Repurposing High-Throughput Screening Reveals Unconventional Drugs with Antimicrobial and Antibiofilm Potential Against Methicillin-Resistant Staphylococcus aureus from a Cystic Fibrosis Patient
Abstract
1. Introduction
2. Results
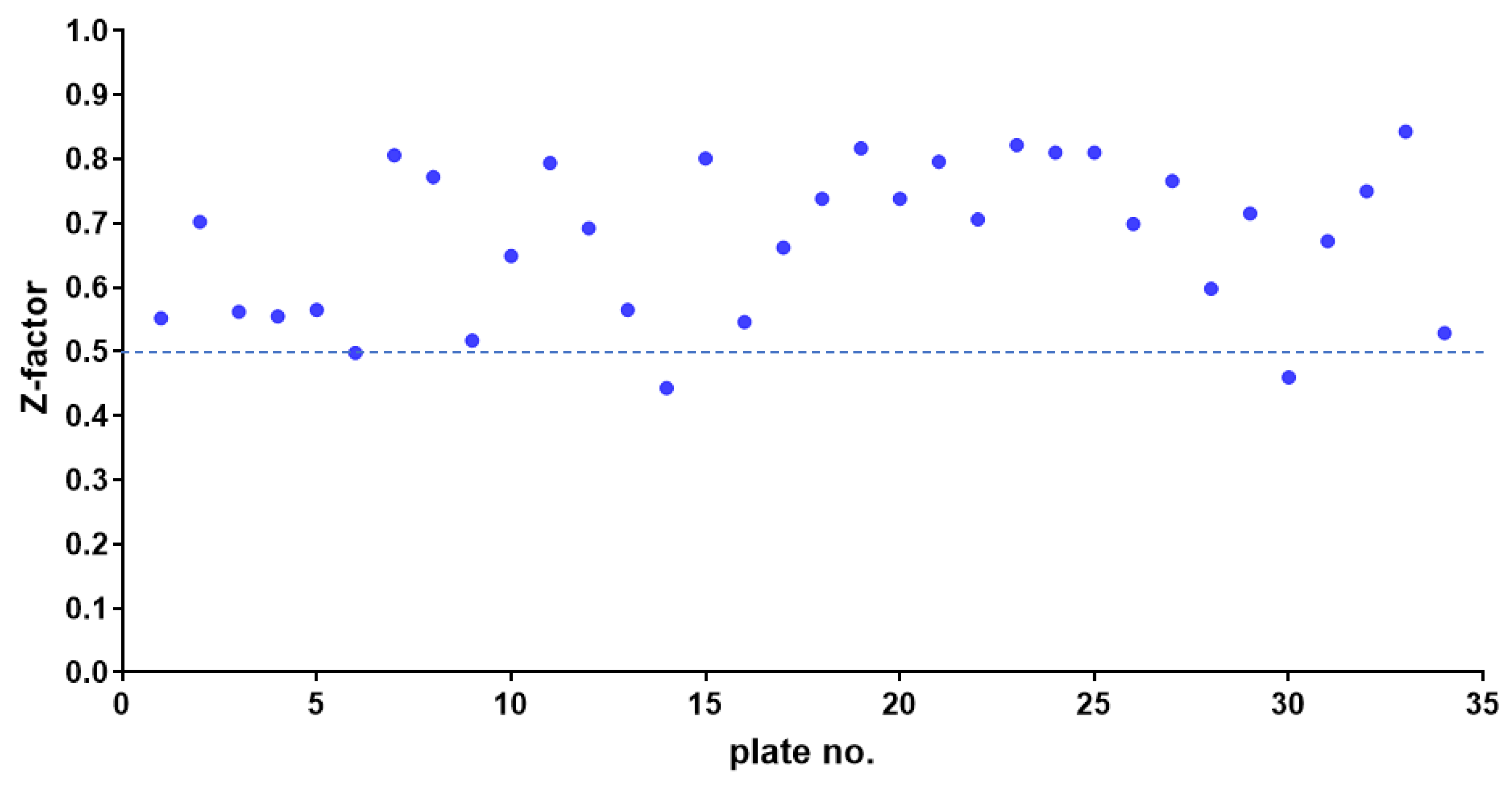
2.1. HTS Assay Validation
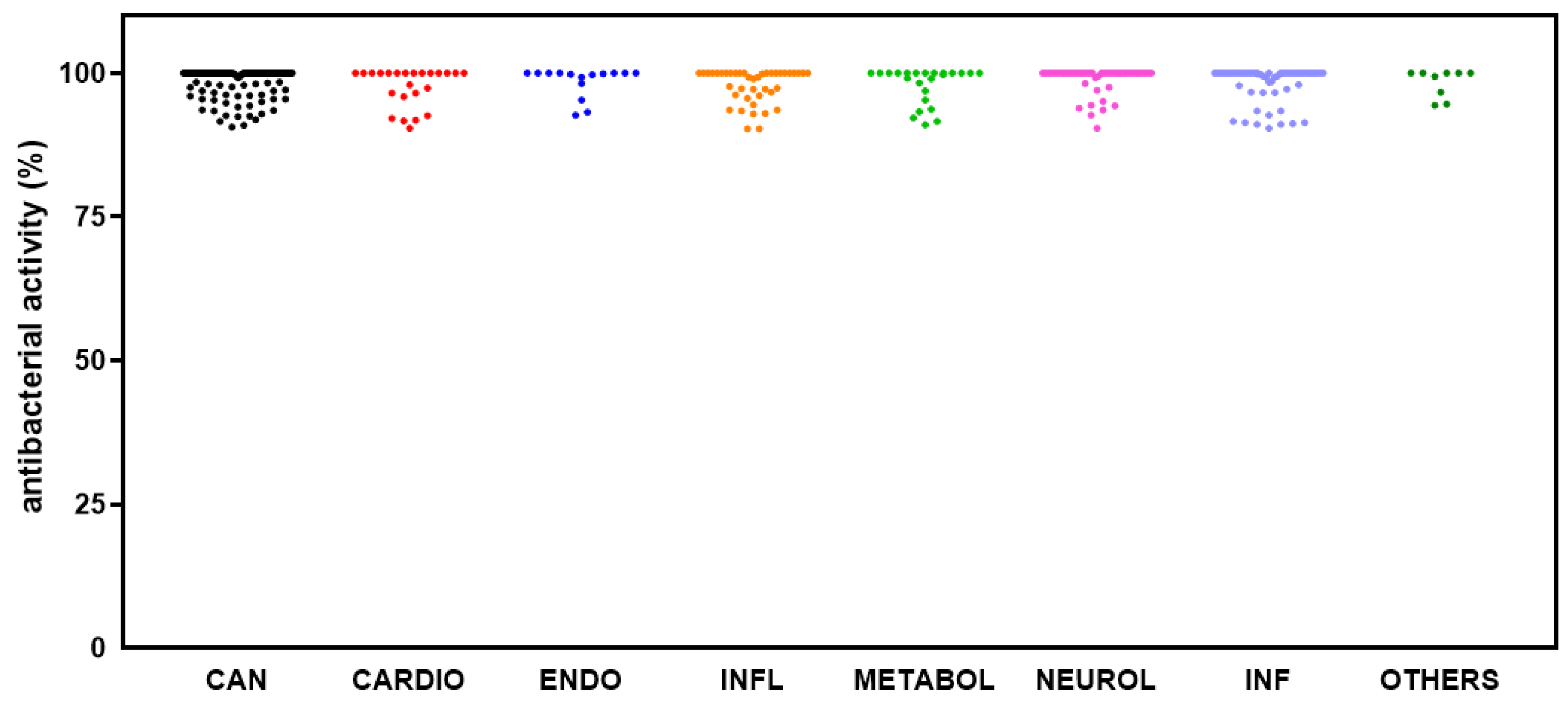
2.2. Identification of Hits Inhibiting S. aureus Growth
2.3. Identification of Hits Active Against S. aureus Biofilm Formation
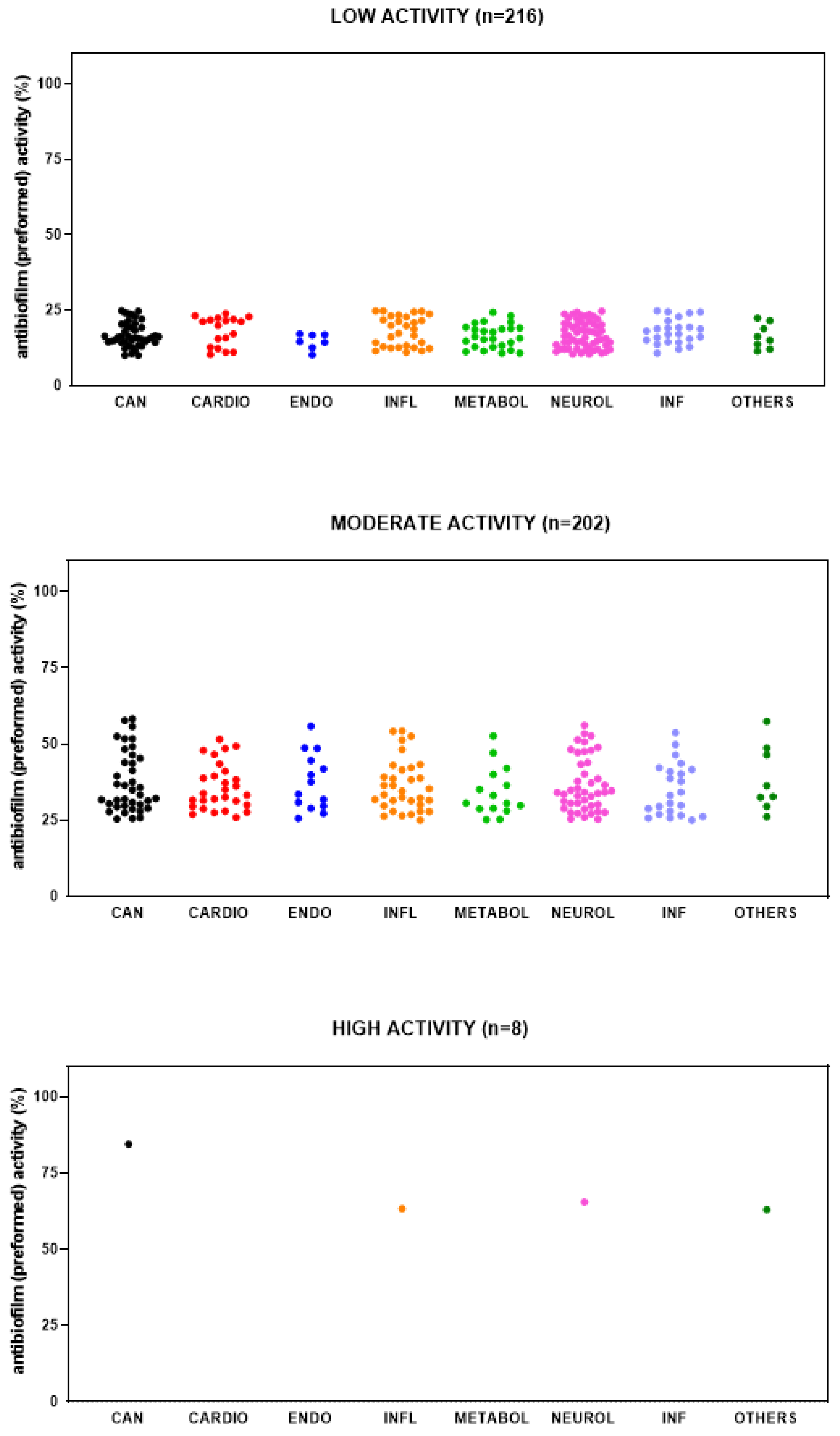
2.4. Identification of Hits Active Against Preformed Biofilm by S. aureus
3. Discussion
| Compound | Classification Therapeutic Category and Indication(s) Mechanism(s) of Action | Antibacterial Activity | Anti-Virulence Activity | Anti-Inflammatory Activity |
|---|---|---|---|---|
| Perphenazine |
|
|
| |
| Miltefosine |
|
| ||
| Diethylstilbestrol |
|
|
| |
| Selexipag |
|
| ||
| AZD-9056 |
| |||
| Vortioxetine |
|
| ||
| Lovastatin |
|
| ||
| Fenretinide |
|
| ||
| Napabucasin |
|
|
| |
| Loratadine |
|
|
| |
| Toremifene |
|
| ||
| Etifoxine |
|
| ||
| 5-(N,N-Hexamethylene)-amiloride |
|
|
| |
| Zafirlukast |
| |||
| Mitoquinone (mesylate) |
|
| ||
| Crisaborole |
|
| ||
| Linsitinib |
|
|
| |
| GW 501516 (Cardarine) |
|
| ||
| Darapladib |
|
| ||
| Bardoxolone |
| |||
| Meisoindigo |
| |||
| Epinastine |
|
| ||
| Salirasib |
| |||
| Omaveloxolone |
|
| ||
| Incyclinide |
|
| ||
| Glecaprevir |
|
| ||
| Isradipine |
|
| ||
| Laurocapram |
|
| ||
| Masitinib |
|
| ||
| Mitotane |
|
| ||
| Montelukast |
| |||
| Ricolinostat |
|
| ||
| Vandetanib |
|
| ||
| Verteporfin |
| |||
| Vigabatrin |
|
| ||
| Diroximel (fumarate) |
|
| ||
| Infigratinib |
|
|
| Compound | Classification Therapeutic Category and Indication(s) Mechanism(s) of Action | Antibacterial Activity | Anti-Virulence Activity | Anti-Inflammatory Activity |
|---|---|---|---|---|
| Tipifarnib |
|
|
| |
| Olaparib |
|
| ||
| Acefylline |
|
|
| |
| Hemin |
|
|
|
|
| TMC647055 (Choline salt) |
|
| Compound | Classification Therapeutic Category and Indication(s) Mechanism(s) of Action | Antibacterial Activity | Anti-Virulence Activity | Anti-Inflammatory Activity |
|---|---|---|---|---|
| Clemastine (fumarate) |
|
|
| |
| Heparin |
|
| ||
| Flumatinib (mesylate) |
| |||
| Bromfenac (sodium hydrate) |
|
|
|
4. Materials and Methods
4.1. Compound Library
4.2. Bacterial Strain and Growth Conditions
4.3. Antibacterial HTS Assay
4.4. HTS Assay Validation
4.5. Biofilm Inhibition and Dispersion HTS Assays
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic Fibrosis |
| MSSA | Methicillin-Sensitive Staphylococcus aureus |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| FEV1 | Forced Expiratory Volume in 1 s |
| HTS | High-Throughput Screening |
| VGS | Viridans Group Streptococci |
References
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Dasenbrook, E.C.; Merlo, C.A.; Diener-West, M.; Lechtzin, N.; Boyle, M.P. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Dasenbrook, E.C.; Checkley, W.; Merlo, C.A.; Konstan, M.W.; Lechtzin, N.; Boyle, M.P. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 2010, 303, 2386–2392. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Zhang, G.; Abbasi Tadi, D. Prevalence of antibiotic resistance of Staphylococcus aureus in cystic fibrosis infection: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2024, 36, 419–425. [Google Scholar] [CrossRef]
- Farha, M.A.; Brown, E.D. Drug repurposing for antimicrobial discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Nilakantan, R.; Immermann, F.; Haraki, K. A novel approach to combinatorial library design. Comb. Chem. High Throughput Screen. 2002, 5, 105–110. [Google Scholar] [CrossRef]
