Prevalence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Dairy Products in Romania
Abstract
1. Introduction
2. Results
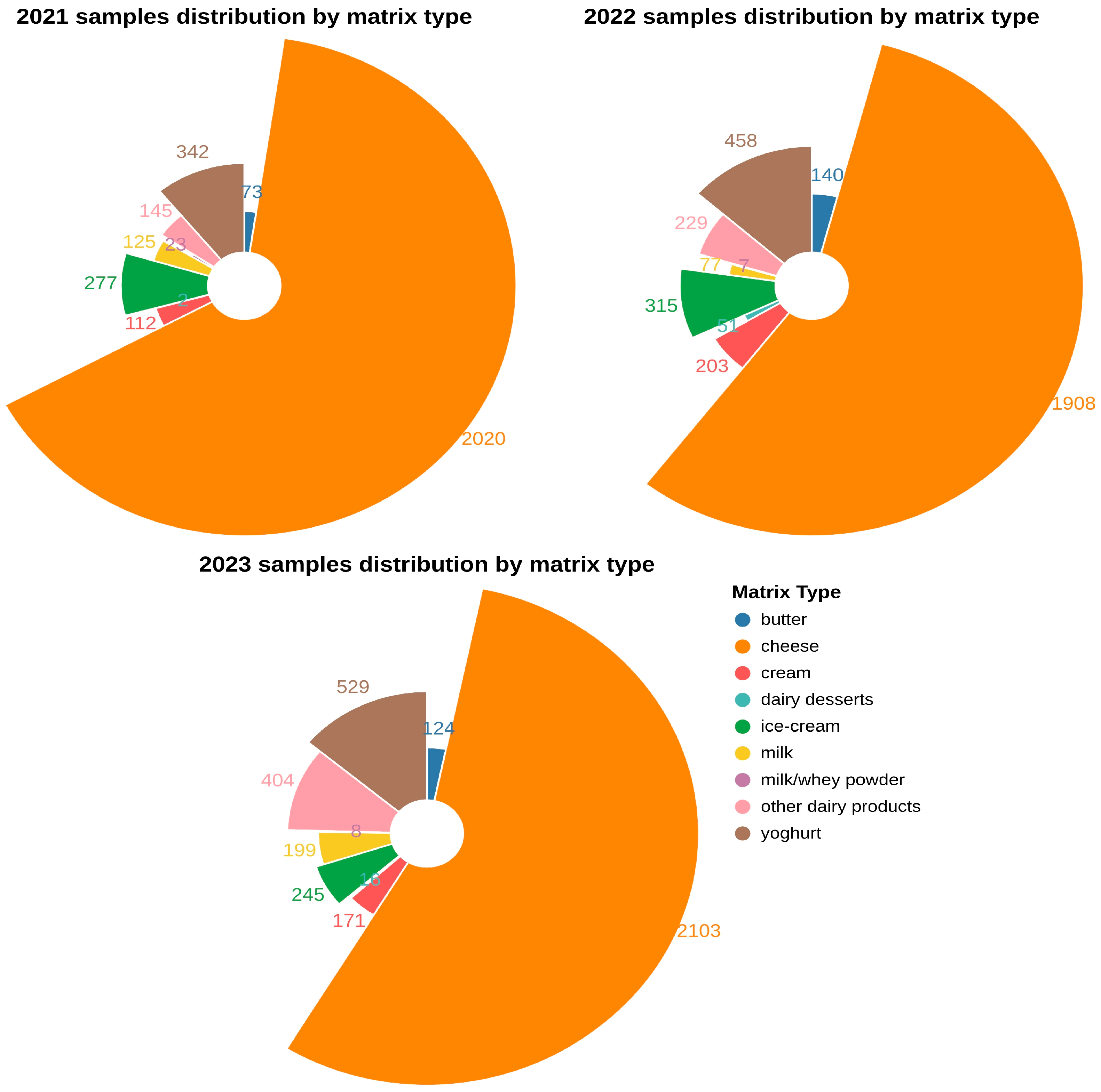
2.1. Prevalence of L. monocytogenes in Dairy Samples
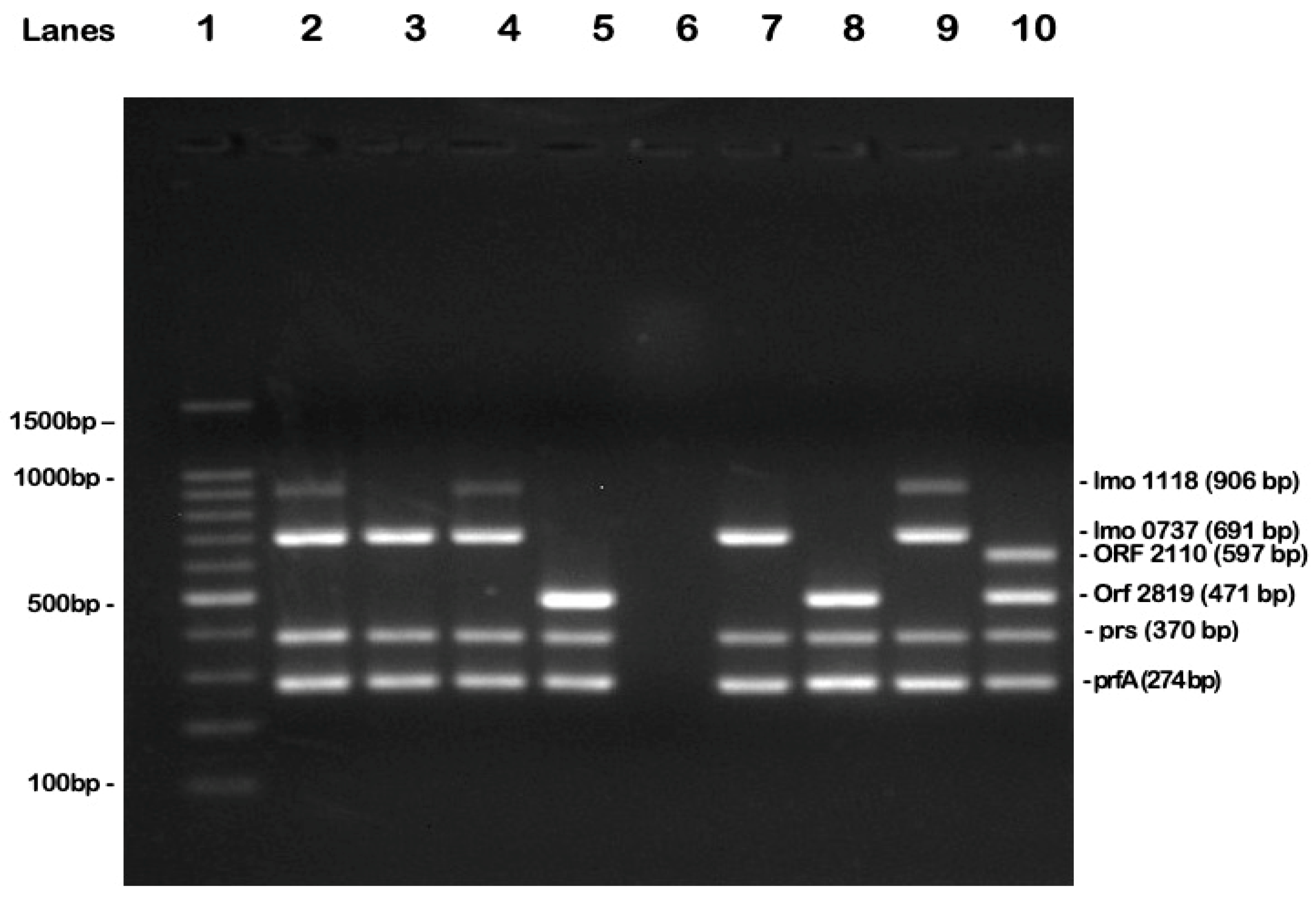
2.2. Molecular Serotyping of L. monocytogenes
2.3. Antimicrobial Susceptibility
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Bacterial Isolation
4.3. Antimicrobial Susceptibility Testing
4.4. Bacterial DNA Extraction
4.5. Polymerase Chain Reaction (PCR) for the Molecular Serotyping of L. monocytogenes
4.6. Data Processing and Visualization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Stantement
Abbreviations
| RTE | Ready-to-eat |
| EU | European Union |
| EFSA | European Food Safety Authority |
| EUCAST | European Committee on Antimicrobial Susceptibility Testing |
| DNA | Deoxyribonucleic acid |
| PCR | Polymerase Chain Reaction |
References
- Félix, B.; Capitaine, K.; Te, S.; Felten, A.; Gillot, G.; Feurer, C.; van den Bosch, T.; Torresi, M.; Lancz, Z.S.; Delannoy, S.; et al. Identification by High-Throughput Real-Time PCR of 30 Major Circulating Listeria monocytogenes Clonal Complexes in Europe. Microbiol. Spectr. 2023, 11, e0395422. [Google Scholar] [CrossRef]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA J. 2018, 16, e05134. [Google Scholar] [CrossRef]
