Serogrouping and Molecular Characterization of ESBL-Producing Avian Pathogenic Escherichia coli from Broilers and Turkeys with Colibacillosis in Algeria
Abstract
1. Introduction
2. Results
2.1. APEC Isolation, Serogrouping, and Antimicrobial Susceptibility Testing
2.2. Prevalence and Antimicrobial Resistance Genotype of ESBL-Producing APEC Isolates
2.3. Molecular Typing and Conjugation Transference
3. Discussion
4. Materials and Methods
4.1. Research Approval
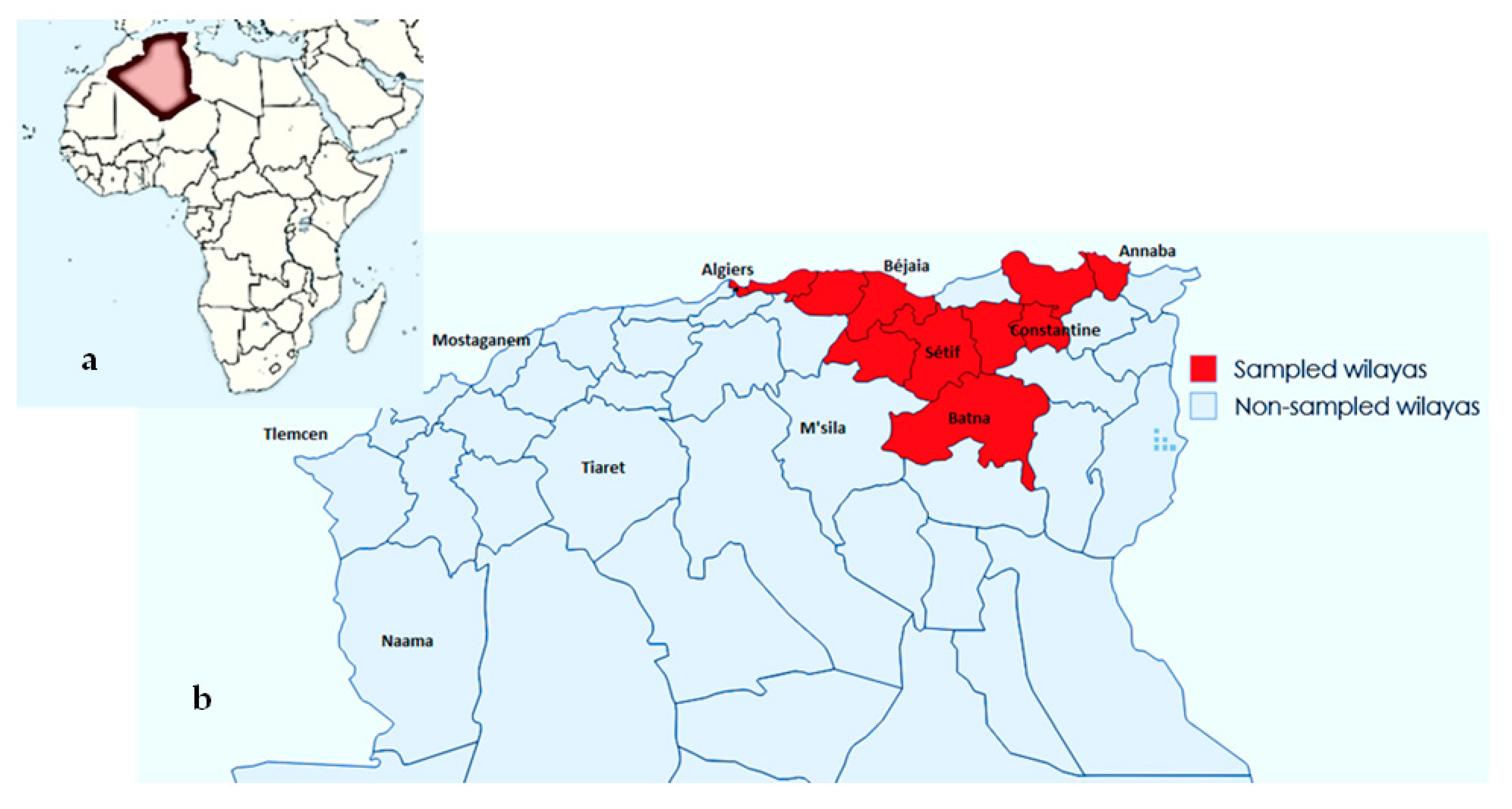
4.2. Sample Collection
4.3. Sample Processing and APEC Strain Isolation
4.4. Serogrouping
4.5. Antimicrobial Susceptibility and ESBL Testing
4.6. Antimicrobial Resistance Genes in ESBL-Producing APEC Isolates
4.7. Phylogrouping, MLST, and Conjugation Experiments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lutful Kabir, S.M. Avian Colibacillosis and Salmonellosis: A Closer Look at Epidemiology, Pathogenesis, Diagnosis, Control and Public Health Concerns. Int. J. Environ. Res. Public. Health 2010, 7, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.K.; Vaillancourt, J.P.; Barbieri, N.L.; Logue, C.M. Colibacillosis. In Diseases of Poultry, 14th ed.; Swayne, D.E., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; Volume 1, pp. 770–830. [Google Scholar]
- Ewers, C.; Janssen, T.; Wieler, L.H. Avian pathogenic Escherichia coli (APEC). Berl. Munch. Tierarztl. Wochenschr. 2003, 116, 381–395. [Google Scholar]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, F. The Serology of the Coli Group. J. Immunol. Baltim. Md 1950 1947, 57, 71–100. [Google Scholar]
- Dziva, F.; Stevens, M.P. Colibacillosis in Poultry: Unravelling the Molecular Basis of Virulence of Avian Pathogenic Escherichia coli in Their Natural Hosts. Avian Pathol. 2008, 37, 355–366. [Google Scholar] [CrossRef]
- Newman, D.M.; Barbieri, N.L.; de Oliveira, A.L.; Willis, D.; Nolan, L.K.; Logue, C.M. Characterizing Avian Pathogenic Escherichia coli (APEC) from Colibacillosis Cases, 2018. PeerJ 2021, 9, e11025. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Mehat, J.W.; van Vliet, A.H.M.; La Ragione, R.M. The Avian Pathogenic Escherichia coli (APEC) Pathotype Is Comprised of Multiple Distinct, Independent Genotypes. Avian Pathol. J. WVPA 2021, 50, 402–416. [Google Scholar] [CrossRef]
- Wirth, T.; Falush, D.; Lan, R.; Colles, F.; Mensa, P.; Wieler, L.H.; Karch, H.; Reeves, P.R.; Maiden, M.C.J.; Ochman, H.; et al. Sex and Virulence in Escherichia coli: An Evolutionary Perspective. Mol. Microbiol. 2006, 60, 1136–1151. [Google Scholar] [CrossRef]
