Phenotypic and Genotypic Characterization of ESBL-, AmpC-, and Carbapenemase-Producing Klebsiella pneumoniae and High-Risk Escherichia coli CC131, with the First Report of ST1193 as a Causative Agent of Urinary Tract Infections in Human Patients in Algeria
Abstract
1. Introduction
2. Results
2.1. Escherichia coli Collection
2.1.1. Antimicrobial Susceptibility Testing (AST) and Genotypic Characterization of bla Genes
2.1.2. Virulence Traits
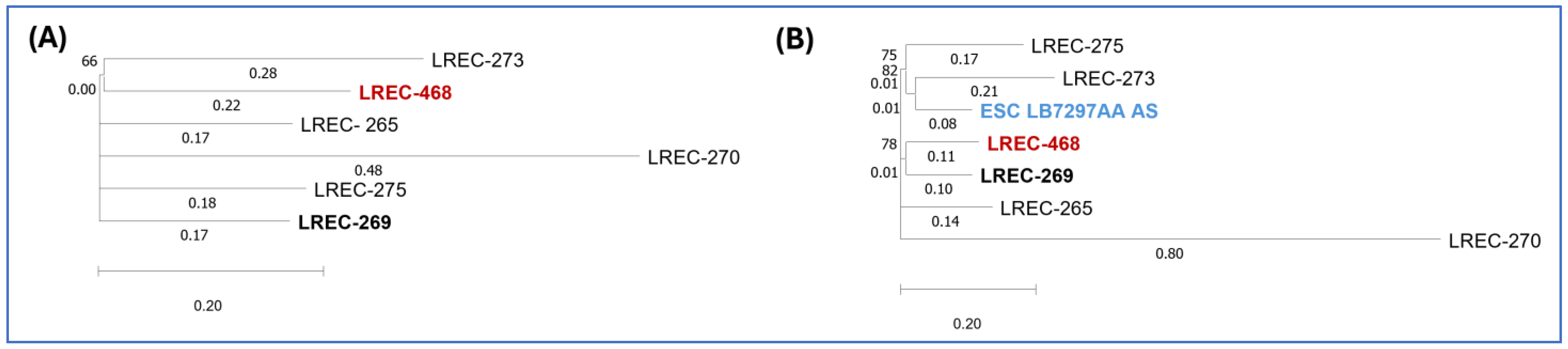
2.1.3. Clonal Groups
| Phylogroup | Clonotype (CH) a | No Isolates | UPEC Status b | ExPEC Status c | FQR |
|---|---|---|---|---|---|
| B2 | CH40-30 | 17 | 15 | 16 | 17 |
| CH14-64 | 1 | 1 | 1 | 1 | |
| CH108-Neg | 2 | 2 | 2 | 1 | |
| CH1012-Neg | 1 | 0 | 0 | 1 | |
| D | CH26-5 | 6 | 0 | 6 | 1 |
| CH26-Neg | 3 | 0 | 3 | 0 | |
| B1 | CH65-27 | 2 | 0 | 0 | 0 |
| CH27-54 | 1 | 0 | 0 | 0 | |
| CH65-32 | 1 | 0 | 0 | 0 | |
| CH7-604 | 1 | 0 | 0 | 0 | |
| A | CH11-54 | 1 | 0 | 1 | 1 |
| CH11-Neg | 1 | 0 | 0 | 1 | |
| CH7-94 | 1 | 0 | 0 | 0 | |
| F | CH88-Neg | 2 | 0 | 0 | 2 |
Characterization of Clonal Complex (CC) 131 Isolates
In Silico Characterization of ST1193 Isolate
2.2. Klebsiella pneumoniae Collection
2.2.1. AST and Genotypic Characterization of bla Genes
2.2.2. Virulence Traits
3. Discussion
4. Materials and Methods
4.1. E. coli and K. pneumoniae Collections
4.2. Antimicrobial Susceptibility Testing (AST)
4.3. Detection and Typing of Antimicrobial Resistance Genes
4.4. Molecular Characterization of E. coli: Virulence Traits, Phylogroup, Clonotype, and Virotype Assignment
4.5. Phenotypic and Genotypic Detection of Hypervirulent K. pneumoniae
4.6. Whole Genome Sequencing (WGS) and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public. Health 2022, 10, 888205. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, R.; Murt, A. Epidemiology of urological infections: A global burden. World J. Urol. 2020, 38, 2669–2679. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- He, Y.; Zhao, J.; Wang, L.; Han, C.; Yan, R.; Zhu, P.; Qian, T.; Yu, S.; Zhu, X.; He, W.H. Epidemiological trends and predictions of urinary tract infections in the global burden of disease study 2021. Sci. Rep. 2025, 15, 4702. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, H.; Zi, H.; Hu, H.; Li, B.; Huang, J.; Luo, P.; Zeng, X. Global and Regional Burden of Bacterial Antimicrobial Resistance in Urinary Tract Infections in 2019. J. Clin. Med. 2022, 11, 2817. [Google Scholar] [CrossRef]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global Extraintestinal Pathogenic Escherichia coli (ExPEC) Lineages. Clin. Microbiol. Rev. 2019, 32, e00135-18. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; Lumbreras, P.; Lestón, L.; Álvarez-Álvarez, M.; García, V.; Hammerl, J.A.; Fernández, J.; Mora, A. Occurrence and Genomic Characterization of Clone ST1193 Clonotype 14-64 in Uncomplicated Urinary Tract Infections Caused by Escherichia coli in Spain. Microbiol. Spectr. 2022, 10, e0004122. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; García, V.; Lumbreras-Iglesias, P.; Fernández, J.; Mora, A. Fluoroquinolone resistance in complicated urinary tract infections: Association with the increased occurrence and diversity of clonal complex 131, together with ST1193. Front. Cell Infect. Microbiol. 2024, 14, 1351618. [Google Scholar] [CrossRef]
- Pitout, J.D.D.; Peirano, G.; Chen, L.; DeVinney, R.; Matsumura, Y. Escherichia coli ST1193: Following in the Footsteps of E. coli ST131. Antimicrob. Agents Chemother. 2022, 66, e0051122. [Google Scholar] [CrossRef]
- Cummins, E.A.; Snaith, A.E.; McNally, A.; Hall, R.J. The role of potentiating mutations in the evolution of pandemic Escherichia coli clones. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- WHO’s List of Medically Important Antimicrobials: A Risk Management Tool for Mitigating Antimicrobial Resistance Due to Non-Human Use; World Health Organization: Geneva, Switzerland, 2024.