- Linke, K.; Rückerl, I.; Brugger, K.; Karpiskova, R.; Walland, J.; Muri-Klinger, S.; Tichy, A.; Wagner, M.; Stessl, B. Reservoirs of Listeria species in three environmental ecosystems. Appl. Environ. Microbiol. 2014, 80, 5583–5592. [Google Scholar] [CrossRef]
- Dowe, M.J.; Jackson, E.D.; Mori, J.G.; Bell, C.R. Listeria monocytogenes Survival in Soil and Incidence in Agricultural Soils. J. Food Prot. 1997, 60, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K.K.; Schukken, Y.H.; Nightingale, C.R.; Fortes, E.D.; Ho, A.J.; Her, Z.; Grohn, Y.T.; McDonough, P.L.; Wiedmann, M. Ecology and transmission of Listena monocytogenes infecting ruminants and in the farm environment. Appl. Environ. Microbiol. 2004, 70, 4458–4467. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar]
- European Food Safety Authority. Multi-Country Outbreak of Listeria Monocytogenes Linked to Cold-Smoked Fish. 2022. Available online: https://www.efsa.europa.eu/en/press/news/190604 (accessed on 5 March 2025).
- Zhang, Y.; Yeh, E.; Hall, G.; Cripe, J.; Bhagwat, A.A.; Meng, J. Characterization of Listeria monocytogenes isolated from retail foods. Int. J. Food Microbiol. 2007, 113, 47–53. [Google Scholar] [CrossRef]
- Duma, M.N.; Ciupescu, L.M.; Dan, S.D.; Crisan-Reget, O.L.; Tabaran, A. Virulence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Ready-to-Eat Food Products in Romania. Microorganisms 2024, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention. The European Union One Health 2023 Zoonoses report. EFSA J. 2014, 22, e9106. [Google Scholar] [CrossRef]
