Pulsed Blue Light and Phage Therapy: A Novel Synergistic Bactericide
Abstract
1. Introduction
1.1. Antibiotic-Resistant Bacteria and Biofilms
1.2. Phage Therapy
1.3. Pulsed Blue Light (PBL)
1.4. Study Rationale and Objective
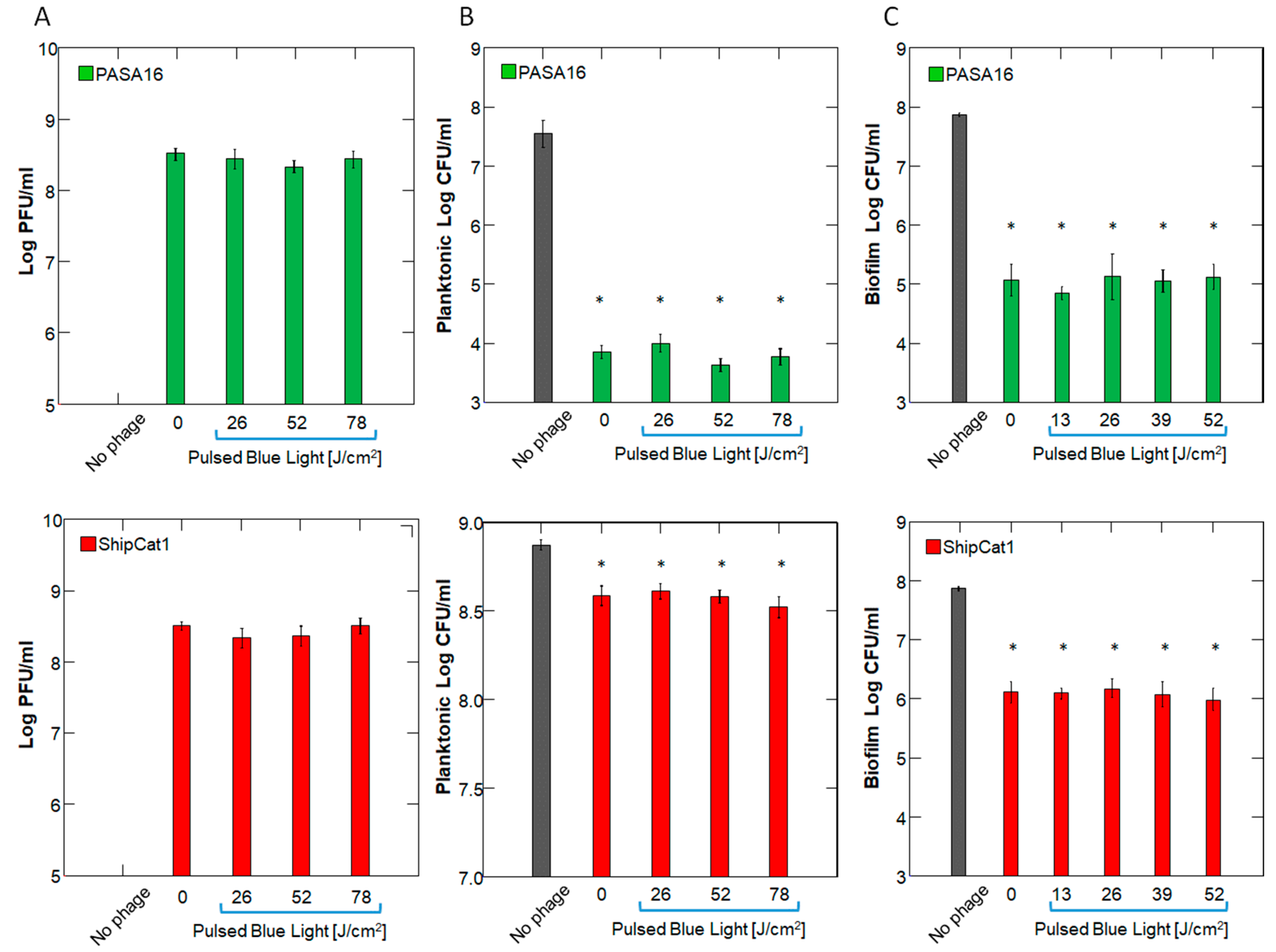
2. Results
2.1. Effect of PBL on Phage Number and Activity
2.2. Timing of Administration and Dose/Concentration Response
2.3. Bacterial Viability and Biomass
2.4. Reactive Oxidant Species
3. Discussion
3.1. Phages and Antibiotics
3.2. Blue Light
3.3. Practical Considerations
3.4. Possible Mechanisms
3.5. Limitations
4. Materials and Methods
4.1. Study Design
4.2. Bacteria and Growth Conditions
4.2.1. Bacterial Strains
4.2.2. Planktonic Bacteria
4.2.3. Biofilm
4.3. Phage Strains
4.4. PBL
4.5. Quantification Methods
4.5.1. CFU
4.5.2. PFU
4.5.3. Growth Curves
4.5.4. Biofilm CFU
4.5.5. Biofilm Biomass Detection
4.5.6. Live-Dead Stain
4.5.7. ROS Determination
4.5.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Schuts, E.C.; Hulscher, M.E.J.L.; Mouton, J.W.; Verduin, C.M.; Stuart, J.W.T.C.; Overdiek, H.W.P.M.; van der Linden, P.D.; Natsch, S.; Hertogh, C.M.P.M.; Wolfs, T.F.W.; et al. Current Evidence on Hospital Antimicrobial Stewardship Objectives: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa Biofilms in Disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage Therapy: From Biological Mechanisms to Future Directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Pires, D.P.; Meneses, L.; Brandão, A.C.; Azeredo, J. An Overview of the Current State of Phage Therapy for the Treatment of Biofilm-Related Infections. Curr. Opin. Virol. 2022, 53, 101209. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef]
- Meile, S.; Du, J.; Dunne, M.; Kilcher, S.; Loessner, M.J. Engineering Therapeutic Phages for Enhanced Antibacterial Efficacy. Curr. Opin. Virol. 2022, 52, 182–191. [Google Scholar] [CrossRef]
- Onallah, H.; Hazan, R.; Nir-Paz, R.; Brownstein, M.J.; Fackler, J.R.; Hopkins, R.; Basu, S.; Yerushalmy, O.; Alkalay-Oren, S.; Braunstein, R. Refractory Pseudomonas aeruginosa Infections Treated with Phage PASA16: A Compassionate Use Case Series. Med 2023, 4, 600–611. [Google Scholar] [CrossRef]
- Technophage, S. Bacteriophage Therapy TP-102 in Diabetic Foot Ulcers (REVERSE). Available online: https://clinicaltrials.gov/ct2/show/NCT04803708 (accessed on 5 May 2025).
- Rimon, A.; Rakov, C.; Lerer, V.; Sheffer-Levi, S.; Oren, S.A.; Shlomov, T.; Shasha, L.; Lubin, R.; Zubeidat, K.; Jaber, N. Topical Phage Therapy in a Mouse Model of Cutibacterium acnes-Induced Acne-like Lesions. Nat. Commun. 2023, 14, 1005. [Google Scholar] [CrossRef]
- Onallah, H.; Hazan, R.; Nir-Paz, R. Compassionate Use of Bacteriophages for Failed Persistent Infections During the First 5 Years of the Israeli Phage Therapy Center. In Open Forum Infectious Diseases; Oxford University Press: New York, NY, USA, 2023; Volume 10, p. ofad221. [Google Scholar]