- Ait-Mimoune, N.; Hassaine, H.; Boulanoir, M. Bacteriological profile of urinary tract infections and antibiotic susceptibility of Escherichia coli in Algeria. Iran. J. Microbiol. 2022, 14, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Bakour, S.; Sahli, F.; Touati, A.; Rolain, J.M. Emergence of KPC-producing Klebsiella pneumoniae ST512 isolated from cerebrospinal fluid of a child in Algeria. New Microbes New Infect. 2015, 3, 34–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brahmia, S.; Lalaoui, R.; Nedjai, S.; Djahmi, N.; Chettibi, S.; Rolain, J.M.; Bakour, S. First Clinical Cases of KPC-2-Producing Klebsiella pneumoniae ST258 in Algeria and Outbreak of Klebsiella pneumoniae ST101 Harboring blaOXA-48 Gene in the Urology Department of Annaba Hospital. Microb. Drug Resist. 2021, 27, 652–659. [Google Scholar] [CrossRef]
- Benbrahim, C.; Barka, M.S.; Benmahdi, L.; Zatout, A.; Khadir, A. Klebsiella pneumoniae producing extended spectrum β-lactamase in Regional Military University Hospital of Oran, Algeria: Antibiotic resistance, biofilm formation, and detection of blaCTX-M and blaTEM genes. Afr. J. Clin. Exper Microbiol. 2021, 22, 28–37. [Google Scholar] [CrossRef]
- Fares, R.; Debabza, M.; Mechai, A. Detection prevalence of extended spectrum β-lactamases production among Enterobacteriaceae isolated from urinary tract infections. Biosyst. Divers. 2023, 31, 163–169. [Google Scholar] [CrossRef]
- Nabti, L.Z.; Sahli, F.; Radji, N.; Mezaghcha, W.; Semara, L.; Aberkane, S.; Lounnas, M.; Solassol, J.; Didelot, M.N.; Jean-Pierre, H.; et al. High Prevalence of Multidrug-Resistant Escherichia coli in Urine Samples from Inpatients and Outpatients at a Tertiary Care Hospital in Sétif, Algeria. Microb. Drug Resist. 2019, 25, 386–393. [Google Scholar] [CrossRef]
- Zenati, F.; Barguigua, A.; Nayme, K.; Benbelaïd, F.; Khadir, A.; Bellahsene, C.; Bendahou, M.; Hafida, H.; Timinouni, M. Characterization of uropathogenic ESBL-producing Escherichia coli isolated from hospitalized patients in western Algeria. J. Infect. Dev. Ctries. 2019, 13, 291–302. [Google Scholar] [CrossRef]
- Touati, A.; Mairi, A. Carbapenemase-Producing Enterobacterales in Algeria: A Systematic Review. Microb. Drug Resist. 2020, 26, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Duarte, O.G.; Arzuza, O.; Urbina, D.; Bai, J.; Guerra, J.; Montes, O.; Puello, M.; Mendoza, K.; Castro, G.Y. Detection of Escherichia coli enteropathogens by multiplex polymerase chain reaction from children’s diarrheal stools in two Caribbean-Colombian cities. Foodborne Pathog. Dis. 2010, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Spurbeck, R.R.; Dinh, P.C.; Walk, S.T.; Stapleton, A.E.; Hooton, T.M.; Nolan, L.K.; Kim, K.S.; Johnson, J.R.; Mobley, H.L.T. Escherichia coli isolates that carry vat, fyuA, chuA, and yfcV efficiently colonize the urinary tract. Infect. Immun. 2012, 80, 4115–4122. [Google Scholar] [CrossRef]
- Johnson, J.R.; Murray, A.C.; Gajewski, A.; Sullivan, M.; Snippes, P.; Kuskowski, M.A.; Smith, K.E. Isolation and molecular characterization of nalidixic acid-resistant extraintestinal pathogenic Escherichia coli from retail chicken products. Antimicrob. Agents Chemother. 2003, 47, 2161–2168. [Google Scholar] [CrossRef]
- Clermont, O.; Christenson, J.K.; Denamur, E.; Gordon, D.M. The Clermont Escherichia coli phylo-typing method revisited: Improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 2013, 5, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Dixit, O.V.A.; Vangchhia, B.; Condamine, B.; Dion, S.; Bridier-Nahmias, A.; Denamur, E.; Gordon, D. Characterization and rapid identification of phylogroup G in Escherichia coli, a lineage with high virulence and antibiotic resistance potential. Environ. Microbiol. 2019, 21, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Alonso, M.P.; Nicolas-Chanoine, M.H.; Dahbi, G.; Mora, A.; Blanco, J.E.; López, C.; Cortés, P.; Llagostera, M.; Leflon-Guibout, V.; et al. Molecular epidemiology of Escherichia coli producing extended-spectrum {beta}-lactamases in Lugo (Spain): Dissemination of clone O25b:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2009, 63, 1135–1141. [Google Scholar] [CrossRef]
- Clermont, O.; Dhanji, H.; Upton, M.; Gibreel, T.; Fox, A.; Boyd, D.; Mulvey, M.R.; Nordmann, P.; Ruppé, E.; Sarthou, J.L.; et al. Rapid detection of the O25b-ST131 clone of Escherichia coli encompassing the CTX-M-15-producing strains. J. Antimicrob. Chemother. 2009, 64, 274–277. [Google Scholar] [CrossRef]
- Dahbi, G.; Mora, A.; Mamani, R.; López, C.; Alonso, M.P.; Marzoa, J.; Blanco, M.; Herrera, A.; Viso, S.; García-Garrote, F.; et al. Molecular epidemiology and virulence of Escherichia coli O16:H5-ST131: Comparison with H30 and H30-Rx subclones of O25b:H4-ST131. Int. J. Med. Microbiol. 2014, 304, 1247–1257. [Google Scholar] [CrossRef]
