (SAPS II) [33], clinical complications, microbiological information, and clinical outcomes, such as ICU length of stay (LOS), hospital LOS, 28-day mortality (28 d), and 90-day mortality (90 d), were included in the dataset. The microbiological diagnosis was confirmed by sputum in non-ventilated patients and using bronchoscopy or blind bronchoalveolar lavage (BAL) or tracheobronchial aspirates (TBA) in ventilated patients. The microbiological threshold was BAL ≥ 104 colony-forming units per mL and ≥105 colony-forming units for sputum or TBAs. Notably, microbiology assessment was performed based on international and local guidelines, not per study protocol. Ventilatory management strategies, treatments, and microbiological evaluations were not standardized across centers. Instead, these decisions were made at the discretion of the attending clinician, guided by local practices and supported by international recommendations.
4.3. Clustering Patients by Shared Demographic and Clinical Characteristics to Enhance Model Accuracy and Generalizability
After obtaining demographic, clinical, and laboratory features, missing data were addressed using the K-nearest neighbors (KNN) imputation method, applied only to variables with less than 30% missing data to maintain data integrity. Variables exceeding this threshold were excluded from the analysis to avoid introducing bias.
Patients were initially categorized into five groups as described in the first publication of this cohort: HAP, ventilated hospital-acquired pneumonia (VHAP), intensive care unit-acquired pneumonia (ICU-AP), VAP, and ventilator-associated tracheobronchitis (VAT) [1]. Two clustering strategies were proposed to enhance statistical power and identify phenotypically similar patient groups. The first strategy involved clustering patients based on the acquisition site of nLRTI (either in the general ward or the ICU), assuming that patients in the ICU were generally more critically ill. The second strategy was based on whether patients required mechanical ventilation at any point during their hospitalization, with the rationale that many patients were already ventilated before nLRTI development, often due to severe underlying conditions like trauma or major surgery. This clustering strategy aimed to reflect the severity of the patient’s pre-existing condition rather than the infection itself. Both methods were designed to create clusters of patients sharing similar clinical and demographic characteristics, optimizing the accuracy of risk factor identification.
Predictor variables were separated from the target variables, and a Random Forest Classifier model, known for its effectiveness with complex datasets and non-linear relationships, was chosen for training and clustering patients into groups that shared similar characteristics. The model underwent training using the Random Forest algorithm with 100 decision trees. Mechanical ventilation status and the place of acquisition, whether in the ward or ICU, were utilized as clinically plausible variables to cluster patients alongside all demographic variables.
Stratified K-fold cross-validation assessed model performance, revealing significant differences between ventilated and non-ventilated patient groups based on the selected variables. Model accuracy was evaluated using traditional accuracy metrics and the area under the receiver operating characteristic (AUROC) curve.
4.4. Statistical Analysis
All categorical variables are presented as relative and absolute frequencies and continuous variables with median (Interquartile Ranges [IQR]) or mean (Standard Deviations [SD]) depending on their distribution according to the Kolmogorov–Smirnov test. The non-normal variables were compared using the Mann–Whitney U and t-test for those with normal distribution. The x2 or Fisher’s exact test was used to compare categorical variables. The variables with 30% or more missing data were not used in the analysis to prevent bias and maintain integrity in the analysis.
First, a bivariate analysis was carried out to identify the factors related to the outcome. Bivariate analyses examine potential correlations between variables, guided by our directed acyclic graph (DAG) (Figure S1) to explore the relationships between covariates and mortality. Variables with a p-value < 0.2 in the bivariate analysis are considered for further inclusion in the multivariate logistic regression model. However, inclusion in the bivariate analysis does not guarantee inclusion in the final model.
The DAG is employed to identify potential collinear variables or those within others’ causal pathways, ensuring that only the most relevant variables are incorporated into the multivariate analysis. A backward elimination method is applied, with a stopping rule based on p-values. Variables with p-values between 0.3 and 0.4 are removed to maintain model parsimony, adjusting from the initially proposed threshold of 0.5 [34]. Combining bivariate analysis, DAG-guided selection, and backward elimination ensures robust and precise identification of risk factors.
