Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Ducks in Hungary Between 2022 and 2023
Abstract
1. Introduction
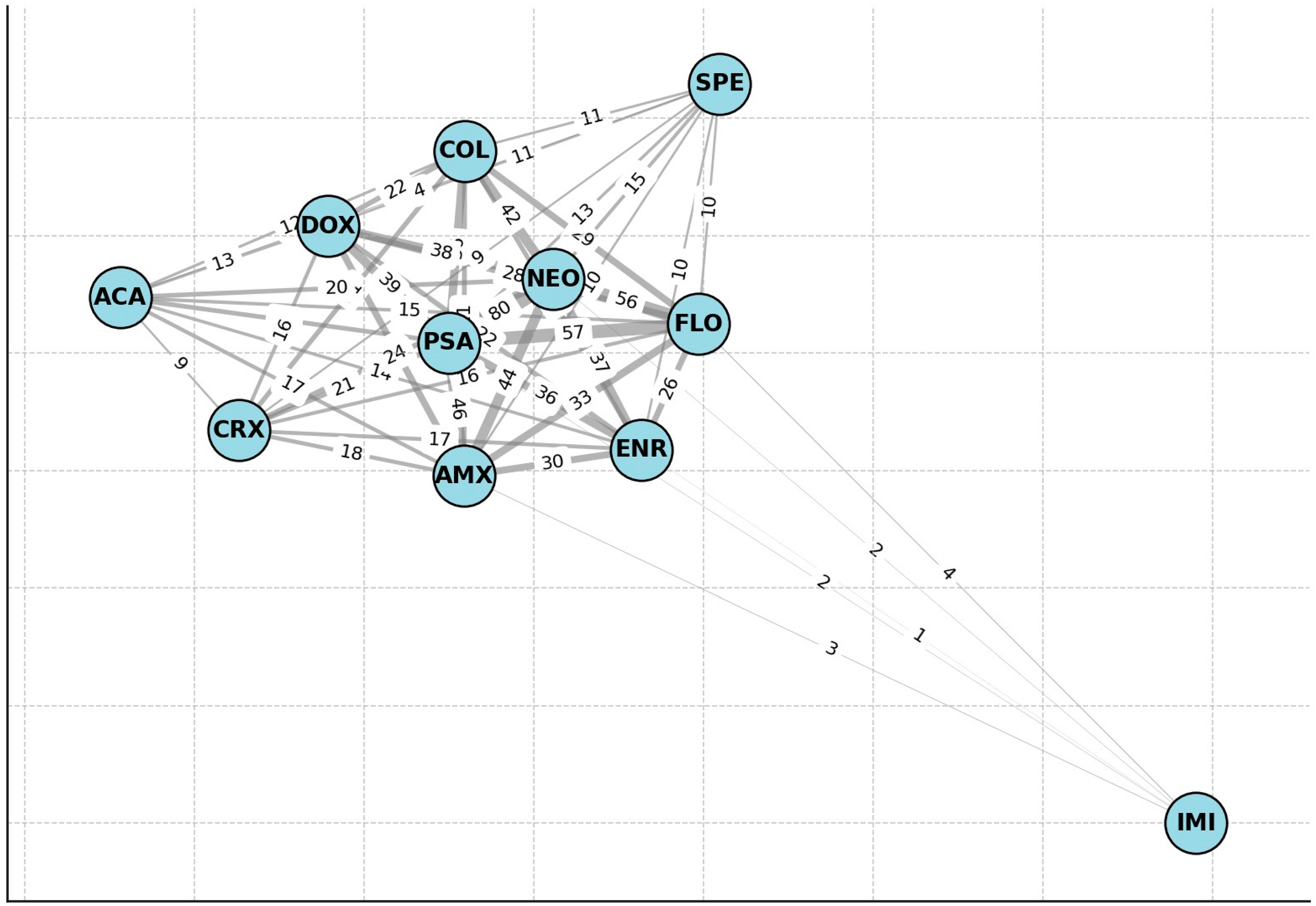
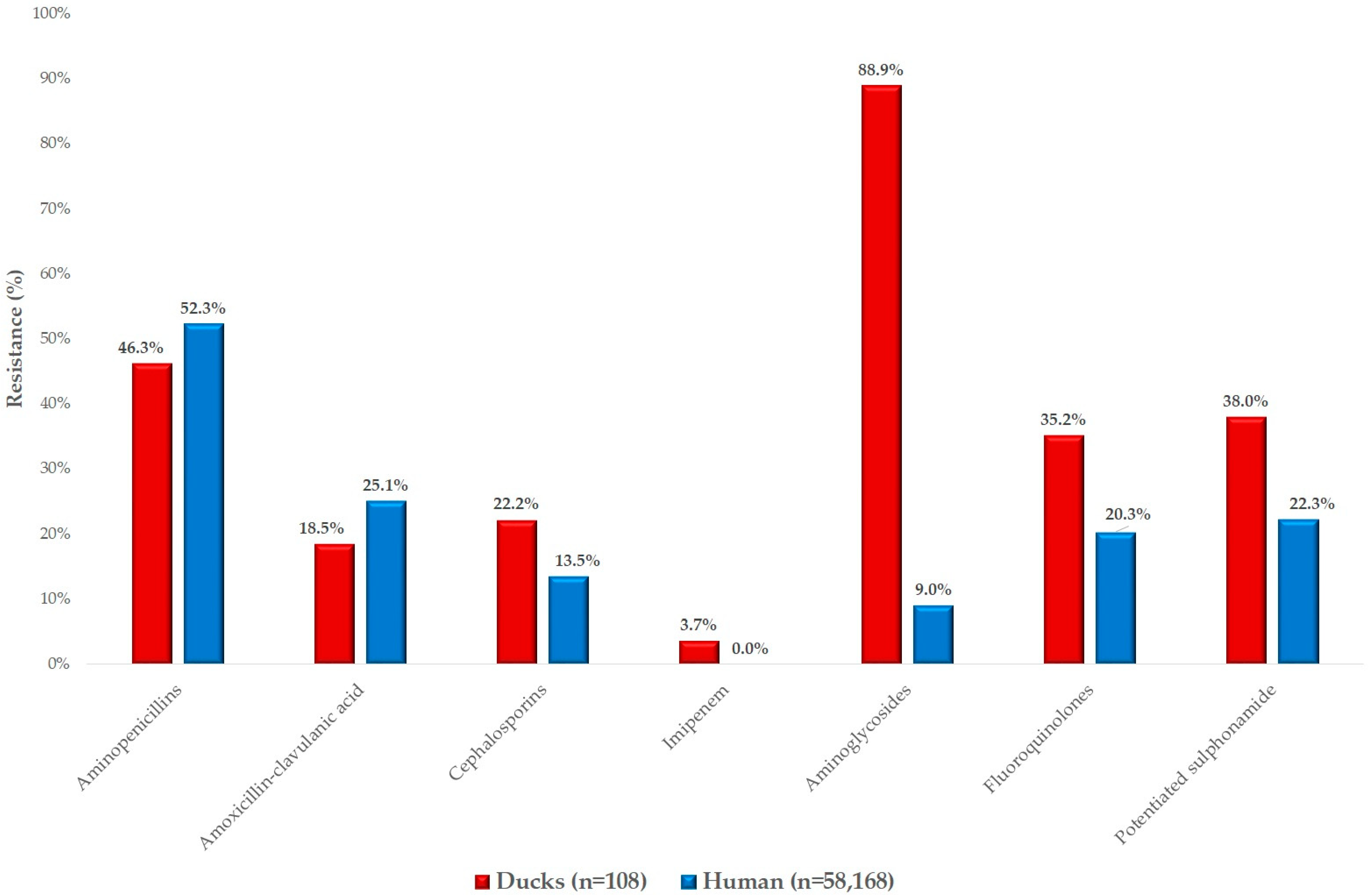
2. Results
3. Discussion
4. Materials and Methods
4.1. Origin of Bacterial Strains and Human Resistance Data
4.2. Minimum Inhibitory Concentration (MIC) Determination
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| CAMHB | Cation-adjusted Mueller Hinton Broth |
| CLSI | Clinical Laboratory Standards Institute |
| ECOFF | Epidemiological cut-off values |
| ESBL | Extended-spectrum beta-lactamases |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| MDR | Multidrug-resistant |
| MIC | Minimum inhibitory concentration |
| WHO | World Health Organization |
| XDR | Extensively drug-resistant |
References
- Akram, F.; Imtiaz, M.; Haq, I. ul Emergent Crisis of Antibiotic Resistance: A Silent Pandemic Threat to 21st Century. Microb. Pathog. 2023, 174, 105923. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, A.; Gupta, S.; Seth, S.; James, S.; Fatima, F.; Chaurasia, P.; Ramachandran, S. Knowledgebase of Potential Multifaceted Solutions to Antimicrobial Resistance. Comput. Biol. Chem. 2022, 101, 107772. [Google Scholar] [CrossRef]
- Zhou, N.; Cheng, Z.; Zhang, X.; Lv, C.; Guo, C.; Liu, H.; Dong, K.; Zhang, Y.; Liu, C.; Chang, Y.-F.; et al. Global Antimicrobial Resistance: A System-Wide Comprehensive Investigation Using the Global One Health Index. Infect. Dis. Poverty 2022, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Benmazouz, I.; Kövér, L.; Kardos, G. The Rise of Antimicrobial Resistance in Wild Birds: Potential AMR Sources and Wild Birds as AMR Reservoirs and Disseminators: Literature Review. Magy. Állatorvosok Lapja 2024, 146, 91–105. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- Eid, H.M.; Algammal, A.M.; Elfeil, W.K.; Youssef, F.M.; Harb, S.M.; Abd-Allah, E.M. Prevalence, Molecular Typing, and Antimicrobial Resistance of Bacterial Pathogens Isolated from Ducks. Vet. World 2019, 12, 677–683. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Abbas, M.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhu, D.; Zhao, X.; Wu, Y.; et al. High Incidence of Multi-Drug Resistance and Heterogeneity of Mobile Genetic Elements in Escherichia coli Isolates from Diseased Ducks in Sichuan Province of China. Ecotoxicol. Environ. Saf. 2021, 222, 112475. [Google Scholar] [CrossRef] [PubMed]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/ pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Kovács, L.; Hejel, P.; Farkas, M.; László, L. Könyves László Study Report on the Effect of a Litter Treatment Product Containing Bacillus licheniformis and Zeolite in Male Fattening Turkey Flock. Magy. Állatorvosok Lapja 2024, 146, 291–305. [Google Scholar] [CrossRef]