- Hof, H.; Nichterlein, T.; Kretschmar, M. Management of listeriosis. Clin. Microbiol. Rev. 1997, 10, 345–357. [Google Scholar] [CrossRef]
- Hof, H. Therapeutic options. FEMS Immunol. Med. Microbiol. 2003, 35, 203–205. [Google Scholar] [CrossRef]
- Hof, H. An update on the medical management of listeriosis. Exp. Opin. Pharmacother. 2004, 5, 1727–1735. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Almeida FA de Medeiros, M.M.; Miranda, B.R.; Pinto, U.M.; Alves, V.F. Listeria monocytogenes: An Inconvenient Hurdle for the Dairy Industry. Dairy 2023, 4, 316–344. [Google Scholar] [CrossRef]
- Matereke, L.T.; Okoh, A.I. Listeria monocytogenes Virulence, Antimicrobial Resistance and Environmental Persistence: A Review. Pathogens 2020, 9, 528. [Google Scholar] [CrossRef] [PubMed]
- Bashiry, M.; Javanmardi, F.; Taslikh, M.; Sheidaei, Z.; Sadeghi, E.; Abedi, A.-S.; Alizadeh, A.M.; Hashempour-Baltork, F.; Beikzadeh, S.; Riahi, S.M.; et al. Listeria monocytogenes in Dairy Products of the Middle East Region: A Systematic Review, Meta-Analysis, and Meta-Regression Study. Iran. J. Public Health 2022, 51, 292. [Google Scholar] [CrossRef]
- Osek, J.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Uses Its Virulence Mechanisms to Infect the Hosts. Pathogens 2022, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Lü, X.F.; Yin, L.; Liu, H.-F.; Zhang, W.-J.; Si, W.; Yu, S.-Y.; Shao, M.-L.; Liu, S.-G. Occurrence and antimicrobial susceptibility of Listeria monocytogenes isolates from retail raw foods. Food Control 2013, 32, 153–158. [Google Scholar] [CrossRef]