The final model is adjusted based on the most significant and relevant covariates, ensuring appropriate adjustment and minimizing bias in estimating associations between risk factors and mortality. Then, multivariable logistic regression models assessed risk factors (HR [95% CI]) associated with 28 d and 90 d mortality among non-ventilated and ventilated patients. In the multivariate test, 28 and 90 days of mortality were taken as the dependent dichotomic variables. The non-IMV model was adjusted by age, taken as a dichotomic variable (>65 years), and severity using the SAPS II score as a continuous variable. The IMV model was adjusted by age and severity, and the SAPS II score was used in both cases as a continuous variable. The goodness of fitness of the multivariable logistic regression models was assessed with the Hosmer–Lemeshow test, and the level of significance considered at two-tailed was a p-value < 0.05. All the analyses were performed in the SPSS statistical package, version 29.
5. Conclusions
This multicenter, multinational study conducted in Europe and Latin America sheds light on the clinical landscape of nLRTI, which continues to be an essential issue in the context of critical care. We provide insights into the higher mortality risk associated with this condition and its risk factors, finding significant differences between non-invasive ventilated and invasive-ventilated patients in the critical care setting. These results highlight the necessity of moving beyond a one-size-fits-all approach, advocating instead for personalized strategies that consider patient severity, demographics, and the specific characteristics of the infection. Early identification of at-risk patients is essential for guiding targeted therapeutic efforts to improve outcomes. Future studies focusing on the distinct groups identified by the ENIRRI group could further refine risk stratification, enabling a more precise and personalized approach for these homogeneous yet distinct patient populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14020127/s1.
Author Contributions
Conceptualization: I.M.-L., A.T., L.F.R. and O.T.R.; Data Curation: L.F.R., I.M.-L., O.T.R., E.D.I.-P. and C.C.S.-M.; Formal Analysis: L.F.R., I.M.-L., J.O.-G., E.D.I.-P. and C.C.S.-M.; Funding Acquisition: I.M.-L., A.T. and L.F.R.; Investigation: L.F.R., I.M.-L., E.D.I.-P., J.O.-G., S.N. (Saad Nseir), O.T.R., P.P., E.D., M.J.S., A.H.R., C.C.S.-M., G.D.P., P.N., S.S., M.E., L.M.C., A.C. (Andrea Cortegiani), S.A., A.C. (Anselmo Caricato), H.J.F.S., A.C. (Adrian Ceccato), R.C., P.M.S., C.-E.L., P.K.E., F.R., J.R.M., J.M., S.I.-M., S.N. (Stefano Nava), D.C., L.D.B., A.A., F.F., D.G., M.P., F.S.T., M.A. and A.T.; Methodology: L.F.R., I.M.-L., E.D.I.-P., J.O.-G., S.N. (Saad Nseir), O.T.R., P.P., E.D., M.J.S., A.H.R., C.C.S.-M., G.D.P., P.N., S.S., M.E., L.M.C., A.C. (Andrea Cortegiani), S.A., A.C. (Anselmo Caricato), H.J.F.S., A.C. (Adrian Ceccato), R.C., P.M.S., C.-E.L., P.K.E., F.R., J.R.M., J.M., S.I.-M., S.N. (Stefano Nava), D.C., L.D.B., A.A., F.F., D.G., M.P., F.S.T., M.A. and A.T.; Project Administration: L.F.R., I.M.