- Langer, T.; Hoffmann, R.; Bryant, S.; Lesur, B. Hit finding: Towards ‘smarter’ approaches. Curr. Opin. Pharmacol. 2009, 9, 589–593. [Google Scholar] [CrossRef]
- Bhagirath, A.Y.; Li, Y.; Somayajula, D.; Dadashi, M.; Badr, S.; Duan, K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016, 16, 174. [Google Scholar] [CrossRef]
- Gerits, E.; Defraine, V.; Vandamme, K.; De Cremer, K.; De Brucker, K.; Thevissen, K.; Cammue, B.P.; Beullens, S.; Fauvart, M.; Verstraeten, N.; et al. Repurposing Toremifene for Treatment of Oral Bacterial Infections. Antimicrob. Agents Chemother. 2017, 61, e01846-16. [Google Scholar] [CrossRef]
- Gerits, E.; Van der Massen, I.; Vandamme, K.; De Cremer, K.; De Brucker, K.; Thevissen, K.; Cammue, B.P.A.; Beullens, S.; Fauvart, M.; Verstraeten, N.; et al. In vitro activity of the antiasthmatic drug zafirlukast against the oral pathogens Porphyromonas gingivalis and Streptococcus mutans. FEMS Microbiol. Lett. 2017, 364, fnx005. [Google Scholar] [CrossRef]
- Mortensen, I.; Kristiansen, J.E.; Christensen, A.V.; Hvidberg, E.F. The antibacterial effect of some neuroleptics on strains isolated from patients with meningitis. Pharmacol. Toxicol. 1992, 71, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.; Yang, T.; Zhang, C.; Peng, X.; Ju, Y.; Li, C.; Zhou, X.; Luo, Y.; Xu, X. Repurposing Napabucasin as an Antimicrobial Agent against Oral Streptococcal Biofilms. BioMed Res. Int. 2020, 2020, 8379526. [Google Scholar] [CrossRef]
- Pinault, L.; Han, J.S.; Kang, C.M.; Franco, J.; Ronning, D.R. Zafirlukast inhibits complexation of Lsr2 with DNA and growth of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2134–2140. [Google Scholar] [CrossRef]
- Wang, H.; Bi, J.; Zhang, Y.; Pan, M.; Guo, Q.; Xiao, G.; Cui, Y.; Hu, S.; Chan, C.K.; Yuan, Y.; et al. Human Kinase IGF1R/IR Inhibitor Linsitinib Controls the In Vitro and Intracellular Growth of Mycobacterium tuberculosis. ACS Infect. Dis. 2022, 8, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- VanDevanter, D.R.; LiPuma, J.J.; Konstan, M.W. Longitudinal bacterial prevalence in cystic fibrosis airways: Fact and artifact. J. Cyst. Fibros. 2024, 23, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.C.; Rudzienski, L. In vitro suppression of Pseudomonas cepacia after limited exposure to subinhibitory concentrations of amiloride and 5-(N,N-hexamethylene) amiloride. Pediatr. Pulmonol. 1994, 17, 366–369. [Google Scholar] [CrossRef]
- LiPuma, J.J. The changing Microbial Epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 2010, 23, 299–323. [Google Scholar] [CrossRef]
- Gutiérrez Santana, J.C.; Coria Jiménez, V.R. Burkholderia cepacia complex in cystic fibrosis: Critical gaps in diagnosis and therapy. Ann. Med. 2024, 56, 2307503. [Google Scholar] [CrossRef]
- Paganin, P.; Fiscarelli, E.V.; Tuccio, V.; Chiancianesi, M.; Bacci, G.; Morelli, P.; Dolce, D.; Dalmastri, C.; De Alessandri, A.; Lucidi, V.; et al. Changes in cystic fibrosis airway microbial community associated with a severe decline in lung function. PLoS ONE 2015, 10, e0124348. [Google Scholar] [CrossRef]
- Rickard, A.H.; Gilbert, P.; High, N.J.; Kolenbrander, P.E.; Handley, P.S. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol. 2003, 11, 94–100. [Google Scholar] [CrossRef]
- Carullo, G.; Di Bonaventura, G.; Rossi, S.; Lupetti, V.; Tudino, V.; Brogi, S.; Butini, S.; Campiani, G.; Gemma, S.; Pompilio, A. Development of Quinazolinone Derivatives as Modulators of Virulence Factors of Pseudomonas aeruginosa Cystic Fibrosis Strains. Molecules 2023, 28, 6535. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, U.; Khullar, L.; Chadha, J.; Prerna Harjai, K. Beyond antibiotics: Emerging antivirulence strategies to combat Pseudomonas aeruginosa in cystic fibrosis. Microb. Pathog. 2024, 193, 106730. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Shang, Y.; Wu, Y.; Zhao, Y.; Chen, Z.; Lin, Z.; Li, P.; Sun, X.; Xu, G.; Wen, Z.; et al. Loratadine inhibits Staphylococcus aureus virulence and biofilm formation. iScience 2022, 25, 103731. [Google Scholar] [CrossRef]