- Weissman, S.J.; Johnson, J.R.; Tchesnokova, V.; Billig, M.; Dykhuizen, D.; Riddell, K.; Peggy, R.; Qin, X.; Butler-Wu, S.; Cookson, B.T.; et al. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Tchesnokova, V.; Johnston, B.; Clabots, C.; Roberts, P.L.; Billig, M.; Riddell, K.; Rogers, P.; Qin, X.; Butler-Wu, S.; et al. Abrupt emergence of a single dominant multidrug-resistant strain of Escherichia coli. J. Infect. Dis. 2013, 207, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef]
- Jaureguy, F.; Landraud, L.; Passet, V.; Diancourt, L.; Frapy, E.; Guigon, G.; Carbonnelle, E.; Lortholary, O.; Clermont, O.; Denamur, E.; et al. Phylogenetic and genomic diversity of human bacteremic Escherichia coli strains. BMC Genom. 2008, 9, 560. [Google Scholar] [CrossRef] [PubMed]
- Shon, A.S.; Bajwa, R.P.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Olson, R.; Fang, C.T.; Stoesser, N.; Miller, M.; MacDonald, U.; Hutson, A.; Barker, J.H.; La Hoz, R.M.; Hohnson, J.R. Identification of Biomarkers for Differentiation of Hypervirulent Klebsiella pneumoniae from Classical K. pneumoniae. J. Clin. Microbiol. 2018, 56, e00776-18. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation (IHME), University of Oxford. MICROBE. Available online: https://vizhub.healthdata.org/microbe (accessed on 23 March 2025).
- Peirano, G.; Pitout, J.D.D. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae: Update on Molecular Epidemiology and Treatment Options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Peirano, G.; Pitout, J.D.D. Rapidly spreading Enterobacterales with OXA-48-like carbapenemases. J. Clin. Microbiol. 2025, 63, e0151524. [Google Scholar] [CrossRef]
- López-Cerero, L.; Salamanca, E.; Delgado-Valverde, M.; Rodríguez-Martínez, J.M.; Rodríguez-Baño, J.; Pascual, Á. Higher prevalence of CTX-M-27-producing Escherichia coli belonging to ST131 clade C1 among residents of two long-term care facilities in Southern Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 335–338. [Google Scholar] [CrossRef]
- Spurbeck, R.R.; Mobley, H.L.T. Chapter 9—Uropathogenic Escherichia coli. In Escherichia coli, Pathotypes and Principles of Pathogenesis, 2nd ed.; Donnenberg, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2013; pp. 275–304. [Google Scholar]
- Wang, M.C.; Fan, Y.H.; Zhang, Y.Z.; Bregente, C.J.B.; Lin, W.H.; Chen, C.A.; Lin, T.L.; Kao, C.Y. Characterization of uropathogenic Escherichia coli phylogroups associated with antimicrobial resistance, virulence factor distribution, and virulence-related phenotypes. Infect. Genet. Evol. 2023, 114, 105493. [Google Scholar] [CrossRef] [PubMed]
- Colpan, A.; Johnston, B.; Porter, S.; Clabots, C.; Anway, R.; Thao, L.; Kuskowski, M.A.; Tchesnokova, V.; Solurenko, E.V.; Johnson, J.R. Escherichia coli sequence type 131 (ST131) subclone H30 as an emergent multidrug-resistant pathogen among US veterans. Clin. Infect. Dis. 2013, 57, 1256–1265. [Google Scholar] [CrossRef]
- Banerjee, R.; Johnson, J.R. A new clone sweeps clean: The enigmatic emergence of Escherichia coli sequence type 131. Antimicrob. Agents Chemother. 2014, 58, 4997–5004. [Google Scholar] [CrossRef] [PubMed]
- Pitout, J.D.; DeVinney, R. ST131: A multidrug-resistant clone primed for global domination. F1000Research 2017, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Baba Ahmed-Kazi Tani, Z.; Decré, D.; Genel, N.; Boucherit-Otmani, Z.; Arlet, G.; Drissi, M. Molecular and epidemiological characterization of enterobacterial multidrug-resistant strains in Tlemcen Hospital (Algeria) (2008–2010). Microb. Drug Resist. 2013, 19, 185–190. [Google Scholar] [CrossRef]
- Agabou, A.; Pantel, A.; Ouchenane, Z.; Lezzar, N.; Khemissi, S.; Satta, D.; Lavigne, J.P. First description of OXA-48-producing Escherichia coli and the pandemic clone ST131 from patients hospitalised at a military hospital in Algeria. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1641–1646. [Google Scholar] [CrossRef]
- Yahiaoui, M.; Robin, F.; Bakour, R.; Hamidi, M.; Bonnet, R.; Messai, Y. Antibiotic Resistance, Virulence, and Genetic Background of Community-Acquired Uropathogenic Escherichia coli from Algeria. Microb. Drug Resist. 2015, 21, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Brahmi, S.; Dunyach-Rémy, C.; Touati, A.; Lavigne, J.P. CTX-M-15-producing Escherichia coli and the pandemic clone O25b-ST131 isolated from wild fish in Mediterranean Sea. Clin. Microbiol. Infect. 2015, 21, e18–e20. [Google Scholar] [CrossRef] [PubMed]