-L. and A.T.; Resources: I.M.-L. and A.T.; Software: L.F.R., I.M.-L., J.O.-G., E.D.I.-P. and C.C.S.-M.; Supervision: L.F.R., I.M.-L., E.D.I.-P., J.O.-G., S.N. (Saad Nseir), O.T.R., P.P., E.D., M.J.S., A.H.R., C.C.S.-M., G.D.P., P.N., S.S., M.E., L.M.C., A.C. (Andrea Cortegiani), S.A., A.C. (Anselmo Caricato), H.J.F.S., A.C. (Adrian Ceccato), R.C., P.M.S., C.-E.L., P.K.E., F.R., J.R.M., J.M., S.I.-M., S.N. (Stefano Nava), D.C., L.D.B., A.A., F.F., D.G., M.P., F.S.T., M.A. and A.T.; Methodology: L.F.R., I.M.-L., E.D.I.-P., J.O.-G., S.N. (Saad Nseir), O.T.R., P.P., E.D., M.J.S., A.H.R., C.C.S.-M., G.D.P., P.N., S.S., M.E., L.M.C., A.C. (Andrea Cortegiani), S.A., A.C. (Anselmo Caricato), H.J.F.S., A.C. (Adrian Ceccato), R.C., P.M.S., C.-E.L., P.K.E., F.R., J.R.M., J.M., S.I.-M., S.N. (Stefano Nava), D.C., L.D.B., A.A., F.F., D.G., M.P., F.S.T., M.A. and A.T.; Validation: L.F.R., I.M.-L., E.D.I.-P., J.O.-G., S.N. (Saad Nseir), O.T.R., P.P., E.D., M.J.S., A.H.R., C.C.S.-M., G.D.P., P.N., S.S., M.E., L.M.C., A.C. (Andrea Cortegiani), S.A., A.C. (Anselmo Caricato), H.J.F.S., A.C. (Adrian Ceccato), R.C., P.M.S., C.-E.L., P.K.E., F.R., J.R.M., J.M., S.I.-M., S.N. (Stefano Nava), D.C., L.D.B., A.A., F.F., D.G., M.P., F.S.T., M.A. and A.T.; Methodology: L.F.R., I.M.-L., E.D.I.-P., J.O.-G., S.N. (Saad Nseir), O.T.R., P.P., E.D., M.J.S., A.H.R., C.C.S.-M., G.D.P., P.N., S.S., M.E., L.M.C., A.C. (Andrea Cortegiani), S.A., A.C. (Anselmo Caricato), H.J.F.S., A.C. (Adrian Ceccato) R.C., P.M.S., C.-E.L., P.K.E., F.R., J.R.M., J.M., S.I.-M., S.N. (Stefano Nava), D.C., L.D.B., A.A., F.F., D.G., M.P., F.S.T., M.A. and A.T.; Visualization: L.F.R., I.M.-L., J.O.-G., E.D.I.-P. and C.C.S.-M.; Writing—Original Draft Preparation: L.F.R., I.M.-L., E.D.I.-P., C.C.S.-M. and A.T.; Writing—Review and Editing: L.F.R., I.M.-L., E.D.I.-P., J.O.-G., S.N. (Saad Nseir), O.T.R., P.P., E.D., M.J.S., A.H.R., C.C.S.-M., G.D.P., P.N., S.S., M.E., L.M.C., A.C. (Andrea Cortegiani), S.A., A.C. (Anselmo Caricato), H.J.F.S., A.C. (Adrian Ceccato), R.C., P.M.S., C.-E.L., P.K.E., F.R., J.R.M., J.M., S.I.-M., S.N. (Stefano Nava), D.C., L.D.B., A.A., F.F., D.G., M.P., F.S.T., M.A. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the IReL Consortium. European Respiratory Society Clinical Research Collaboration (Prof Ignacio Martin-Loeches) for The European Network for ICU-Related Respiratory Infections (ENIRRIs) and Universidad de La Sabana [MED-244-2018].
Institutional Review Board Statement
The study received approval from the Institutional Review Board (Comité Ètic d’Investigació Clínica, registry number HCB/2020/0370). The IRB waived the requirement for informed consent as the study involved only prospective chart reviews. The study was registered in ClinicalTrials.gov with the Identifier NCT03183921.
Informed Consent Statement
Informed consent was waived by the IRB under the approved protocol (HCB/2020/0370) due to the nature of the study, which included only prospective chart reviews. All clinical data were anonymized and securely transferred to the coordinating center for data curation and analysis.