- Such, N.; Molnár, A.; Pál, L.; Farkas, V.; Menyhárt, L.; Husvéth, F.; Dublecz, K. The Effect of Pre- and Probiotic Treatment on the Gumboro-Titer Values of Broilers. Magy. Állatorvosok Lapja 2021, 143, 119–127. [Google Scholar]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of Fermented Wheat Germ Extract on Artificial Salmonella Typhimurium Infection in Broiler Chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antiprotozoal and Antifungal Efficiency of Propolis—Part 2. Magy. Állatorvosok Lapja 2022, 144, 691–704. [Google Scholar]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils–Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In Vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Petrilla, J.; Mátis, G.; Molnár, A.; Jerzsele, Á.; Pál, L.; Gálfi, P.; Neogrády, Z.; Dublecz, K. In Vitro Investigation of the Antibacterial Efficacy of Butyrate on Various Campylobacter jejuni Strains. MÁL 2021, 143, 57–64. [Google Scholar]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Jócsák, G.; Schilling-Tóth, B.; Bartha, T.; Tóth, I.; Ondrašovičová, S.; Kiss, D.S. Metal Nanoparticles-Immersion in the “tiny” World of Medicine. Magy. Állatorvosok Lapja 2025, 147, 115–127. [Google Scholar] [CrossRef]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of Antibiotic Alternatives in Pig Farming: Literature Review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Bintsis, T. Foodborne Pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Rehman, M.U.; Yang, H.; Yang, Z.; Wang, M.; Jia, R.; Chen, S.; Liu, M.; Zhu, D.; et al. Distribution and Association of Antimicrobial Resistance and Virulence Traits in Escherichia coli Isolates from Healthy Waterfowls in Hainan, China. Ecotoxicol. Environ. Saf. 2021, 220, 112317. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Delavari, P.; O’Bryan, T.T.; Smith, K.E.; Tatini, S. Contamination of Retail Foods, Particularly Turkey, from Community Markets (Minnesota, 1999-2000) with Antimicrobial-Resistant and Extraintestinal Pathogenic Escherichia coli. Foodborne Pathog. Dis. 2005, 2, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Arbab, S.; Ullah, H.; Wang, W.; Zhang, J. Antimicrobial Drug Resistance against Escherichia coli and Its Harmful Effect on Animal Health. Vet. Med. Sci. 2022, 8, 1780–1786. [Google Scholar] [CrossRef]