- Chenouf, N.S.; Carvalho, I.; Messaï, C.R.; Ruiz-Ripa, L.; Mama, O.M.; Titouche, Y.; Zitouni, A.; Hakem, A.; Torres, C. Extended Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Brolier Liver in the Center of Algeria, with Detection of CTX-M-55 and B2/ST131-CTX-M-15 in Escherichia coli. Microb. Drug Resist. 2021, 27, 268–276. [Google Scholar] [CrossRef]
- Bachiri, T.; Bakour, S.; Ladjouzi, R.; Thongpan, L.; Rolain, J.M.; Touati, A. High rates of CTX-M-15-producing Escherichia coli and Klebsiella pneumoniae in wild boars and Barbary macaques in Algeria. J. Glob. Antimicrob. Resist. 2017, 8, 35–40. [Google Scholar] [CrossRef]
- Johnson, T.J.; Elnekave, E.; Miller, E.A.; Munoz-Aguayo, J.; Flores Figueroa, C.; Johnston, B.; Nielson, D.W.; Logue, C.M.; Johnson, J.R. Phylogenomic Analysis of Extraintestinal Pathogenic Escherichia coli Sequence Type 1193, an Emerging Multidrug-Resistant Clonal Group. Antimicrob. Agents Chemother. 2019, 63, e01913-18. [Google Scholar] [CrossRef] [PubMed]
- Wyrsch, E.R.; Bushell, R.N.; Marenda, M.S.; Browning, G.F.; Djordjevic, S.P. Global Phylogeny and F Virulence Plasmid Carriage in Pandemic Escherichia coli ST1193. Microbiol. Spectr. 2022, 10, e0255422. [Google Scholar] [CrossRef]
- Birgy, A.; Madhi, F.; Jung, C.; Levy, C.; Cointe, A.; Bidet, P.; Hobson, C.A.; Bechet, S.; Sobral, E.; Vuthien, H.; et al. Diversity and trends in population structure of ESBL-producing Enterobacteriaceae in febrile urinary tract infections in children in France from 2014 to 2017. J. Antimicrob. Chemother. 2020, 75, 96–105. [Google Scholar] [CrossRef]
- Valenza, G.; Werner, M.; Eisenberger, D.; Nickel, S.; Lehner-Reindl, V.; Höller, C.; Lehner-Reindl, V.; Höller, C.; Bagdan, C. First report of the new emerging global clone ST1193 among clinical isolates of extended-spectrum β-lactamase (ESBL)-producing Escherichia coli from Germany. J. Glob. Antimicrob. Resist. 2019, 17, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, J.; Yao, K.; Gao, W.; Wang, Y. Molecular characteristics of the new emerging global clone ST1193 among clinical isolates of Escherichia coli from neonatal invasive infections in China. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Oh, T.; Nam, Y.S.; Cho, S.Y.; Lee, H.J. Prevalence of ST131 and ST1193 Among Bloodstream Isolates of Escherichia coli not Susceptible to Ciprofloxacin in a Tertiary Care University Hospital in Korea, 2013–2014. Clin. Lab. 2017, 63, 1541–1543. [Google Scholar] [CrossRef]
- Nguyen, Q.; Nguyen, T.T.N.; Pham, P.; Chau, V.; Nguyen, L.P.H.; Nguyen, T.D.; Ha, T.T.; Le, N.T.Q.; Vu, D.T.; Baker, S.; et al. Genomic insights into the circulation of pandemic fluoroquinolone-resistant extra-intestinal pathogenic Escherichia coli ST1193 in Vietnam. Microb. Genom. 2021, 7, 000733. [Google Scholar] [CrossRef]
- Tchesnokova, V.L.; Rechkina, E.; Larson, L.; Ferrier, K.; Weaver, J.L.; Schroeder, D.W.; She, R.; Butler-Wu, S.M.; Aguero-Rosenfeld, M.E.; Zerr, D.; et al. Rapid and Extensive Expansion in the United States of a New Multidrug-resistant Escherichia coli Clonal Group, Sequence Type 1193. Clin. Infect. Dis. 2019, 68, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Yehouenou, C.L.; Bogaerts, B.; De Keersmaecker, S.C.J.; Roosens, N.H.C.; Marchal, K.; Tchiakpe, E.; Affolabi, D.; Simon, A.; Dossou, F.M.; Vanneste, K.; et al. Whole-Genome Sequencing-Based Antimicrobial Resistance Characterization and Phylogenomic Investigation of 19 Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Positive Escherichia coli Strains Collected From Hospital Patients in Benin in 2019. Front. Microbiol. 2021, 12, 752883. [Google Scholar] [CrossRef] [PubMed]
- Byarugaba, D.K.; Erima, B.; Wokorach, G.; Alafi, S.; Kibuuka, H.; Mworozi, E.; Musinguzi, A.K.; Kiyengo, J.; Najjuka, F.; Wabwire-Mangen, F. Resistome and virulome of high-risk pandemic clones of multidrug-resistant extra-intestinal pathogenic Escherichia coli (ExPEC) isolated from tertiary healthcare settings in Uganda. PLoS ONE 2023, 18, e0294424. [Google Scholar] [CrossRef]
- Gomi, R.; Matsumura, Y.; Yamamoto, M.; Tanaka, M.; Komakech, A.J.; Matsuda, T.; Harada, H. Genomic surveillance of antimicrobial-resistant Escherichia coli in fecal sludge and sewage in Uganda. Water Res. 2024, 248, 120830. [Google Scholar] [CrossRef] [PubMed]
- Achtman, M.; Zhou, Z.; Charlesworth, J.; Baxter, L. EnteroBase: Hierarchical clustering of 100 000s of bacterial genomes into species/subspecies and populations. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022, 377, 20210240. [Google Scholar] [CrossRef]
- Falgenhauer, L.; Nordmann, P.; Imirzalioglu, C.; Yao, Y.; Falgenhauer, J.; Hauri, A.M.; Heinmüller, P.; Chakraborty, T. Cross-border emergence of clonal lineages of ST38 Escherichia coli producing the OXA-48-like carbapenemase OXA-244 in Germany and Switzerland. Int. J. Antimicrob. Agents 2020, 56, 106157. [Google Scholar] [CrossRef] [PubMed]