Data Availability Statement
Data and materials will be available upon request to the corresponding author.
Acknowledgments
ENIRRI Collaborators: Esteban Garcia-Gallo, Sara Duque, Natalia Sanabria, Yuli Viviana Fuentes, Francesco Blasi, Marta Di Pasquale, Paolo Maurizio Soave, Giorgia Spinazzola, Anselmo Caricato, Serena Silva, Mariachiara Ippolito, Federico Longhini, Andrea Bruni, Eugenio Garofalo, Vittoria Comellini, Luca Fasano, Angelo Pezzi, Valeria A Enciso-Prieto.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Martin-Loeches, I.; Reyes, L.F.; Nseir, S.; Ranzani, O.; Povoa, P.; Diaz, E.; Schultz, M.J.; Rodriguez, A.H.; Serrano-Mayorga, C.C.; De Pascale, G.; et al. European Network for ICU-Related Respiratory Infections (ENIRRIs): A multinational, prospective, cohort study of nosocomial LRTI. Intensive Care Med. 2023, 49, 1212–1222. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratala, J.; et al. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, e61–e111. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef]
- Ferrer, M.; Torres, A. Epidemiology of ICU-acquired pneumonia. Curr. Opin. Crit. Care 2018, 24, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Bekaert, M.; Timsit, J.F.; Vansteelandt, S.; Depuydt, P.; Vesin, A.; Garrouste-Orgeas, M.; Decruyenaere, J.; Clec’h, C.; Azoulay, E.; Benoit, D.; et al. Attributable mortality of ventilator-associated pneumonia: A reappraisal using causal analysis. Am. J. Respir. Crit. Care Med. 2011, 184, 1133–1139. [Google Scholar] [CrossRef]
- Costa, R.D.; Baptista, J.P.; Freitas, R.; Martins, P.J. Hospital-Acquired Pneumonia in a Multipurpose Intensive Care Unit: One-Year Prospective Study. Acta Med. Port. 2019, 32, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.; Grant, C.; Cooke, R.P.; Dempsey, G. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst. Rev. 2015, 2015, CD007577. [Google Scholar] [CrossRef]
- Magill, S.S.; Klompas, M.; Balk, R.; Burns, S.M.; Deutschman, C.S.; Diekema, D.; Fridkin, S.; Greene, L.; Guh, A.; Gutterman, D.; et al. Developing a new, national approach to surveillance for ventilator-associated events: Executive summary. Chest 2013, 144, 1448–1452. [Google Scholar] [CrossRef][Green Version]
- Papajk, J.; Uvizl, R.; Kolar, M. Effect of previous antibiotic therapy on the epidemiology of ventilator-associated pneumonia. Klin. Mikrobiol. Infekc. Lek. 2019, 25, 7–11. [Google Scholar] [PubMed]
- Parker, C.M.; Kutsogiannis, J.; Muscedere, J.; Cook, D.; Dodek, P.; Day, A.G.; Heyland, D.K. Canadian Critical Care Trials Group. Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: Prevalence, incidence, risk factors, and outcomes. J. Crit. Care 2008, 23, 18–26. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Klompas, M.; Kyeremanteng, K.; Mehta, S.; English, S.W.; Muscedere, J.; Cook, D.J.; Torres, A.; et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients-a systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-associated pneumonia in adults: A narrative review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef]
- Vincent, J.L.; Rello, J.; Marshall, J.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef]
- Shebl, E.; Gulick, P.G. Nosocomial Pneumonia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kumar, S.T.; Yassin, A.; Bhowmick, T.; Dixit, D. Recommendations from the 2016 Guidelines for the Management of Adults with Hospital-Acquired or Ventilator-Associated Pneumonia. Pharm. Ther. 2017, 42, 767–772. [Google Scholar]