- Koch, G.; Wermser, C.; Acosta, I.C.; Kricks, L.; Stengel, S.T.; Yepes, A.; Lopez, D. Attenuating Staphylococcus aureus Virulence by Targeting Flotillin Protein Scaffold Activity. Cell Chem. Biol. 2017, 24, 845–857.e6. [Google Scholar] [CrossRef] [PubMed]
- Yotis, W. Effects of diethylstilbestrol on the production of various extracellular products of Staphylococcus aureus. Experientia 1977, 33, 325–326. [Google Scholar] [CrossRef]
- Balogh, H.; Anthony, A.K.; Stempel, R.; Vossen, L.; Federico, V.A.; Valenzano, G.Z.; Blackledge, M.S.; Miller, H.B. Novel anti-virulence compounds disrupt exotoxin expression in MRSA. Microbiol. Spectr. 2024, 12, e0146424. [Google Scholar] [CrossRef]
- De Cremer, K.; Delattin, N.; De Brucker, K.; Peeters, A.; Kucharíková, S.; Gerits, E.; Verstraeten, N.; Michiels, J.; Van Dijck, P.; Cammue, B.P.; et al. Oral administration of the broad-spectrum antibiofilm compound toremifene inhibits Candida albicans and Staphylococcus aureus biofilm formation in vivo. Antimicrob. Agents Chemother. 2014, 58, 7606–7610. [Google Scholar] [CrossRef]
- Wargo, M.J.; Gross, M.J.; Rajamani, S.; Allard, J.L.; Lundblad, L.K.; Allen, G.B.; Vasil, M.L.; Leclair, L.W.; Hogan, D.A. Hemolytic phospholipase C inhibition protects lung function during Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 2011, 184, 345–354. [Google Scholar] [CrossRef]
- Hennessy, E.; Mooij, M.J.; Legendre, C.; Reen, F.J.; O’Callaghan, J.; Adams, C.; O’Gara, F. Statins inhibit in vitro virulence phenotypes of Pseudomonas aeruginosa. J. Antibiot. 2013, 66, 99–101. [Google Scholar] [CrossRef]
- Hussein, M.H.; Schneider, E.K.; Elliott, A.G.; Han, M.; Reyes-Ortega, F.; Morris, F.; Blastovich, M.A.T.; Jasim, R.; Currie, B.; Mayo, M.; et al. From Breast Cancer to Antimicrobial: Combating Extremely Resistant Gram-Negative “Superbugs” Using Novel Combinations of Polymyxin B with Selective Estrogen Receptor Modulators. Microb. Drug Resist. 2017, 23, 640–650. [Google Scholar] [CrossRef]
- Tran, T.B.; Wang, J.; Doi, Y.; Velkov, T.; Bergen, P.J.; Li, J. Novel Polymyxin Combination With Antineoplastic Mitotane Improved the Bacterial Killing Against Polymyxin-Resistant Multidrug-Resistant Gram-Negative Pathogens. Front. Microbiol. 2018, 9, 721. [Google Scholar] [CrossRef]
- Wang, N.; Li, W.; Yu, H.; Huang, W.; Qiao, Y.; Wang, Q.; Wei, Y.; Deng, X.; Wang, J.; Cui, M.; et al. Laurocapram, a transdermal enhancer, boosts cephalosporin’s antibacterial activity against Methicillin-resistant Staphylococcus aureus. Biochem. Pharmacol. 2024, 227, 116404. [Google Scholar] [CrossRef]
- Verhaar, A.P.; Wildenberg, M.E.; Velde, A.A.; Meijer, S.L.; Vos, A.C.; Duijvestein, M.; Peppelenbosch, M.P.; Hommes, D.W.; van den Brink, G.R. Miltefosine suppresses inflammation in a mouse model of inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.J.; Choi, S.Y.; Lee, C.; Choi, Y.M.; An, I.S.; Bae, S.; An, S.; Jung, J.H. Perphenazine Attenuates the Pro-Inflammatory Responses in Mouse Models of Th2-Type Allergic Dermatitis. Int. J. Mol. Sci. 2020, 21, 3241. [Google Scholar] [CrossRef] [PubMed]
- Geerdink, L.M.; Bertram, H.; Hansmann, G. First-in-child use of the oral selective prostacyclin IP receptor agonist selexipag in pulmonary arterial hypertension. Pulm. Circ. 2017, 7, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Aizman, E.; Blacher, E.; Ben-Moshe, O.; Kogan, T.; Kloog, Y.; Mor, A. Therapeutic effect of farnesylthiosalicylic acid on adjuvant-induced arthritis through suppressed release of inflammatory cytokines. Clin. Exp. Immunol. 2014, 175, 458–467. [Google Scholar] [CrossRef]