- Grevskott, D.H.; Radisic, V.; Salvà-Serra, F.; Moore, E.R.B.; Akervold, K.S.; Victor, M.P.; Marathe, N.P. Emergence and dissemination of epidemic-causing OXA-244 carbapenemase-producing Escherichia coli ST38 through hospital sewage in Norway, 2020–2022. J. Hosp. Infect. 2024, 145, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, P.; Hastak, P.; DeMaere, M.; Wyrsch, E.; Li, D.; Elankumaran, P.; Dolejska, M.; Browning, G.F.; Marenda, M.S.; Gottlieb, T.; et al. Phylogenomic analysis of a global collection of Escherichia coli ST38: Evidence of interspecies and environmental transmission? mSystems 2023, 8, e0123622. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, A.; Loucif, L.; Ayachi, A.; Guehaz, K.; Bendjama, E.; Rolain, J.M. Migratory White Stork (Ciconia ciconia): A Potential Vector of the OXA-48-Producing Escherichia coli ST38 Clone in Algeria. Microb. Drug Resist. 2018, 24, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Tafoukt, R.; Touati, A.; Leangapichart, T.; Bakour, S.; Rolain, J.M. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res. 2017, 120, 185–189. [Google Scholar] [CrossRef]
- Yagoubat, M.; Ould El-Hadj-Khelil, A.; Malki, A.; Bakour, S.; Touati, A.; Rolain, J.M. Genetic characterisation of carbapenem-resistant Gram-negative bacteria isolated from the University Hospital Mohamed Boudiaf in Ouargla, southern Algeria. J. Glob. Antimicrob. Resist. 2017, 8, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Belmahdi, M.; Bakour, S.; Al Bayssari, C.; Touati, A.; Rolain, J.M. Molecular characterisation of extended-spectrum β-lactamase- and plasmid AmpC-producing Escherichia coli strains isolated from broilers in Béjaïa, Algeria. J. Glob. Antimicrob. Resist. 2016, 6, 108–112. [Google Scholar] [CrossRef]
- Abderrahim, A.; Djahmi, N.; Pujol, C.; Nedjai, S.; Bentakouk, M.C.; Kirane-Gacemi, D.; Dekhil, M.; Sotto, A.; Lavigne, J.P.; Pantel, A. First Case of NDM-1-Producing Klebsiella pneumoniae in Annaba University Hospital, Algeria. Microb. Drug Resist. 2017, 23, 895–900. [Google Scholar] [CrossRef]
- Zemmour, A.; Dali-Yahia, R.; Maatallah, M.; Saidi-Ouahrani, N.; Rahmani, B.; Benhamouche, N.; Al-Farsi, H.M.; Giske, C.G. High-risk clones of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from the University Hospital Establishment of Oran, Algeria (2011–2012). PLoS ONE 2021, 16, e0254805. [Google Scholar] [CrossRef]
- Liu, C.; Guo, J.; Lu, M.; Shen, N.; Du, P. Dissemination of the mobilised RND efflux pump gene cluster tmexCD-toprJ among Klebsiella pneumoniae. Lancet Microbe 2023, 4, e135. [Google Scholar] [CrossRef]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32, e00001-19. [Google Scholar] [CrossRef] [PubMed]
- Al Ismail, D.; Campos-Madueno, E.I.; Donà, V.; Endimiani, A. Hypervirulent Klebsiella pneumoniae (hvKP): Overview, Epidemiology, and Laboratory Detection. Pathog. Immun. 2025, 10, 80–119. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, G.; Yu, Y.; Li, N.; Chen, M.; Jin, R.; Jiao, Y.; Wu, H. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin. Infect. Dis. 2014, 58, 225–232. [Google Scholar] [CrossRef]
- Russo, T.A.; MacDonald, U. The Galleria mellonella Infection Model Does Not Accurately Differentiate between Hypervirulent and Classical Klebsiella pneumoniae. mSphere 2020, 5, e00850-19. [Google Scholar] [CrossRef]
- Russo, T.A.; Alvarado, C.L.; Davies, C.J.; Drayer, Z.J.; Carlino-MacDonald, U.; Hutson, A.; Luo, T.L.; Martin, M.J.; Corey, B.W.; Moser, K.A.; et al. Differentiation of hypervirulent and classical Klebsiella pneumoniae with acquired drug resistance. mBio 2024, 15, e0286723. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Du, P.; Du, C.; Yang, P.; Shen, N.; Russo, T.A.; Liu, C. Genomically defined hypervirulent Klebsiella pneumoniae contributed to early-onset increased mortality. Nat. Commun. 2025, 16, 2096. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Emergence of Hypervirulent Klebsiella pneumoniae ST23 Carrying Carbapenemase Genes in EU/EEA Countries, First Update; ECDC: Stockholm, Sweden, 2024; ISBN 978-92-9498-691-7. [Google Scholar] [CrossRef]
- Institut Pasteur d’Algérie. Surveillance de la Résistance des Bactéries Aux Antibiotiques; 23ème Rapport D’évaluation 2023; Algerian Antimicrobial Resistance Network: Algiers, Algeria, 2023. [Google Scholar]
- Bialek-Davenet, S.; Criscuolo, A.; Ailloud, F.; Passet, V.; Nicolas-Chanoine, M.H.; Decré, D.; Brisse, S. Development of a multiplex PCR assay for identification of Klebsiella pneumoniae hypervirulent clones of capsular serotype K2. J. Med. Microbiol. 2014, 63 Pt 12, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Clinical Breakpoints. 2025. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 10 January 2025).