- Bhosale, S.; Desale, R.J.; Fulpagare, Y.G. Biofilm: An Overview with Respect to Dairy Industry. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 150–160. [Google Scholar] [CrossRef]
- Gopal, N.; Hill, C.; Ross, P.R.; Beresford, T.P.; Fenelon, M.A.; Cotter, P.D. The Prevalence and Control of Bacillus and Related Spore-Forming Bacteria in the Dairy Industry. Front. Microbiol. 2015, 6, 1418. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Akabanda, F.; Agyei, D.; Jespersen, L. Microbial Safety of Milk Production and Fermented Dairy Products in Africa. Microorganisms 2020, 8, 752. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Matle, I.; Mbatha, K.R.; Madoroba, E. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet. Res. 2020, 87, 20. [Google Scholar] [CrossRef]
- dos Santos, J.S.; Biduski, B.; dos Santos, L.R. Listeria monocytogenes: Health risk and a challenge for food processing establishments. Arch. Microbiol. 2021, 203, 5907–5919. [Google Scholar] [CrossRef] [PubMed]
- Osek, J.; Lachtara, B.; Wieczorek, K. Listeria monocytogenes—How This Pathogen Survives in Food-Production Environments? Front. Microbiol. 2022, 13, 866462. [Google Scholar] [CrossRef]
- Food and Drug Administration. Get the Facts About Listeria. 2022. Available online: https://www.fda.gov/animal-veterinary/animal-health-literacy/get-facts-about-listeria (accessed on 26 March 2025).