- Kravik, I.H.; Kaspersen, H.; Sjurseth, S.K.; Dean, K.R.; David, B.; Aspholm, M.; Sekse, C. A Molecular Epidemiological Study on Escherichia coli in Young Chicks with Colibacillosis Identified Two Possible Outbreaks across Farms. Vet. Res. 2023, 54, 10. [Google Scholar] [CrossRef]
- Cordoni, G.; Woodward, M.J.; Wu, H.; Alanazi, M.; Wallis, T.; La Ragione, R.M. Comparative Genomics of European Avian Pathogenic E. Coli (APEC). BMC Genomics 2016, 17, 960. [Google Scholar] [CrossRef] [PubMed]
- Chenouf, N.S.; Carvalho, I.; Messaï, C.R.; Ruiz-Ripa, L.; Mama, O.M.; Titouche, Y.; Zitouni, A.; Hakem, A.; Torres, C. Extended Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Broiler Liver in the Center of Algeria, with Detection of CTX-M-55 and B2/ST131-CTX-M-15 in Escherichia coli. Microb. Drug Resist. Larchmt. N 2021, 27, 268–276. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kathayat, D.; Closs, G.J.; Galgozy, K.; Fuchs, J.R.; Rajashekara, G. Efficacy of Quorum Sensing and Growth Inhibitors Alone and in Combination against Avian Pathogenic Escherichia coli Infection in Chickens. Poult. Sci. 2023, 102, 102543. [Google Scholar] [CrossRef] [PubMed]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Ur Rahman, S.; Ali, T.; Ali, I.; Khan, N.A.; Han, B.; Gao, J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. BioMed Res. Int. 2018, 2018, 9519718. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-Spectrum β-Lactamases: An Update on Their Characteristics, Epidemiology and Detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef]
- Meguenni, N.; Chanteloup, N.; Tourtereau, A.; Ahmed, C.A.; Bounar-Kechih, S.; Schouler, C. Virulence and Antibiotic Resistance Profile of Avian Escherichia coli Strains Isolated from Colibacillosis Lesions in Central of Algeria. Vet. World 2019, 12, 1840–1848. [Google Scholar] [CrossRef]
- Mohamed, L.; Ge, Z.; Yuehua, L.; Yubin, G.; Rachid, K.; Mustapha, O.; Junwei, W.; Karine, O. Virulence Traits of Avian Pathogenic (APEC) and Fecal (AFEC) E. coli Isolated from Broiler Chickens in Algeria. Trop. Anim. Health Prod. 2018, 50, 547–553. [Google Scholar] [CrossRef]
- Meguenni, N.; Le Devendec, L.; Jouy, E.; Le Corvec, M.; Bounar-Kechih, S.; Rabah Bakour, D.; Kempf, I. First Description of an Extended-Spectrum Cephalosporin- and Fluoroquinolone- Resistant Avian Pathogenic Escherichia coli Clone in Algeria. Avian Dis. 2015, 59, 20–23. [Google Scholar] [CrossRef]