- Hu, H.; Yang, B.; Li, Y.; Zhang, S.; Li, Z. Blocking of the P2X7 receptor inhibits the activation of the MMP-13 and NF-κB pathways in the cartilage tissue of rats with osteoarthritis. Int. J. Mol. Med. 2016, 38, 1922–1932. [Google Scholar] [CrossRef]
- Townsend, E.A.; Guadarrama, A.; Shi, L.; Roti Roti, E.; Denlinger, L.C. P2X7 signaling influences the production of pro-resolving and pro-inflammatory lipid mediators in alveolar macrophages derived from individuals with asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L399–L410. [Google Scholar] [CrossRef]
- Tian, Q.; Yang, X.; Du, J.; Huang, H.; Liu, W.; Zhao, P. Translocator Protein Ligand Etifoxine Attenuates MPTP-Induced Neurotoxicity. Front. Mol. Neurosci. 2022, 15, 850904. [Google Scholar] [CrossRef]
- Yang, D.; Xu, D.; Wang, T.; Yuan, Z.; Liu, L.; Shen, Y.; Wen, F. Mitoquinone ameliorates cigarette smoke-induced airway inflammation and mucus hypersecretion in mice. Int. Immunopharmacol. 2021, 90, 107149. [Google Scholar] [CrossRef]
- Fan, T.; Wang, W.; Wang, Y.; Zeng, M.; Liu, Y.; Zhu, S.; Yang, L. PDE4 inhibitors: Potential protective effects in inflammation and vascular diseases. Front. Pharmacol. 2024, 15, 1407871. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, A.; Horstmann, M.; Daser, A.; Flögel, U.; Oeverhaus, M.; Bechrakis, N.E.; Banga, J.P.; Keitsch, S.; Wilker, B.; Krause, G.; et al. Linsitinib, an IGF-1R inhibitor, attenuates disease development and progression in a model of thyroid eye disease. Front. Endocrinol. 2023, 14, 1211473. [Google Scholar] [CrossRef] [PubMed]
- Ketabforoush, A.H.M.E.; Chegini, R.; Barati, S.; Tahmasebi, F.; Moghisseh, B.; Joghataei, M.T.; Faghihi, F.; Azedi, F. Masitinib: The promising actor in the next season of the Amyotrophic Lateral Sclerosis treatment series. Biomed. Pharmacother. 2023, 160, 114378. [Google Scholar] [CrossRef]
- Haddad, J.J.; Land, S.C. Amiloride blockades lipopolysaccharide-induced proinflammatory cytokine biosynthesis in an IkappaB-alpha/NF-kappaB-dependent mechanism. Evidence for the amplification of an antiinflammatory pathway in the alveolar epithelium. Am. J. Respir. Cell Mol. Biol. 2002, 26, 114–126. [Google Scholar] [CrossRef]
- Xue, T.; Zhang, Q.; Zhang, T.; Meng, L.; Liu, J.; Chai, D.; Liu, Y.; Yang, Z.; Jiao, R.; Cui, Y.; et al. Zafirlukast ameliorates lipopolysaccharide and bleomycin-induced lung inflammation in mice. BMC Pulm. Med. 2024, 24, 456. [Google Scholar] [CrossRef]
- Zhang, W.B.; Yang, F.; Wang, Y.; Jiao, F.Z.; Zhang, H.Y.; Wang, L.W.; Gong, Z.J. Inhibition of HDAC6 attenuates LPS-induced inflammation in macrophages by regulating oxidative stress and suppressing the TLR4-MAPK/NF-κB pathways. Biomed. Pharmacother. 2019, 117, 109166. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Kwak, H.J. Selective PPARδ Agonist GW501516 Protects Against LPS-Induced Macrophage Inflammation and Acute Liver Failure in Mice via Suppressing Inflammatory Mediators. Molecules 2024, 29, 5189. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, X.J.; Chen, H.M. Bardoxolone treatment alleviates lipopolysaccharide (LPS)-induced acute lung injury through suppressing inflammation and oxidative stress regulated by Nrf2 signaling. Biochem. Biophys. Res. Commun. 2019, 516, 270–277. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Ge, L.; He, S.; Zhang, Y.; Chen, D.; Nie, Y.; Zhu, M.; Pang, Q. RTA408 alleviates lipopolysaccharide-induced acute lung injury via inhibiting Bach1-mediated ferroptosis. Int. Immunopharmacol. 2024, 142 Pt B, 113250. [Google Scholar] [CrossRef]