- Yerushalmy, O.; Braunstein, R.; Alkalay-Oren, S.; Rimon, A.; Coppenhagn-Glazer, S.; Onallah, H.; Nir-Paz, R.; Hazan, R. Towards Standardization of Phage Susceptibility Testing: The Israeli Phage Therapy Center “Clinical Phage Microbiology”—A Pipeline Proposal. Clin. Infect. Dis. 2023, 77, S337–S351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and in Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Can Light-based Approaches Overcome Antimicrobial Resistance? Drug Dev. Res. 2019, 80, 48–67. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Inactivation of Pseudomonas aeruginosa by Photo-excitation of Endogenous Porphyrins: In Vitro and in Vivo Studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Bumah, V.V.; Niesman, I.R.; Cortez, P.; Enwemeka, C.S. Structural Membrane Changes Induced by Pulsed Blue Light on Methicillin-Resistant Staphylococcus aureus (MRSA). J. Photochem. Photobiol. B 2021, 216, 112150. [Google Scholar] [CrossRef]
- Biener, G.; Masson-Meyers, D.S.; Bumah, V.V.; Hussey, G.; Stoneman, M.R.; Enwemeka, C.S.; Raicu, V. Blue/Violet Laser Inactivates Methicillin-Resistant Staphylococcus aureus by Altering Its Transmembrane Potential. J. Photochem. Photobiol. B 2017, 170, 118–124. [Google Scholar] [CrossRef]
- Hadi, J.; Wu, S.; Brightwell, G. Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods 2020, 9, 1895. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Bumah, V.V.; Castel, C.; Castel, D.; Enwemeka, C.S. Pulsed 450 Nm Blue Light Significantly Inactivates Propionibacterium acnes More than Continuous Wave Blue Light. J. Photochem. Photobiol. B 2020, 202, 111719. [Google Scholar] [CrossRef]
- Bumah, V.V.; Masson-Meyers, D.S.; Enwemeka, C.S. Pulsed 450 Nm Blue Light Suppresses MRSA and Propionibacterium acnes in Planktonic Cultures and Bacterial Biofilms. J. Photochem. Photobiol. B 2020, 202, 111702. [Google Scholar] [CrossRef]
- Robertson, J.; McGoverin, C.; Vanholsbeeck, F.; Swift, S. Optimisation of the Protocol for the LIVE/DEAD® BacLightTM Bacterial Viability Kit for Rapid Determination of Bacterial Load. Front. Microbiol. 2019, 10, 801. [Google Scholar] [CrossRef]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Nina, P.B.; Jp, D.; Kumar, S.; Singh, B.; Tiwari, R.R. Futuristic Non-Antibiotic Therapies to Combat Antibiotic Resistance: A Review. Front. Microbiol. 2021, 12, 609459. [Google Scholar] [CrossRef]
- Chen, X.; Mendes, B.G.; Alves, B.S.; Duan, Y. Phage Therapy in Gut Microbiome. Prog. Mol. Biol. Transl. Sci. 2023, 201, 93–118. [Google Scholar] [PubMed]
- Stacey, H.J.; De Soir, S.; Jones, J.D. The Safety and Efficacy of Phage Therapy: A Systematic Review of Clinical and Safety Trials. Antibiotics 2022, 11, 1340. [Google Scholar] [CrossRef] [PubMed]
- Segall, A.M.; Roach, D.R.; Strathdee, S.A. Stronger Together? Perspectives on Phage-Antibiotic Synergy in Clinical Applications of Phage Therapy. Curr. Opin. Microbiol. 2019, 51, 46–50. [Google Scholar] [CrossRef]