- Messaï, C.; Khelef, D.; Boukhors, K.; Radji, N.; Goucem, R.; Hamdi, T. Antimicrobial Susceptibility of Escherichia coli Strains Isolated from Broiler Chickens Affected by Colibacillosis in Setif. Afr. J. Microbiol. Res. 2013, 7, 2668–2672. [Google Scholar] [CrossRef]
- Messaï, C.R.; Aït-Oudhia, K.; Khelef, D.; Hamdi, T.M.; Chenouf, N.S.; Messaï, M.R. Serogroups and Antibiotic Susceptibility Pattern of Avian Pathogenic Escherichia coli Strains Responsible for Colibacillosis in Broiler Breeding Farms in the East of Algeria. Afr. J. Microbiol. Res. 2015, 9, 2358–2363. [Google Scholar] [CrossRef]
- Halfaoui, Z.; Menoueri, N.M.; Bendali, L.M. Serogrouping and Antibiotic Resistance of Escherichia coli Isolated from Broiler Chicken with Colibacillosis in Center of Algeria. Vet. World 2017, 10, 830–835. [Google Scholar] [CrossRef]
- Aberkane, C.; Messaï, A.; Messaï, C.R.; Boussaada, T. Antimicrobial Resistance Pattern of Avian Pathogenic Escherichia coli with Detection of Extended-Spectrum β-Lactamase-Producing Isolates in Broilers in East Algeria. Vet. World 2023, 16, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Goudarztalejerdi, A.; Mohammadzadeh, A.; Najafi, S.V.; Nargesi, F.; Joudari, S. Serogrouping, Phylotyping, and Virulence Genotyping of Commensal and Avian Pathogenic Escherichia coli Isolated from Broilers in Hamedan, Iran. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101558. [Google Scholar] [CrossRef]
- Delago, J.; Miller, E.A.; Flores-Figueroa, C.; Munoz-Aguayo, J.; Cardona, C.; Smith, A.H.; Johnson, T.J. Survey of Clinical and Commensal Escherichia coli from Commercial Broilers and Turkeys, with Emphasis on High-Risk Clones Using APECTyper. Poult. Sci. 2023, 102, 102712. [Google Scholar] [CrossRef]
- Mellata, M.; Dho-Moulin, M.; Dozois, C.M.; Curtiss, R., 3rd; Lehoux, B.; Fairbrother, J.M. Role of Avian Pathogenic Escherichia coli Virulence Factors in Bacterial Interaction with Chicken Heterophils and Macrophages. Infect. Immun. 2003, 71, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Yoon, M.Y.; Ha, J.S.; Seo, K.W.; Noh, E.B.; Son, S.H.; Lee, Y.J. Molecular Characterization of Avian Pathogenic Escherichia coli from Broiler Chickens with Colibacillosis. Poult. Sci. 2020, 99, 1088–1095. [Google Scholar] [CrossRef]