- Li, X.; Zheng, J.; Zhao, W.; Wu, Y. Prevalence of Listeria monocytogenes in Milk and Dairy Product Supply Chains: A Global Systematic Review and Meta-analysis. Foodborne Pathog. Dis. 2024, 21, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lawrence, M.L.; Austin, F.W.; Ainsworth, A.J. A multiplex PCR for species-and virulence-specific determination of Listeria monocytogenes. J. Microbiol. Methods 2007, 71, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Burall, L.S.; Macarisin, D.; Pouillot, R.; Strain, E.; De Jesus, A.J.; Laasri, A.; Wang, H.; Ali, L.; Tatavarthy, A.; et al. Prevalence and Level of Listeria monocytogenes in Ice Cream Linked to a Listeriosis Outbreak in the United States. J. Food Prot. 2016, 79, 1828–1832. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Gonzales-Barron, U.; Pilão Cadavez, V.A.; Sant’ana, A.S.; Schaffner, D.W. Quantitative risk assessment of Listeria monocytogenes in traditional Minas cheeses: The cases of artisanal semi-hard and fresh soft cheeses. Food Control 2018, 92, 370–379. [Google Scholar] [CrossRef]
- Pouillot, R.; Klontz, K.C.; Chen, Y.; Burall, L.S.; Macarisin, D.; Doyle, M.; Bally, K.M.; Strain, E.; Datta, A.R.; Hammack, T.S.; et al. Infectious Dose of Listeria monocytogenes in Outbreak Linked to Ice Cream, United States, 2015. Emerg. Infect. Dis. 2016, 22, 2113–2119. [Google Scholar] [CrossRef]
- Doyle, M.P.; Glass, K.A.; Beery, J.T.; A Garcia, G.; Pollard, D.J.; Schultz, R.D. Survival of Listeria monocytogenes in milk during high-temperature, short-time pasteurization. Appl. Environ. Microbiol. 1987, 53, 1433–1438. [Google Scholar] [CrossRef]
- Kuenne, C.; Billion, A.; Mraheil, M.A.; Strittmatter, A.; Daniel, R.; Goesmann, A.; Barbuddhe, S.; Hain, T.; Chakraborty, T. Reassessment of the Listeria monocytogenes pan-genome reveals dynamic integration hotspots and mobile genetic elements as major components of the accessory genome. BMC Genom. 2013, 12, 47. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Wang, J.; Wu, Q.; Cheng, J.; Zhang, J.; Sun, Q.; Xue, L.; Zeng, H.; Lei, T.; et al. Heterogeneity, Characteristics, and Public Health Implications of Listeria monocytogenes in Ready-to-Eat Foods and Pasteurized Milk in China. Front. Microbiol. 2020, 11, 486158. [Google Scholar] [CrossRef] [PubMed]
- Haase, J.K.; Didelot, X.; Lecuit, M.; Korkeala, H.; L. monocytogenes MLST Study Group; Achtman, M. The ubiquitous nature of Listeria monocytogenes clones: A large-scale Multilocus Sequence Typing study. Environ. Microbiol. 2014, 16, 405–416. [Google Scholar] [CrossRef]
- Varsaki, A.; Ortiz, S.; Santorum, P.; López, P.; López-Alonso, V.; Hernández, M.; Abad, D.; Rodríguez-Grande, J.; Ocampo-Sosa, A.A.; Martínez-Suárez, J.V. Prevalence and Population Diversity of Listeria monocytogenes Isolated from Dairy Cattle Farms in the Cantabria Region of Spain. Animals 2022, 12, 2477. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 7.0. 2017. Available online: http://www.eucast.org (accessed on 21 March 2025).
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, M100S, 29th ed.; Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2019; p. 39. [Google Scholar]
- Reback, J.; McKinney, W.; Jbrockmendel; Van den Bossche, J.; Augspurger, T.; Cloud, P.; Hawkins, S.; gfyoung; Sinhrks; Roeschke, M.; et al. pandas-dev/pandas: Pandas 1.2.4, version 1.2.4; Zenodo CERN: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- VanderPlas, J.; Granger, B.E.; Heer, J.; Moritz, D.; Wongsuphasawat, K.; Satyanarayan, A.; Lees, E.; Timofeev, I.; Welsh, B.; Sievert, S. Altair: Interactive Statistical Visualizations for Python. J. Open Source Softw. 2018, 3, 1057. [Google Scholar] [CrossRef]