- Palaniappan, R.U.M.; Zhang, Y.; Chiu, D.; Torres, A.; DebRoy, C.; Whittam, T.S.; Chang, Y.-F. Differentiation of Escherichia coli Pathotypes by Oligonucleotide Spotted Array. J. Clin. Microbiol. 2006, 44, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Tivendale, K.A.; Logue, C.M.; Kariyawasam, S.; Jordan, D.; Hussein, A.; Li, G.; Wannemuehler, Y.; Nolan, L.K. Avian-Pathogenic Escherichia coli Strains Are Similar to Neonatal Meningitis E. coli Strains and Are Able to Cause Meningitis in the Rat Model of Human Disease. Infect. Immun. 2010, 78, 3412–3419. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.-G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and Public Health Implications—A Review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef]
- Anjum, M.F.; Schmitt, H.; Börjesson, S.; Berendonk, T.U.; Donner, E.; Stehling, E.G.; Boerlin, P.; Topp, E.; Jardine, C.; Li, X.; et al. The Potential of Using E. coli as an Indicator for the Surveillance of Antimicrobial Resistance (AMR) in the Environment. Curr. Opin. Microbiol. 2021, 64, 152–158. [Google Scholar] [CrossRef]
- Schrijver, R.; Stijntjes, M.; Rodríguez-Baño, J.; Tacconelli, E.; Rajendran, N.B.; Voss, A. Review of Antimicrobial Resistance Surveillance Programmes in Livestock and Meat in EU with Focus on Humans. Clin. Microbiol. Infect. 2018, 24, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Franiek, N.; Orth, D.; Grif, K.; Ewers, C.; Wieler, L.H.; Thalhammer, J.G.; Wuerzner, R. ESBL-producing E. coli and EHEC in dogs and cats in the Tyrol as possible source of human infection. Berl. Munch. Tierarztl. Wochenschr. 2012, 125, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Adorján, A.; Makrai, L.; Könyves, L.; Tóth, I. Enteropathogenic Escherichia coli (EPEC): Short Literature Summary. Magy. Állatorvosok Lapja 2021, 143, 429–438. [Google Scholar]
- da Silva, G.J.; Mendonça, N. Association between Antimicrobial Resistance and Virulence in Escherichia coli. Virulence 2012, 3, 18–28. [Google Scholar] [CrossRef]
- Dziva, F.; Stevens, M.P. Colibacillosis in Poultry: Unravelling the Molecular Basis of Virulence of Avian Pathogenic Escherichia coli in Their Natural Hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef]
- Soares, B.D.; de Brito, K.C.T.; Grassotti, T.T.; Filho, H.C.K.; de Camargo, T.C.L.; Carvalho, D.; Dorneles, I.C.; Otutumi, L.K.; Cavalli, L.S.; de Brito, B.G. Respiratory Microbiota of Healthy Broilers Can Act as Reservoirs for Multidrug-Resistant Escherichia coli. Comp. Immunol. Microbiol. Infect. Dis. 2021, 79, 101700. [Google Scholar] [CrossRef]