- Cen, H.; Sun, M.; Zheng, B.; Peng, W.; Wen, Q.; Lin, Z.; Zhang, X.; Zhou, N.; Zhu, G.; Yu, X.; et al. Hyaluronic acid modified nanocarriers for aerosolized delivery of verteporfin in the treatment of acute lung injury. Int. J. Biol. Macromol. 2024, 267 Pt 1, 131386. [Google Scholar] [CrossRef]
- Puhl, A.C.; Gomes, G.F.; Damasceno, S.; Fritch, E.J.; Levi, J.A.; Johnson, N.J.; Scholle, F.; Premkumar, L.; Hurst, B.L.; Lee-Montiel, F.; et al. Vandetanib Blocks the Cytokine Storm in SARS-CoV-2-Infected Mice. ACS Omega 2022, 7, 31935–31944. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, C.; De Sanctis, J.B.; Wojewodka, G.; Saeed, Z.; Lachance, C.; Skinner, T.A.; Vilela, R.M.; Kubow, S.; Lands, L.C.; Hajduch, M.; et al. Fenretinide corrects newly found ceramide deficiency in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 38, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, C.; Wojewodka, G.; Saeed, Z.; Hajduch, M.; Matouk, E.; De Sanctis, J.B.; Radzioch, D. Cystic fibrosis fatty acid imbalance is linked to ceramide deficiency and corrected by fenretinide. Am. J. Respir. Cell Mol. Biol. 2009, 41, 100–106. [Google Scholar] [CrossRef]
- Conway, S.P.; Etherington, C.; Peckham, D.G.; Whitehead, A. A pilot study of zafirlukast as an anti-inflammatory agent in the treatment of adults with cystic fibrosis. J. Cyst. Fibros. 2003, 2, 25–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmitt-Grohé, S.; Zielen, S. Leukotriene receptor antagonists in children with cystic fibrosis lung disease: Anti-inflammatory and clinical effects. Paediatr. Drugs 2005, 7, 353–363. [Google Scholar] [CrossRef]
- Jean-Pierre, V.; Boudet, A.; Sorlin, P.; Menetrey, Q.; Chiron, R.; Lavigne, J.P.; Marchandin, H. Biofilm Formation by Staphylococcus aureus in the Specific Context of Cystic Fibrosis. Int. J. Mol. Sci. 2022, 24, 597. [Google Scholar] [CrossRef]
- Wieneke, M.K.; Dach, F.; Neumann, C.; Görlich, D.; Kaese, L.; Thißen, T.; Dübbers, A.; Kessler, C.; Große-Onnebrink, J.; Küster, P.; et al. Association of Diverse Staphylococcus aureus Populations with Pseudomonas aeruginosa Coinfection and Inflammation in Cystic Fibrosis Airway Infection. mSphere 2021, 6, e0035821. [Google Scholar] [CrossRef]
- Kapoor, K.; Singla, E.; Sahu, B.; Naura, A.S. PARP inhibitor, olaparib ameliorates acute lung and kidney injury upon intratracheal administration of LPS in mice. Mol. Cell. Biochem. 2015, 400, 153–162. [Google Scholar] [CrossRef]
- Elgazar, A.A.; El-Domany, R.A.; Eldehna, W.M.; Badria, F.A. Theophylline-based hybrids as acetylcholinesterase inhibitors endowed with anti-inflammatory activity: Synthesis, bioevaluation, in silico and preliminary kinetic studies. RSC Adv. 2023, 13, 25616–25634. [Google Scholar] [CrossRef]
- Cheng, X.; Yin, M.; Sun, X.; Zhang, Z.; Yao, X.; Liu, H.; Xia, H. Hemin attenuated LPS-induced acute lung injury in mice via protecting pulmonary epithelial barrier and regulating HO-1/NLRP3-mediated pyroptosis. Shock 2023, 59, 744–753. [Google Scholar] [CrossRef]
- Schmitt, J.; Joost, I.; Skaar, E.P.; Herrmann, M.; Bischoff, M. Haemin represses the haemolytic activity of Staphylococcus aureus in an Sae-dependent manner. Microbiology 2012, 158 Pt 10, 2619–2631. [Google Scholar] [CrossRef]
- Ladan, H.; Nitzan, Y.; Malik, Z. The antibacterial activity of haemin compared with cobalt, zinc and magnesium protoporphyrin and its effect on potassium loss and ultrastructure of Staphylococcus aureus. FEMS Microbiol. Lett. 1993, 112, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Hagemann, A.; Kaltenhäuser, J.; Besser, M.; Rockenfeller, P.; Ehrhardt, A.; Stuermer, E.; Bachmann, H.S. Bacteria Are New Targets for Inhibitors of Human Farnesyltransferase. Front. Microbiol. 2021, 12, 628283. [Google Scholar] [CrossRef]