- CLSI Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- García-Meniño, I.; García, V.; Alonso, M.P.; Blanco, J.E.; Blanco, J.; Mora, A. Clones of enterotoxigenic and Shiga toxin-producing Escherichia coli implicated in swine enteric colibacillosis in Spain and rates of antibiotic resistance. Vet. Microbiol. 2021, 252, 108924. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Viso, S.; López, C.; Alonso, M.P.; García-Garrote, F.; Dabhi, G.; Mamani, R.; Herrera, A.; Marzoa, J.; Blanco, M.; et al. Poultry as reservoir for extraintestinal pathogenic Escherichia coli O45:K1:H7-B2-ST95 in humans. Vet Microbiol. 2013, 167, 506–512. [Google Scholar] [CrossRef]
- Saladin, M.; Cao, V.T.; Lambert, T.; Donay, J.L.; Herrmann, J.L.; Ould-Hocine, Z.; Verdet, C.; Delisle, F.; Philippon, A.; Arlet, G. Diversity of CTX-M beta-lactamases and their promoter regions from Enterobacteriaceae isolated in three Parisian hospitals. FEMS Microbiol. Lett. 2002, 209, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Simarro, E.; Navarro, F.; Ruiz, J.; Miró, E.; Gómez, J.; Mirelis, B. Salmonella enterica serovar virchow with CTX-M-like beta-lactamase in Spain. J. Clin. Microbiol. 2000, 38, 4676–4678. [Google Scholar] [CrossRef] [PubMed]
- García-Meniño, I.; García, V.; Mora, A.; Díaz-Jiménez, D.; Flament-Simon, S.C.; Alonso, M.P.; Blanco, J.E.; Blanco, M.; Blanco, J. Swine enteric colibacillosis in Spain: Pathogenic potential of mcr-1 ST10 and ST131 E. coli isolates. Front. Microbiol. 2018, 9, 2659. [Google Scholar] [CrossRef]
- Pérez-Pérez, F.J.; Hanson, N.D. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 2002, 40, 2153–2162. [Google Scholar] [CrossRef]
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. 2018, 23, 17-00672. [Google Scholar] [CrossRef]
- Borowiak, M.; Fischer, J.; Hammerl, J.A.; Hendriksen, R.S.; Szabo, I.; Malorny, B. Identification of a novel transposon-associated phosphoethanolamine transferase gene, mcr-5, conferring colistin resistance in d-tartrate fermenting Salmonella enterica subsp. enterica serovar Paratyphi B. J. Antimicrob. Chemother. 2017, 72, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Le Bouguenec, C.; Archambaud, M.; Labigne, A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1189–1193. [Google Scholar] [CrossRef]
- Lescat, M.; Clermont, O.; Woerther, P.L.; Glodt, J.; Dion, S.; Skurnik, D.; Djossou, F.; Dupont, C.; Perroz, G.; Picard, B.; et al. Commensal Escherichia coli strains in Guiana reveal a high genetic diversity with host-dependant population structure. Environ. Microbiol. Rep. 2013, 5, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Herrrera, A.; Lopez, C.; Dahbi, G.; Mamani, R.; Pita, J.M.; Alonso, M.P.; Llovo, J.; Bernardez, M.I.; Blanco, J.E.; et al. Characteristics of the Shiga-toxin-producing enteroaggregative Escherichia coli O104:H4 German outbreak strain and of STEC strains isolated in Spain. Int. Microbiol. 2011, 4, 121–141. [Google Scholar] [CrossRef]
- Johnson, J.R.; Russo, T.A.; Tarr, P.I.; Carlino, U.; Bilge, S.S.; Vary, J.C.; Stell, A.L. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN (E. coli), among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 2000, 68, 3040–3047. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Gajewski, A.; Lesse, A.J.; Russo, T.A. Extraintestinal pathogenic Escherichia coli as a cause of invasive non urinary infections. J. Clin. Microbiol. 2003, 41, 5798–5802. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Terai, A.; Yuri, K.; Kurazono, H.; Takeda, Y.; Yoshida, O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 1995, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tóth, I.; Hérault, F.; Beutin, L.; Oswald, E. Production of cytolethal distending toxins by pathogenic Escherichia coli strains isolated from human and animal sources: Establishment of the existence of a new cdt variant (Type IV). J. Clin. Microbiol. 2003, 41, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Moulin-Schouleur, M.; Schouler, C.; Tailliez, P.; Kao, M.-R.; Brée, A.; Germon, P.; Oswald, E.; Mainil, J.; Blanco, M.; Blanco, J. Common virulence factors and genetic relationships between O18:K1:H7 Escherichia coli isolates of human and avian origin. J Clin. Microbiol. 2006, 44, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; O’Bryan, T.T. Detection of the Escherichia coli group 2 polysaccharide capsule synthesis Gene kpsM by a rapid and specific PCR-based assay. J. Clin. Microbiol. 2004, 42, 1773–1776. [Google Scholar] [CrossRef]