- de Oliveira, A.L.; Newman, D.M.; Sato, Y.; Noel, A.; Rauk, B.; Nolan, L.K.; Barbieri, N.L.; Logue, C.M. Characterization of Avian Pathogenic Escherichia coli (APEC) Associated With Turkey Cellulitis in Iowa. Front. Vet. Sci. 2020, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Janßen, T.; Wieler, L.H. Avian pathogenic Escherichia coli (APEC). Berl. Munch. Tierarztl. Wochenschr. 2003, 116, 381–395. [Google Scholar]
- Watts, A.; Wigley, P. Avian Pathogenic Escherichia coli: An Overview of Infection Biology, Antimicrobial Resistance and Vaccination. Antibiotics 2024, 13, 809. [Google Scholar] [CrossRef]
- Kong, H.; Hong, X.; Li, X. Current Perspectivesin Pathogenesis and Antimicrobial Resistance of Enteroaggregative Escherichia coli. Microb. Pathog. 2015, 85, 44–49. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Day, M.; Doumith, M.; Jenkins, C.; Dallman, T.J.; Hopkins, K.L.; Elson, R.; Godbole, G.; Woodford, N. Antimicrobial Resistance in Shiga Toxin-Producing Escherichia coli Serogroups O157 and O26 Isolated from Human Cases of Diarrhoeal Disease in England, 2015. J. Antimicrob. Chemother. 2017, 72, 145–152. [Google Scholar] [CrossRef]
- Lalak, A.; Wasyl, D.; Zając, M.; Skarżyńska, M.; Hoszowski, A.; Samcik, I.; Woźniakowski, G.; Szulowski, K. Mechanisms of Cephalosporin Resistance in Indicator Escherichia coli Isolated from Food Animals. Vet. Microbiol. 2016, 194, 69–73. [Google Scholar] [CrossRef]
- Aerts, M.; Battisti, A.; Hendriksen, R.; Kempf, I.; Teale, C.; Tenhagen, B.-A.; Veldman, K.; Wasyl, D.; Guerra, B.; Liebana, E.; et al. Technical Specifications on Harmonised Monitoring of Antimicrobial Resistance in Zoonotic and Indicator Bacteria from Food-Producing Animals and Food. EFSA J. 2019, 17, 5709. [Google Scholar] [CrossRef]
- Afayibo, D.J.A.; Zhu, H.; Zhang, B.; Yao, L.; Abdelgawad, H.A.; Tian, M.; Qi, J.; Liu, Y.; Wang, S. Isolation, Molecular Characterization, and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. Vet. Sci. 2022, 9, 319. [Google Scholar] [CrossRef]
- Yassin, A.K.; Gong, J.; Kelly, P.; Lu, G.; Guardabassi, L.; Wei, L.; Han, X.; Qiu, H.; Price, S.; Cheng, D.; et al. Antimicrobial Resistance in Clinical Escherichia coli Isolates from Poultry and Livestock, China. PLoS ONE 2017, 12, e0185326. [Google Scholar] [CrossRef] [PubMed]