- Shang, Y.; Guo, J.; Zhao, Y.; Chen, J.; Meng, Q.; Qu, D.; Zheng, J.; Yu, Z.; Wu, Y.; Deng, Q. Clemastine Inhibits the Biofilm and Hemolytic of Staphylococcus aureus through the GdpP Protein. Microbiol. Spectr. 2022, 10, e0054121. [Google Scholar] [CrossRef]
- Shanks, R.M.; Donegan, N.P.; Graber, M.L.; Buckingham, S.E.; Zegans, M.E.; Cheung, A.L.; O’Toole, G.A. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 2005, 73, 4596–4606. [Google Scholar] [CrossRef] [PubMed]
- Najarzadeh, Z.; Zaman, M.; Sereikaite, V.; Strømgaard, K.; Andreasen, M.; Otzen, D.E. Heparin promotes fibrillation of most phenol-soluble modulin virulence peptides from Staphylococcus aureus. J. Biol. Chem. 2021, 297, 100953. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Horswill, A.R. Heparin Mimics Extracellular DNA in Binding to Cell Surface-Localized Proteins and Promoting Staphylococcus aureus Biofilm Formation. mSphere 2017, 2, e00135-17. [Google Scholar] [CrossRef]
- Wu, D.; Li, X.; Yu, Y.; Gong, B.; Zhou, X. Heparin stimulates biofilm formation of Escherichia coli strain Nissle 1917. Biotechnol. Lett. 2021, 43, 235–246. [Google Scholar] [CrossRef]
- Chen, Q.; Xu, L.; Wu, T.; Li, J.; Hua, L. Analysis of abnormal intestinal flora on risk of intestinal cancer and effect of heparin on formation of bacterial biofilm. Bioengineered 2022, 13, 894–904. [Google Scholar] [CrossRef]
- Hamada, S.; Minami, S.; Gomi, M. Heparinoid enhances the efficacy of a bactericidal agent by preventing Cutibacterium acnes biofilm formation via quorum sensing inhibition. J. Microorg. Control 2024, 29, 27–31. [Google Scholar] [CrossRef]
- Kida, T.; Kozai, S.; Takahashi, H.; Isaka, M.; Tokushige, H.; Sakamoto, T. Pharmacokinetics and efficacy of topically applied nonsteroidal anti-inflammatory drugs in retinochoroidal tissues in rabbits. PLoS ONE 2014, 9, e96481. [Google Scholar] [CrossRef] [PubMed]
- Yi, N.Y.; Newman, D.R.; Zhang, H.; Morales Johansson, H.; Sannes, P.L. Heparin and LPS-induced COX-2 expression in airway cells: A link between its anti-inflammatory effects and GAG sulfation. Exp. Lung Res. 2015, 41, 499–513. [Google Scholar] [CrossRef]
- Chimenti, L.; Camprubí-Rimblas, M.; Guillamat-Prats, R.; Gomez, M.N.; Tijero, J.; Blanch, L.; Artigas, A. Nebulized Heparin Attenuates Pulmonary Coagulopathy and Inflammation through Alveolar Macrophages in a Rat Model of Acute Lung Injury. Thromb. Haemost. 2017, 117, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; El-Maraghy, S.A.; Kamel, A.S.; Said, S.E.; Kortam, M.A. Modulation of p38 MAPK and Nrf2/HO-1/NLRP3 inflammasome signaling and pyroptosis outline the anti-neuroinflammatory and remyelinating characters of Clemastine in EAE rat model. Biochem. Pharmacol. 2023, 209, 115435. [Google Scholar] [CrossRef] [PubMed]
- Tou, J.S.; Urbizo, C. Diethylstilbestrol inhibits phospholipase D activity and degranulation by stimulated human neutrophils. Steroids 2008, 73, 216–221. [Google Scholar] [CrossRef]
- Talmon, M.; Rossi, S.; Pastore, A.; Cattaneo, C.I.; Brunelleschi, S.; Fresu, L.G. Vortioxetine exerts anti-inflammatory and immunomodulatory effects on human monocytes/macrophages. Br. J. Pharmacol. 2018, 175, 113–124. [Google Scholar] [CrossRef]
- Planagumà, A.; Pfeffer, M.A.; Rubin, G.; Croze, R.; Uddin, M.; Serhan, C.N.; Levy, B.D. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal Immunol. 2010, 3, 270–279. [Google Scholar] [CrossRef]
- Williams, J.P.; Hernady, E.; Johnston, C.J.; Reed, C.M.; Fenton, B.; Okunieff, P.; Finkelstein, J.N. Effect of administration of lovastatin on the development of late pulmonary effects after whole-lung irradiation in a murine model. Radiat. Res. 2004, 161, 560–567. [Google Scholar] [CrossRef]