- Erb, C.T.; Patel, B.; Orr, J.E.; Bice, T.; Richards, J.B.; Metersky, M.L.; Wilson, K.C.; Thomson, C.C. Management of Adults with Hospital-acquired and Ventilator-associated Pneumonia. Ann. Am. Thorac. Soc. 2016, 13, 2258–2260. [Google Scholar] [CrossRef]
- Zaragoza, R.; Vidal-Cortes, P.; Aguilar, G.; Borges, M.; Diaz, E.; Ferrer, R.; Maseda, E.; Nieto, M.; Nuvials, F.X.; Ramirez, P.; et al. Update of the treatment of nosocomial pneumonia in the ICU. Crit. Care 2020, 24, 383. [Google Scholar] [CrossRef]
- Shah, H.; Ali, A.; Patel, A.A.; Abbagoni, V.; Goswami, R.; Kumar, A.; Velasquez Botero, F.; Otite, E.; Tomar, H.; Desai, M.; et al. Trends and Factors Associated with Ventilator-Associated Pneumonia: A National Perspective. Cureus 2022, 14, e23634. [Google Scholar] [CrossRef]
- Bouza, E.; Guillen-Zabala, H.; Rojas, A.; Canada, G.; Cercenado, E.; Sanchez-Carrillo, C.; Martin-Rabadan, P.; Diez, C.; Puente, L.; Munoz, P.; et al. Comparative study of the etiology of nosocomial bacteremic pneumonia in ventilated and non-ventilated patients: A 10-year experience in an institution. Microbiol. Spectr. 2023, 11, e0151723. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.B.S.; Sacillotto, G.H.; Neves, M.F.B.; Silva, A.; Moimaz, T.A.; Gandolfi, J.V.; Nogueira, M.C.L.; Lobo, S.M. Prevalence, outcomes, and predictors of multidrug-resistant nosocomial lower respiratory tract infections among patients in an ICU. J. Bras. Pneumol. 2023, 49, e20220235. [Google Scholar] [PubMed]
- Equils, O.; da Costa, C.; Wible, M.; Lipsky, B.A. The effect of diabetes mellitus on outcomes of patients with nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus: Data from a prospective double-blind clinical trial comparing treatment with linezolid versus vancomycin. BMC Infect. Dis. 2016, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Yakoub, M.; Elkhwsky, F.; El Tayar, A.; El Sayed, I. Hospital-acquired pneumonia pattern in the intensive care units of a governmental hospital: A prospective longitudinal study. Ann. Afr. Med. 2023, 22, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, M.; Esperatti, M.; Crisafulli, E.; Ferrer, M.; Bassi, G.L.; Rinaudo, M.; Escorsell, A.; Fernandez, J.; Mas, A.; Blasi, F.; et al. Impact of chronic liver disease in intensive care unit acquired pneumonia: A prospective study. Intensive Care Med. 2013, 39, 1776–1784. [Google Scholar] [CrossRef]
- Maruyama, T.; Fujisawa, T.; Ishida, T.; Ito, A.; Oyamada, Y.; Fujimoto, K.; Yoshida, M.; Maeda, H.; Miyashita, N.; Nagai, H.; et al. A Therapeutic Strategy for All Pneumonia Patients: A 3-Year Prospective Multicenter Cohort Study Using Risk Factors for Multidrug-resistant Pathogens to Select Initial Empiric Therapy. Clin. Infect. Dis. 2019, 68, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Shetty, N.; Gadekari, S.; Thunga, G.; Rao, K.; Kunhikatta, V. Comparison of the Nosocomial Pneumonia Mortality Prediction (NPMP) model with standard mortality prediction tools. J. Hosp. Infect. 2017, 96, 250–255. [Google Scholar] [CrossRef]
- Iwashyna, T.J.; Hodgson, C.L.; Pilcher, D.; Bailey, M.; van Lint, A.; Chavan, S.; Bellomo, R. Timing of onset and burden of persistent critical illness in Australia and New Zealand: A retrospective, population-based, observational study. Lancet Respir. Med. 2016, 4, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Lameire, N.; KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit. Care 2013, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.T.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Executive Summary: Surviving Sepsis Campaign: International Guidelines for the Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).