- Varga, C.; Guerin, M.T.; Brash, M.L.; Slavic, D.; Boerlin, P.; Susta, L. Antimicrobial Resistance in Fecal Escherichia coli and Salmonella enterica Isolates: A Two-Year Prospective Study of Small Poultry Flocks in Ontario, Canada. BMC Vet. Res. 2019, 15, 464. [Google Scholar] [CrossRef]
- Jeong, J.; Lee, J.-Y.; Kang, M.-S.; Lee, H.-J.; Kang, S.-I.; Lee, O.-M.; Kwon, Y.-K.; Kim, J.-H. Comparative Characteristics and Zoonotic Potential of Avian Pathogenic Escherichia coli (APEC) Isolates from Chicken and Duck in South Korea. Microorganisms 2021, 9, 946. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Jarlier, V.; Nicolas, M.H.; Fournier, G.; Philippon, A. Extended Broad-Spectrum Beta-Lactamases Conferring Transferable Resistance to Newer Beta-Lactam Agents in Enterobacteriaceae: Hospital Prevalence and Susceptibility Patterns. Rev. Infect. Dis. 1988, 10, 867–878. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Saleha, A.A.; Jalila, A.; Zunita, Z. Risk Factors and Spatial Distribution of Extended Spectrum Beta-Lactamase-Producing-Escherichia coli at Retail Poultry Meat Markets in Malaysia: A Cross-Sectional Study. BMC Public Health 2016, 16, 699. [Google Scholar] [CrossRef]
- Cen, D.-J.; Sun, R.-Y.; Mai, J.-L.; Jiang, Y.-W.; Wang, D.; Guo, W.-Y.; Jiang, Q.; Zhang, H.; Zhang, J.-F.; Zhang, R.-M.; et al. Occurrence and Transmission of BlaNDM-Carrying Enterobacteriaceae from Geese and the Surrounding Environment on a Commercial Goose Farm. Appl. Environ. Microbiol. 2021, 87, e00087-21. [Google Scholar] [CrossRef]
- Mitra, S.; Sultana, S.A.; Prova, S.R.; Uddin, T.M.; Islam, F.; Das, R.; Nainu, F.; Sartini, S.; Chidambaram, K.; Alhumaydhi, F.A.; et al. Investigating Forthcoming Strategies to Tackle Deadly Superbugs: Current Status and Future Vision. Expert. Rev. Anti Infect. Ther. 2022, 20, 1309–1332. [Google Scholar] [CrossRef]
- Munguia, J.; Nizet, V. Pharmacological Targeting of the Host-Pathogen Interaction: Alternatives to Classical Antibiotics to Combat Drug-Resistant Superbugs. Trends Pharmacol. Sci. 2017, 38, 473–488. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Collineau, L.; Bourély, C.; Rousset, L.; Berger-Carbonne, A.; Ploy, M.-C.; Pulcini, C.; Colomb-Cotinat, M. Towards One Health Surveillance of Antibiotic Resistance: Characterisation and Mapping of Existing Programmes in Humans, Animals, Food and the Environment in France, 2021. Euro Surveill. 2023, 28, 2200804. [Google Scholar] [CrossRef] [PubMed]