- García, V.; García-Meniño, I.; Gómez, V.; Jiménez-Orellana, M.; Méndez, A.; Aguarón, A.; Roca, E.; Mora, A. Mobile colistin resistance (MCR), extended-spectrum beta-lactamase (ESBL) and multidrug resistance monitoring in Escherichia coli (commensal and pathogenic) in pig farming: Need of harmonized guidelines and clinical breakpoints. Front. Microbiol. 2022, 13, 1042612. [Google Scholar] [CrossRef]
- García-Meniño, I.; Díaz-Jiménez, D.; García, V.; de Toro, M.; Flament-Simon, S.C.; Blanco, J.; Mora, A. Genomic Characterization of Prevalent mcr-1, mcr-4, and mcr-5 Escherichia coli Within Swine Enteric Colibacillosis in Spain. Front. Microbiol. 2019, 10, 2469. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoer, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Clausen, P.T.L.C.; Aarestrup, F.M.; Lund, O. Rapid and precise alignment of raw reads against redundant databases with KMA. BMC Bioinform. 2018, 19, 307. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Voldby Larsen, M.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Roer, L.; Johannesen, T.B.; Hansen, F.; Stegger, M.; Tchesnokova, V.; Sokurenko, E.; Garibay, N.; Allesoe, R.; Thomsen, M.C.F.; Lund, O.; et al. CHTyper, a Web Tool for Subtyping of Extraintestinal Pathogenic Escherichia coli Based on the fumC and fimH alleles. J. Clin. Microbiol. 2018, 56, e00063-18. [Google Scholar] [CrossRef] [PubMed]
- Joensen, K.G.; Tetzschner, A.M.; Iguchi, A.; Aarestrup, F.M.; Scheutz, F. Rapid and Easy In Silico Serotyping of Escherichia coli Isolates by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2015, 53, 2410–2426. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and virulence in Escherichia coli: An evolutionary perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Alikhan, N.F.; Mohamed, K.; Fan, Y.; Achtman, M. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020, 30, 138–152. [Google Scholar] [CrossRef]
- Kaas, R.S.; Leekitcharoenphon, P.; Aarestrup, F.M.; Lund, O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS ONE 2014, 9, e104984. [Google Scholar] [CrossRef] [PubMed]
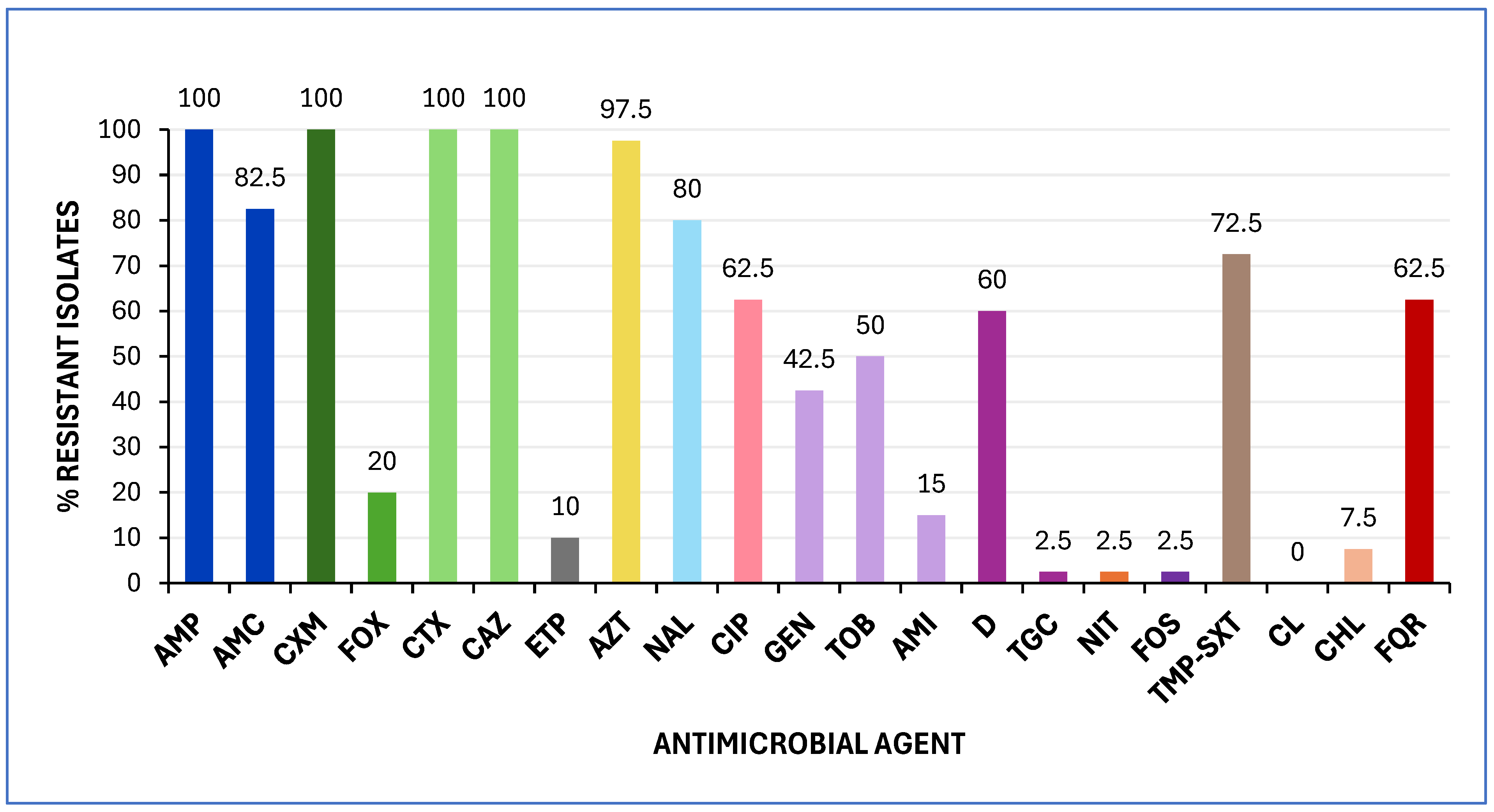

| Isolate | UPEC Status a | ExPEC Status b | Virotype c | Phenotypic Resistance d | Virulence Genes Profile | bla Genes |
|---|---|---|---|---|---|---|
| Ec4 | + | + | NT | AMP, CXM, CTX, CAZ, AZT, NAL, CIP, TOB, AMI, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, papGII | CTX-M-15 |
| Ec5 | + | + | NT | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, TOB, AMI, D, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, papGII | CTX-M-15 |
| Ec8 | + | + | F | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB, AMI, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII | CTX-M-15 |
| Ec10 | + | + | F | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII | CTX-M-15 |
| Ec19 a | + | + | F | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII | CTX-M-15 |
| Ec20 a | + | + | E | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII, cnfI, hlyA | CTX-M-15 |
| Ec22 | + | + | F | AMP, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII | CTX-M-15 |
| Ec23 | + | − | NT | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, TOB, AMI, D, NIT, TMP-SXT | fyuA, yfcV, chuA, papAH, papGII | CTX-M-15 |
| Ec28 | + | + | NT | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, sat, papGII | CTX-M-15 |
| Ec30 | + | + | NT | AMP, AMC, CXM, CTX, FOX, CAZ, ETP, AZT, NAL, CIP, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, sat, papGII | CTX-M-15 |
| Ec31 | + | + | NT | AMP, AMC, CXM, CTX, FOX, CAZ, AZT, NAL, CIP, GEN, TOB, D, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, sat, papGII | CTX-M-15 |