| Antimicrobial Agent | Resistant L. monocytogenes Isolates (%) | Intermediate Resistant L. monocytogenes Isolates (%) |
|---|---|---|
| Ampicillin | 5 (11.62%) | 1 (2.32%) |
| Cephalothin | 3 (6.97%) | 0 |
| Penicillin G | 4 (9.30%) | 1 (2.32%) |
| Oxacillin | 6 (13.95%) | 2 (4.65%) |
| Methicillin | 1 (2.32%) | 0 |
| Ciprofloxacin | 0 | 0 |
| Levofloxacin | 0 | 0 |
| Moxifloxacin | 0 | 0 |
| Clindamycin | 1 (2.32%) | 0 |
| Tetracycline | 5 (11.62%) | 0 |
| Gentamicin | 1 (2.32%) | 0 |
| Chloramphenicol | 1 (2.32%) | 0 |
| Rifampicin | 1 (2.32%) | 0 |
| Trimethoprim-sulfamethoxazole | 6 (13.95%) | 1 (2.32%) |
| Target Gene | Primer Sequence (5′-3′) | Product Size (bp) | Serovar Specificity | Protein Encoded by the Target Gene |
|---|---|---|---|---|
| lmo0737 | F:AGGGCTTCAAGGACTTACCC R: ACGATTTCTGCTTGCCATTC | 691 | L. monocytogenes serovars 1/2a, 1/2c, 3a, and 3c | Unknown, no similarity |
| lmo1118 | F: AGGGGTCTTAAATCCTGGAA R: CGGCTTGTTCGGCATACTTA | 906 | L. monocytogenes serovars 1/2c and 3c | Unknown, no similarity |
| orf2819 | F: AGCAAAATGCCAAAACTCGT R: CATCACTAAAGCCTCCCATTG | 471 | L. monocytogenes serovars 1/2b, 3b, 4b, 4d, and 4e | Putative transcriptional regulator |
| orf2110 | F: AGTGGACAATTGATTGGTGAA R: CATCCATCCCTTACTTTGGAC | 597 | L. monocytogenes serovars 1/2b, 3b, 4b, 4d, and 4e | Putative transcriptional regulator |
| prs | F: GCTGAAGAGATTGCGAAAGAAG R: CAAAGAAACCTTGGATTTGCGG | 370 | L. monocytogenes serovars 4b, 4d, and 4e | Putative secreted protein |
| prfA | F: GATACAGAAACATCGGTTGGC R: GTGTAATCTTGATGCCATCAGG | 274 | L. monocytogenes serovars 4b, 4d, and 4e | Putative secreted protein |
| Gene | Serogroup IIa | Serogroup IIb | Serogroup IIc | Serogroup IVb |
|---|---|---|---|---|
| lmo0737 | + | − | + | − |
| ORF2819 | − | + | − | + |
| lmo1118 | − | − | + | − |
| ORF2110 | − | − | − | + |
| prs | + | + | + | + |
| prfA | + | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaou, F.G.; Colobatiu, L.M.; Ciupescu, L.M.; Tabaran, A.; Hategan, A.R.; Mihaiu, R.; Tanasuica, R.; Poenaru, M.M.; Mihaiu, M. Prevalence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Dairy Products in Romania. Antibiotics 2025, 14, 482. https://doi.org/10.3390/antibiotics14050482
Nikolaou FG, Colobatiu LM, Ciupescu LM, Tabaran A, Hategan AR, Mihaiu R, Tanasuica R, Poenaru MM, Mihaiu M. Prevalence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Dairy Products in Romania. Antibiotics. 2025; 14(5):482. https://doi.org/10.3390/antibiotics14050482
Chicago/Turabian StyleNikolaou, Filippos Georgios, Liora Mihaela Colobatiu, Laurentiu Mihai Ciupescu, Alexandra Tabaran, Ariana Raluca Hategan, Romolica Mihaiu, Radu Tanasuica, Magdalena Maria Poenaru, and Marian Mihaiu. 2025. "Prevalence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Dairy Products in Romania" Antibiotics 14, no. 5: 482. https://doi.org/10.3390/antibiotics14050482
APA StyleNikolaou, F. G., Colobatiu, L. M., Ciupescu, L. M., Tabaran, A., Hategan, A. R., Mihaiu, R., Tanasuica, R., Poenaru, M. M., & Mihaiu, M. (2025). Prevalence and Antimicrobial Resistance of Listeria monocytogenes Isolated from Dairy Products in Romania. Antibiotics, 14(5), 482. https://doi.org/10.3390/antibiotics14050482