- Lin, Y.; Chang, R.Y.K.; Britton, W.J.; Morales, S.; Kutter, E.; Chan, H.-K. Synergy of Nebulized Phage PEV20 and Ciprofloxacin Combination against Pseudomonas aeruginosa. Int. J. Pharm. 2018, 551, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, P.; Curcin, S.; Aleksic, V.; Petrusic, M.; Vlaski, L. Phage-Antibiotic Synergism: A Possible Approach to Combatting Pseudomonas aeruginosa. Res. Microbiol. 2013, 164, 55–60. [Google Scholar] [CrossRef]
- Chaudhry, W.N.; Concepcion-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef]
- Ciofu, O.; Rojo-Molinero, E.; Macià, M.D.; Oliver, A. Antibiotic Treatment of Biofilm Infections. Apmis 2017, 125, 304–319. [Google Scholar] [CrossRef]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; Van Erp, P.E.J.; Van De Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and Histological Effects of Blue Light on Normal Skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- Rupel, K.; Zupin, L.; Ottaviani, G.; Bertani, I.; Martinelli, V.; Porrelli, D.; Vodret, S.; Vuerich, R.; Passos da Silva, D.; Bussani, R. Blue Laser Light Inhibits Biofilm Formation in Vitro and in Vivo by Inducing Oxidative Stress. NPJ Biofilms Microbiomes 2019, 5, 29. [Google Scholar] [CrossRef]
- Ryan, E.M.; Gorman, S.P.; Donnelly, R.F.; Gilmore, B.F. Recent Advances in Bacteriophage Therapy: How Delivery Routes, Formulation, Concentration and Timing Influence the Success of Phage Therapy. J. Pharm. Pharmacol. 2011, 63, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Bumah, V.V.; Masson-Meyers, D.S.; Enwemeka, C.S. Blue 470 Nm Light Suppresses the Growth of Salmonella enterica and Methicillin-resistant Staphylococcus aureus (MRSA) in Vitro. Lasers Surg. Med. 2015, 47, 595–601. [Google Scholar] [CrossRef]
- Turner, P.E.; Azeredo, J.; Buurman, E.T.; Green, S.; Haaber, J.K.; Haggstrom, D.; Kameda de Figueiredo Carvalho, K.; Kirchhelle, C.; Gonzalez Moreno, M.; Pirnay, J.-P. Addressing the Research and Development Gaps in Modern Phage Therapy. Phage 2024, 5, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Wang, N.; Wang, C.; Yao, Y.; Fu, X.; Yu, W.; Cai, R.; Yao, M. 460nm Visible Light Irradiation Eradicates MRSA via Inducing Prophage Activation. J. Photochem. Photobiol. B 2017, 166, 311–322. [Google Scholar] [CrossRef]
- Godley, B.F.; Shamsi, F.A.; Liang, F.-Q.; Jarrett, S.G.; Davies, S.; Boulton, M. Blue Light Induces Mitochondrial DNA Damage and Free Radical Production in Epithelial Cells. J. Biol. Chem. 2005, 280, 21061–21066. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F. Catalase A Is Involved in the Response to Photooxidative Stress in Pseudomonas aeruginosa. Photodiagn. Photodyn. Ther. 2018, 22, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Abedon, S.T. Ecology and Evolutionary Biology of Hindering Phage Therapy: The Phage Tolerance vs. Phage Resistance of Bacterial Biofilms. Antibiotics 2023, 12, 245. [Google Scholar] [CrossRef]