| Ec41 | − | + | F | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB, D, TMP-SXT | chuA, iutA, kpsMII-K5, sat, papGII | CTX-M-15 |
| Ec43 | + | + | F | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB, D TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII | CTX-M-15 |
| Ec45 | + | + | NT | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB, AMI, D, TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, sat, papGII | CTX-M-15 |
| Ec52 | + | + | F | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB, D TMP-SXT | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII | CTX-M-15 |
| Ec55 a | + | + | E | AMP, AMC, CXM, CTX, CAZ, AZT, NAL, CIP, GEN, TOB | fyuA, yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII, cnfI, hlyA | CTX-M-15 |
| Ec57 | − | + | E | AMP, AMC, CXM, CTX, FOX, CAZ, AZT, NAL, CIP, GEN, TOB, D, TGC, FOS, TMP-SXT, CHL | yfcV, chuA, papAH, iutA, kpsMII-K5, sat, papGII, cnfI, hlyA | CTX-M-15 |
| ID Code for Isolate/Genome a | Ec1a/LREC-468 |
|---|---|
| O:H antigens b | O75:H5 |
| ST#1/ST#2 c | 1193/53 |
| cgMLST d | 140226 |
| Acquired resistance and point mutations [in brackets] e | aac(3)-IIa, aac(6′)-Ib-cr, blaCTX-M-15, blaOXA-1, catB3 [gyrA p.S83L, gyrA p.D87N, parC p.S80I, parE p.L416F] |
| Plasmid content: Inc. group [pMLST] f | IncF [F-:A1:B10], IncI1-I [ST Unknown], ColBS512-like |
| Virulence genes g | aslA, chuA, cia, csgA, fdeC, fimH, fyuA, gad, iha, irp2, iucC, iutA, kpsE, kpsMII_K1, neuC, nlpI, ompT, papA_F43, sat, shiA, sitA, terC, tia, usp, vat, yehA, yehB, yehC, yehD, yfcV |
| Phenotypic resistance h | AMP, AMC, CXM, CAZ, CTX, ATM, NAL, CIP, GEN |
| Hypermucoviscous Phenotype (HMV) a | |||||||
|---|---|---|---|---|---|---|---|
| Isolate | ML | MH | TSA | CA | Phenotypic Resistance b | Virulence Genes c | bla Genes |
| KP6b | + | – | + | – | AMP, CXM, CTX, CAZ, AZT, CIP, FOS | terB | CTX-M-15 |
| KP10c | + | + | + | + | AMP, AMC, CXM, FOX, CTX, CAZ, ETP, AZT, NAL, CIP, GEN, TOB, NIT, FOS | iucA, peg-344, rmpA | NDM, CTX-M-15 |
| KP16 | + | + | + | + | AMP, AMC, CXM, FOX, CTX, CAZ, ETP, AZT, NAL, CIP, GEN, TOB, TGC, NIT, FOS, TMP-SXT, CHL | iucA, peg-344, rmpA | NDM, CTX-M-15 |
| KP20a | + | + | + | – | AMP, CXM, CTX, CAZ, AZT, CIP, FOS, TMP-SXT | terB | CTX-M-15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziadi, H.; Chougrani, F.; Cheriguene, A.; Carballeira, L.; García, V.; Mora, A. Phenotypic and Genotypic Characterization of ESBL-, AmpC-, and Carbapenemase-Producing Klebsiella pneumoniae and High-Risk Escherichia coli CC131, with the First Report of ST1193 as a Causative Agent of Urinary Tract Infections in Human Patients in Algeria. Antibiotics 2025, 14, 485. https://doi.org/10.3390/antibiotics14050485
Ziadi H, Chougrani F, Cheriguene A, Carballeira L, García V, Mora A. Phenotypic and Genotypic Characterization of ESBL-, AmpC-, and Carbapenemase-Producing Klebsiella pneumoniae and High-Risk Escherichia coli CC131, with the First Report of ST1193 as a Causative Agent of Urinary Tract Infections in Human Patients in Algeria. Antibiotics. 2025; 14(5):485. https://doi.org/10.3390/antibiotics14050485
Chicago/Turabian StyleZiadi, Hajer, Fadela Chougrani, Abderrahim Cheriguene, Leticia Carballeira, Vanesa García, and Azucena Mora. 2025. "Phenotypic and Genotypic Characterization of ESBL-, AmpC-, and Carbapenemase-Producing Klebsiella pneumoniae and High-Risk Escherichia coli CC131, with the First Report of ST1193 as a Causative Agent of Urinary Tract Infections in Human Patients in Algeria" Antibiotics 14, no. 5: 485. https://doi.org/10.3390/antibiotics14050485
APA StyleZiadi, H., Chougrani, F., Cheriguene, A., Carballeira, L., García, V., & Mora, A. (2025). Phenotypic and Genotypic Characterization of ESBL-, AmpC-, and Carbapenemase-Producing Klebsiella pneumoniae and High-Risk Escherichia coli CC131, with the First Report of ST1193 as a Causative Agent of Urinary Tract Infections in Human Patients in Algeria. Antibiotics, 14(5), 485. https://doi.org/10.3390/antibiotics14050485