- Ravi, L.I.; Li, L.; Wong, P.S.; Sutejo, R.; Tan, B.H.; Sugrue, R.J. Lovastatin treatment mitigates the pro-inflammatory cytokine response in respiratory syncytial virus infected macrophage cells. Antivir. Res. 2013, 98, 332–343. [Google Scholar] [CrossRef]
- Yu, H.; Valerio, M.; Bielawski, J. Fenretinide inhibited de novo ceramide synthesis and proinflammatory cytokines induced by Aggregatibacter actinomycetemcomitans. J. Lipid Res. 2013, 54, 189–201. [Google Scholar] [CrossRef]
- Wang, J.; Feng, H.; Li, Z.; Zhang, X. Napabucasin prevents brain injury in neuronal neonatal rat cells through suppression of apoptosis and inflammation. Microb. Pathog. 2019, 128, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Hsu, J.Y.; Fu, L.S.; Chang, W.C.; Chu, J.J.; Chi, C.S. Influence of cetirizine and loratadine on granulocyte-macrophage colony-stimulating factor and interleukin-8 release in A549 human airway epithelial cells stimulated with interleukin-1beta. J. Microbiol. Immunol. Infect. 2006, 39, 206–211. [Google Scholar] [PubMed]
- Lv, S.L.; Zeng, Z.F.; Gan, W.Q.; Wang, W.Q.; Li, T.G.; Hou, Y.F.; Yan, Z.; Zhang, R.X.; Yang, M. Lp-PLA2 inhibition prevents Ang II-induced cardiac inflammation and fibrosis by blocking macrophage NLRP3 inflammasome activation. Acta Pharmacol. Sin. 2021, 42, 2016–2032. [Google Scholar] [CrossRef]
- He, J.X.; Zhu, C.Q.; Liang, G.F.; Mao, H.B.; Shen, W.Y.; Hu, J.B. Targeted-lung delivery of bardoxolone methyl using PECAM-1 antibody-conjugated nanostructure lipid carriers for the treatment of lung inflammation. Biomed. Pharmacother. 2024, 178, 116992. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Xiong, X.; Deng, X.; Gu, L.; Wang, Q.; Zeng, Z.; Gao, X.; Gao, Q.; Wang, Y. Meisoindigo, but not its core chemical structure indirubin, inhibits zebrafish interstitial leukocyte chemotactic migration. Pharm. Biol. 2017, 55, 673–679. [Google Scholar] [CrossRef]
- Ye, Y.; Jin, T.; Zhang, X.; Zeng, Z.; Ye, B.; Wang, J.; Zhong, Y.; Xiong, X.; Gu, L. Meisoindigo Protects Against Focal Cerebral Ischemia-Reperfusion Injury by Inhibiting NLRP3 Inflammasome Activation and Regulating Microglia/Macrophage Polarization via TLR4/NF-κB Signaling Pathway. Front. Cell. Neurosci. 2019, 13, 553. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sawamoto, A.; Okuyama, S.; Nakajima, M. T-Cell Activation-Inhibitory Assay to Screen Caloric Restriction Mimetics Drugs for Drug Repositioning. Biol. Pharm. Bull. 2021, 44, 550–556. [Google Scholar] [CrossRef]
- Wu, J.J.; Yuan, X.M.; Huang, C.; An, G.Y.; Liao, Z.L.; Liu, G.A.; Chen, R.X. Farnesyl thiosalicylic acid prevents iNOS induction triggered by lipopolysaccharide via suppression of iNOS mRNA transcription in murine macrophages. Int. Immunopharmacol. 2019, 68, 218–225. [Google Scholar] [CrossRef]
- Alyousef, A.A.; Divakar, D.D.; Muzaheed. Chemically modified tetracyclines an emerging host modulator in chronic periodontitis patients: A randomized, double-blind, placebo-controlled, clinical trial. Microb. Pathog. 2017, 110, 279–284. [Google Scholar] [CrossRef]
- Farghaly, T.A.; Alqurashi, R.M.; Masaret, G.S.; Abdulwahab, H.G. Recent Methods for the Synthesis of Quinoxaline Derivatives and their Biological Activities. Mini Rev. Med. Chem. 2024, 24, 920–982. [Google Scholar] [CrossRef]
- Capecchi, P.L.; Laghi Pasini, F.; Ceccatelli, L.; Di Perri, T. Isradipine inhibits PMN leukocyte function. A possible interference with the adenosine system. Immunopharmacol. Immunotoxicol. 1993, 15, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Alnfakh, Z.A.; Al-Mudhafar, D.H.; Al-Nafakh, R.T.; Jasim, A.E.; Hadi, N.R. The anti-inflammatory and antioxidant effects of Montelukast on lung sepsis in adult mice. J. Med. Life 2022, 15, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.; Rapún-Araiz, B.; Gil, C.; Penadés, J.R.; Lasa, I.; Latasa, C. Inhibiting the two-component system GraXRS with verteporfin to combat Staphylococcus aureus infections. Sci. Rep. 2020, 10, 17939. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.; Al-Shabanah, O.A.; El-Hadiyah, T.M.H.; Qureshi, S. Thermoregulatory and In-vivo Anti-inflammatory Effects of Vigabatrin In Rat and Mice. Sci. Pharm. 2000, 68, 379–388. [Google Scholar] [CrossRef]