- Coculescu, B.-I. Antimicrobial Resistance Induced by Genetic Changes. J. Med. Life 2009, 2, 114–123. [Google Scholar]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Whittaker, A.; Do, T.T.; Davis, M.D.M.; Barr, J. AMR Survivors? Chronic Living with Antimicrobial Resistant Infections. Glob. Public Health 2023, 18, 2217445. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial Resistance: A Global Emerging Threat to Public Health Systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef]
- Hossain, A.; Habibullah-Al-Mamun, M.; Nagano, I.; Masunaga, S.; Kitazawa, D.; Matsuda, H. Antibiotics, Antibiotic-Resistant Bacteria, and Resistance Genes in Aquaculture: Risks, Current Concern, and Future Thinking. Environ. Sci. Pollut. Res. Int. 2022, 29, 11054–11075. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Espinosa-Gongora, C.; Guardabassi, L. Human Health Risks Associated with Antimicrobial-Resistant Enterococci and Staphylococcus aureus on Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 130–140. [Google Scholar] [CrossRef]
- Sun, W.; Wang, D.; Yan, S.; Xue, Y. Characterization of Escherichia coli Strains Isolated from Geese by Detection of Integron-Mediated Antimicrobial Resistance. J. Glob. Antimicrob. Resist. 2022, 31, 10–14. [Google Scholar] [CrossRef] [PubMed]
- de Jong, A.; Garch, F.E.; Simjee, S.; Moyaert, H.; Rose, M.; Youala, M.; Siegwart, E. VetPath Study Group Monitoring of Antimicrobial Susceptibility of Udder Pathogens Recovered from Cases of Clinical Mastitis in Dairy Cows across Europe: VetPath Results. Vet. Microbiol. 2018, 213, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Elbediwi, M.; Nambiar, R.B.; Yang, H.; Lin, J.; Yue, M. Genomic Characterization of Antimicrobial-Resistant Salmonella enterica in Duck, Chicken, and Pig Farms and Retail Markets in Eastern China. Microbiol. Spectr. 2022, 10, e0125722. [Google Scholar] [CrossRef]
- Wang, X.-R.; Lian, X.-L.; Su, T.-T.; Long, T.-F.; Li, M.-Y.; Feng, X.-Y.; Sun, R.-Y.; Cui, Z.-H.; Tang, T.; Xia, J.; et al. Duck Wastes as a Potential Reservoir of Novel Antibiotic Resistance Genes. Sci. Total Environ. 2021, 771, 144828. [Google Scholar] [CrossRef]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food-Producing Animals: 2020 to 2030. PLoS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef]
- Munk, P.; Yang, D.; Röder, T.; Maier, L.; Petersen, T.N.; Duarte, A.S.R.; Clausen, P.T.L.C.; Brinch, C.; Van Gompel, L.; Luiken, R.; et al. The European Livestock Resistome. mSystems 2024, 9, e0132823. [Google Scholar] [CrossRef]
- ISO 16649-2:2001. Available online: https://www.iso.org/standard/29824.html (accessed on 27 April 2025).