- Chua, S.L.; Ding, Y.; Liu, Y.; Cai, Z.; Zhou, J.; Swarup, S.; Drautz-Moses, D.I.; Schuster, S.C.; Kjelleberg, S.; Givskov, M. Reactive Oxygen Species Drive Evolution of Pro-Biofilm Variants in Pathogens by Modulating Cyclic-Di-GMP Levels. Open Biol. 2016, 6, 160162. [Google Scholar] [CrossRef]
- Hazan, R.; Que, Y.A.; Maura, D.; Strobel, B.; Majcherczyk, P.A.; Hopper, L.R.; Wilbur, D.J.; Hreha, T.N.; Barquera, B.; Rahme, L.G. Auto Poisoning of the Respiratory Chain by a Quorum-Sensing-Regulated Molecule Favors Biofilm Formation and Antibiotic Tolerance. Curr. Biol. 2016, 26, 195–206. [Google Scholar] [CrossRef]
- Müsken, M.; Di Fiore, S.; Römling, U.; Häussler, S. A 96-Well-Plate–Based Optical Method for the Quantitative and Qualitative Evaluation of Pseudomonas aeruginosa Biofilm Formation and Its Application to Susceptibility Testing. Nat. Protoc. 2010, 5, 1460–1469. [Google Scholar] [CrossRef]
- Alkalay-Oren, S.; Yerushalmy, O.; Adler, K.; Khalifa, L.; Gelman, D.; Coppenhagen-Glazer, S.; Nir-Paz, R.; Hazan, R. Complete Genome Sequence of Pseudomonas aeruginosa Bacteriophage PASA16, Used in Multiple Phage Therapy Treatments Globally. Microbiol. Resour. Announc. 2022, 11, e00092-22. [Google Scholar] [CrossRef] [PubMed]
- Rimon, A.; Yerushalmy, O.; Belin, J.; Alkalay-Oren, S.; Gavish, L.; Coppenhagen-Glazer, S.; Hazan, R. Six Novel Pseudomonas aeruginosa Phages: Genomic Insights and Therapeutic Potential. Phage 2025, 6, 32–40. [Google Scholar] [CrossRef]
- Shteindel, N.; Yankelev, D.; Gerchman, Y. High-Throughput Quantitative Measurement of Bacterial Attachment Kinetics on Seconds Time Scale. Microb. Ecol. 2019, 77, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ghosh, D.; Vishakha, K.; Das, S.; Mondal, S.; Ganguli, A. Photodynamic Antimicrobial Chemotherapy (PACT) Using Riboflavin Inhibits the Mono and Dual Species Biofilm Produced by Antibiotic Resistant Staphylococcus aureus and Escherichia coli. Photodiagn. Photodyn. Ther. 2020, 32, 102002. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimon, A.; Belin, J.; Yerushalmy, O.; Eavri, Y.; Shapochnikov, A.; Coppenhagen-Glazer, S.; Hazan, R.; Gavish, L. Pulsed Blue Light and Phage Therapy: A Novel Synergistic Bactericide. Antibiotics 2025, 14, 481. https://doi.org/10.3390/antibiotics14050481
Rimon A, Belin J, Yerushalmy O, Eavri Y, Shapochnikov A, Coppenhagen-Glazer S, Hazan R, Gavish L. Pulsed Blue Light and Phage Therapy: A Novel Synergistic Bactericide. Antibiotics. 2025; 14(5):481. https://doi.org/10.3390/antibiotics14050481
Chicago/Turabian StyleRimon, Amit, Jonathan Belin, Ortal Yerushalmy, Yonatan Eavri, Anatoly Shapochnikov, Shunit Coppenhagen-Glazer, Ronen Hazan, and Lilach Gavish. 2025. "Pulsed Blue Light and Phage Therapy: A Novel Synergistic Bactericide" Antibiotics 14, no. 5: 481. https://doi.org/10.3390/antibiotics14050481
APA StyleRimon, A., Belin, J., Yerushalmy, O., Eavri, Y., Shapochnikov, A., Coppenhagen-Glazer, S., Hazan, R., & Gavish, L. (2025). Pulsed Blue Light and Phage Therapy: A Novel Synergistic Bactericide. Antibiotics, 14(5), 481. https://doi.org/10.3390/antibiotics14050481