- He, Y.; Gong, G.; Quijas, G.; Lee, S.M.; Chaudhuri, R.K.; Bojanowski, K. Comparative activity of dimethyl fumarate derivative IDMF in three models relevant to multiple sclerosis and psoriasis. FEBS Open Bio 2025. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Rajendran, V.; Böttiger, G.; Stadelmann, C.; Shirvanchi, K.; von Au, L.; Bhushan, S.; Wallendszus, N.; Schunin, D.; Westbrock, V.; et al. The small molecule fibroblast growth factor receptor inhibitor infigratinib exerts anti-inflammatory effects and remyelination in a model of multiple sclerosis. Br. J. Pharmacol. 2023, 180, 2989–3007. [Google Scholar] [CrossRef]
- Marcuzzi, A.; De Leo, L.; Decorti, G.; Crovella, S.; Tommasini, A.; Pontillo, A. The farnesyltransferase inhibitors tipifarnib and lonafarnib inhibit cytokines secretion in a cellular model of mevalonate kinase deficiency. Pediatr. Res. 2011, 70, 78–82. [Google Scholar] [CrossRef]
- Voynikov, Y.; Valcheva, V.; Momekov, G.; Peikov, P.; Stavrakov, G. Theophylline-7-acetic acid derivatives with amino acids as anti-tuberculosis agents. Bioorg. Med. Chem. Lett. 2014, 24, 3043–3045. [Google Scholar] [CrossRef]
- Rosett, W.; Hodges, G.R. Antimicrobial activity of heparin. J. Clin. Microbiol. 1980, 11, 30–34. [Google Scholar] [CrossRef]
- Zappala, C.; Chandan, S.; George, N.; Faoagali, J.; Boots, R.J. The antimicrobial effect of heparin on common respiratory pathogens. Crit. Care Resusc. 2007, 9, 157–160. [Google Scholar] [CrossRef]
- Patti, J.M.; Allen, B.L.; McGavin, M.J.; Höök, M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 1994, 48, 585–617. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, Y.; Whittell, L.R.; Jergic, S.; Liu, M.; Harry, E.; Dixon, N.E.; Kelso, M.J.; Beck, J.L.; Oakley, A.J. DNA replication is the target for the antibacterial effects of nonsteroidal anti-inflammatory drugs. Chem. Biol. 2014, 21, 481–487. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompilio, A.; Lupetti, V.; Puca, V.; Di Bonaventura, G. Repurposing High-Throughput Screening Reveals Unconventional Drugs with Antimicrobial and Antibiofilm Potential Against Methicillin-Resistant Staphylococcus aureus from a Cystic Fibrosis Patient. Antibiotics 2025, 14, 402. https://doi.org/10.3390/antibiotics14040402
Pompilio A, Lupetti V, Puca V, Di Bonaventura G. Repurposing High-Throughput Screening Reveals Unconventional Drugs with Antimicrobial and Antibiofilm Potential Against Methicillin-Resistant Staphylococcus aureus from a Cystic Fibrosis Patient. Antibiotics. 2025; 14(4):402. https://doi.org/10.3390/antibiotics14040402
Chicago/Turabian StylePompilio, Arianna, Veronica Lupetti, Valentina Puca, and Giovanni Di Bonaventura. 2025. "Repurposing High-Throughput Screening Reveals Unconventional Drugs with Antimicrobial and Antibiofilm Potential Against Methicillin-Resistant Staphylococcus aureus from a Cystic Fibrosis Patient" Antibiotics 14, no. 4: 402. https://doi.org/10.3390/antibiotics14040402
APA StylePompilio, A., Lupetti, V., Puca, V., & Di Bonaventura, G. (2025). Repurposing High-Throughput Screening Reveals Unconventional Drugs with Antimicrobial and Antibiofilm Potential Against Methicillin-Resistant Staphylococcus aureus from a Cystic Fibrosis Patient. Antibiotics, 14(4), 402. https://doi.org/10.3390/antibiotics14040402