- Clinical and Laboratory Standards Institute CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume CLSI standards M07. [Google Scholar]
- Brian, V.L. VET01SEd5 | Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 5th Edition. Available online: https://clsi.org/standards/products/veterinary-medicine/documents/vet01s/ (accessed on 8 May 2022).
- EUCAST: MIC and Zone Distributions and ECOFFs. Available online: https://www.eucast.org/mic_distributions_and_ecoffs/ (accessed on 1 May 2022).
- Boulianne, M.; Arsenault, J.; Daignault, D.; Archambault, M.; Letellier, A.; Dutil, L. Drug Use and Antimicrobial Resistance among Escherichia coli and Enterococcus spp. Isolates from Chicken and Turkey Flocks Slaughtered in Quebec, Canada. Can. J. Vet. Res. 2016, 80, 49–59. [Google Scholar]
- Lima-Filho, J.V.; Martins, L.V.; de Oliveira Nascimento, D.C.; Ventura, R.F.; Batista, J.E.C.; Silva, A.F.B.; Ralph, M.T.; Vaz, R.V.; Rabello, C.B.-V.; da Silva, I.d.M.M.; et al. Zoonotic Potential of Multidrug-Resistant Extraintestinal Pathogenic Escherichia coli Obtained from Healthy Poultry Carcasses in Salvador, Brazil. Braz. J. Infect. Dis. 2013, 17, 54–61. [Google Scholar] [CrossRef]
- Hesp, A.; van Schaik, G.; Wiegel, J.; Heuvelink, A.; Mevius, D.; Veldman, K. Antimicrobial Resistance Monitoring in Commensal and Clinical Escherichia coli from Broiler Chickens: Differences and Similarities. Prev. Vet. Med. 2022, 204, 105663. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-Test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]

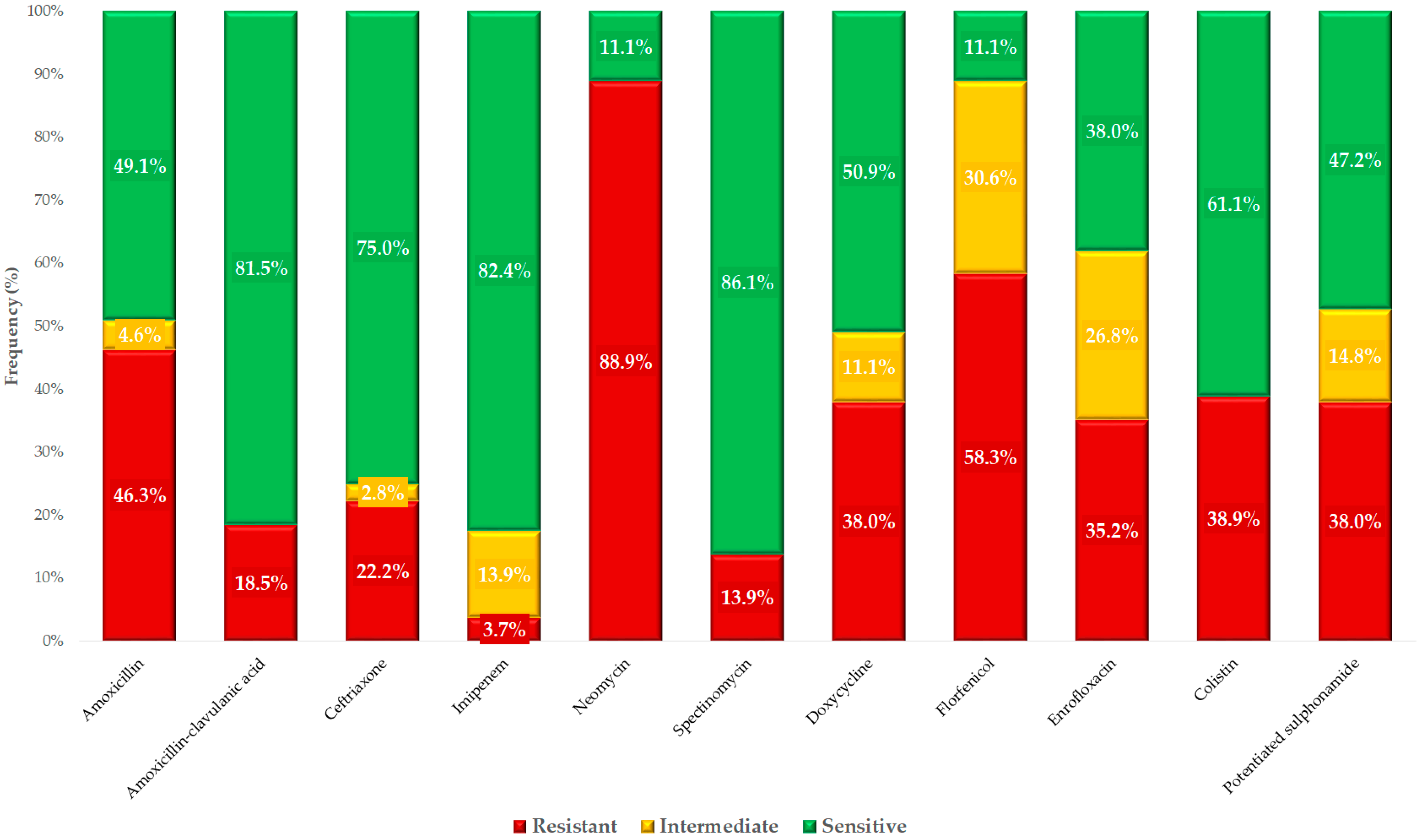




| Antibiotics | Breakpoint | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.031 | 0.063 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | ECOFF 3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (µg/mL) | |||||||||||||||||||||||||
| Amoxicillin | 32 | 5 | 0 | 4 | 22 | 22 | 5 | 2 | 27 | 3 | 5 | 5 | 8 | 16 | 512 | 8 | |||||||||
| 4.6% | 0.0% | 3.7% | 20.4% | 20.4% | 4.6% | 1.9% | 25.0% | 2.8% | 4.6% | 4.6% | 7.4% | ||||||||||||||
| Amoxicillin-clavulanic acid 1 | 32 | 1 | 0 | 0 | 0 | 5 | 1 | 7 | 29 | 25 | 20 | 7 | 10 | 1 | 1 | 1 | 8 | 64 | 8 | ||||||
| 0.9% | 0.0% | 0.0% | 0.0% | 4.6% | 0.9% | 6.5% | 26.9% | 23.1% | 18.5% | 6.5% | 9.3% | 0.9% | 0.9% | 0.9% | |||||||||||
| Ceftriaxone | 4 | 5 | 23 | 28 | 7 | 8 | 4 | 6 | 3 | 0 | 2 | 3 | 8 | 3 | 3 | 3 | 1 | 1 | 0.06 | 32 | 0.125 | ||||
| 4.6% | 21.3% | 25.9% | 6.5% | 7.4% | 3.7% | 5.6% | 2.8% | 0.0% | 1.9% | 2.8% | 7.4% | 2.8% | 2.8% | 2.8% | 0.9% | 0.9% | |||||||||
| Colistin | 2 | 3 | 2 | 6 | 28 | 16 | 11 | 5 | 1 | 2 | 1 | 16 | 0 | 2 | 1 | 4 | 10 | 0.5 | 512 | 2 | |||||
| 2.8% | 1.9% | 5.6% | 25.9% | 14.8% | 10.2% | 4.6% | 0.9% | 1.9% | 0.9% | 14.8% | 0.0% | 1.9% | 0.9% | 3.7% | 9.3% | ||||||||||
| Doxycycline | 16 | 1 | 2 | 6 | 33 | 13 | 12 | 10 | 18 | 7 | 6 | 4 | 64 | 8 | |||||||||||
| 0.9% | 1.9% | 5.6% | 30.6% | 12.0% | 11.1% | 9.3% | 16.7% | 6.5% | 5.6% | ||||||||||||||||
| Enrofloxacin | 2 | 3 | 22 | 6 | 7 | 3 | 22 | 7 | 3 | 4 | 2 | 4 | 14 | 2 | 8 | 0 | 1 | 0.5 | 32 | 0.125 | |||||
| 2.8% | 20.4% | 5.6% | 6.5% | 2.8% | 20.4% | 6.5% | 2.8% | 3.7% | 1.9% | 3.7% | 13.0% | 1.9% | 7.4% | 0.0% | 0.9% | ||||||||||
| Florfenicol | 16 | 2 | 10 | 33 | 34 | 13 | 3 | 10 | 2 | 1 | 16 | 128 | 16 | ||||||||||||
| 1.9% | 9.3% | 30.6% | 31.5% | 12.0% | 2.8% | 9.3% | 1.9% | 0.9% | |||||||||||||||||
| Imipenem | 4 | 1 | 0 | 1 | 4 | 8 | 21 | 26 | 28 | 15 | 1 | 2 | 1 | 0.5 | 16 | 0.5 | |||||||||
| 0.9% | 0.0% | 0.9% | 3.7% | 7.4% | 19.4% | 24.1% | 25.9% | 13.9% | 0.9% | 1.9% | 0.9% | ||||||||||||||
| Neomycin | 32 | 1 | 2 | 9 | 39 | 43 | 5 | 1 | 6 | 2 | 64 | 128 | 8 | ||||||||||||
| 0.9% | 1.9% | 8.3% | 36.1% | 39.8% | 4.6% | 0.9% | 5.6% | 1.9% | |||||||||||||||||
| Potentiated sulphonamide 2 | 4 | 4 | 14 | 21 | 12 | 12 | 4 | 2 | 2 | 19 | 5 | 13 | 16 | 1024 | 0.5 | ||||||||||
| 3.7% | 13.0% | 19.4% | 11.1% | 11.1% | 3.7% | 1.9% | 1.9% | 17.6% | 4.6% | 12.0% | |||||||||||||||
| Spectinomycin | 128 | 1 | 0 | 0 | 23 | 69 | 8 | 4 | 0 | 3 | 64 | 128 | 64 | ||||||||||||
| 0.9% | 0.0% | 0.0% | 21.3% | 63.9% | 7.4% | 3.7% | 0.0% | 2.8% | |||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerek, Á.; Szabó, Á.; Jerzsele, Á. Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Ducks in Hungary Between 2022 and 2023. Antibiotics 2025, 14, 491. https://doi.org/10.3390/antibiotics14050491
Kerek Á, Szabó Á, Jerzsele Á. Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Ducks in Hungary Between 2022 and 2023. Antibiotics. 2025; 14(5):491. https://doi.org/10.3390/antibiotics14050491
Chicago/Turabian StyleKerek, Ádám, Ábel Szabó, and Ákos Jerzsele. 2025. "Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Ducks in Hungary Between 2022 and 2023" Antibiotics 14, no. 5: 491. https://doi.org/10.3390/antibiotics14050491
APA StyleKerek, Á., Szabó, Á., & Jerzsele, Á. (2025). Antimicrobial Susceptibility Profiles of Escherichia coli Isolates from Clinical Cases of Ducks in Hungary Between 2022 and 2023. Antibiotics, 14(5), 491. https://doi.org/10.3390/antibiotics14050491