- Hu, R.; Li, J.; Zhao, Y.; Lin, H.; Liang, L.; Wang, M.; Liu, H.; Min, Y.; Gao, Y.; Yang, M. Exploiting Bacterial Outer Membrane Vesicles as a Cross-Protective Vaccine Candidate against Avian Pathogenic Escherichia coli (APEC). Microb. Cell Factories 2020, 19, 119. [Google Scholar] [CrossRef]
- Durso, L.M.; Bono, J.L.; Keen, J.E. Molecular Serotyping of Escherichia coli O26:H11. Appl. Environ. Microbiol. 2005, 71, 4941–4944. [Google Scholar] [CrossRef]
- Schouler, C.; Schaeffer, B.; Brée, A.; Mora, A.; Dahbi, G.; Biet, F.; Oswald, E.; Mainil, J.; Blanco, J.; Moulin-Schouleur, M. Diagnostic Strategy for Identifying Avian Pathogenic Escherichia coli Based on Four Patterns of Virulence Genes. J. Clin. Microbiol. 2012, 50, 1673–1678. [Google Scholar] [CrossRef]
- Frydendahl, K. Prevalence of Serogroups and Virulence Genes in Escherichia coli Associated with Postweaning Diarrhoea and Edema Disease in Pigs and a Comparison of Diagnostic Approaches. Vet. Microbiol. 2002, 85, 169–182. [Google Scholar] [CrossRef]
- La Ragione, R.M.; Woodward, M.J. Virulence Factors of Escherichia coli Serotypes Associated with Avian Colisepticaemia. Res. Vet. Sci. 2002, 73, 27–35. [Google Scholar] [CrossRef]
- Dziva, F.; Hauser, H.; Connor, T.R.; van Diemen, P.M.; Prescott, G.; Langridge, G.C.; Eckert, S.; Chaudhuri, R.R.; Ewers, C.; Mellata, M.; et al. Sequencing and Functional Annotation of Avian Pathogenic Escherichia coli Serogroup O78 Strains Reveal the Evolution of E. coli Lineages Pathogenic for Poultry via Distinct Mechanisms. Infect. Immun. 2013, 81, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Belmahdi, M.; Bakour, S.; Al Bayssari, C.; Touati, A.; Rolain, J.-M. Molecular Characterisation of Extended-Spectrum β-Lactamase- and Plasmid AmpC-Producing Escherichia coli Strains Isolated from Broilers in Béjaïa, Algeria. J. Glob. Antimicrob. Resist. 2016, 6, 108–112. [Google Scholar] [CrossRef]
- Maamar, E.; Hammami, S.; Alonso, C.A.; Dakhli, N.; Abbassi, M.S.; Ferjani, S.; Hamzaoui, Z.; Saidani, M.; Torres, C.; Boubaker, I.B.-B. High Prevalence of Extended-Spectrum and Plasmidic AmpC Beta-Lactamase-Producing Escherichia coli from Poultry in Tunisia. Int. J. Food Microbiol. 2016, 231, 69–75. [Google Scholar]
- Misumi, W.; Magome, A.; Okuhama, E.; Uchimura, E.; Tamamura-Andoh, Y.; Watanabe, Y.; Kusumoto, M. CTX-M-55-Type ESBL-Producing Fluoroquinolone-Resistant Escherichia Coli Sequence Type 23 Repeatedly Caused Avian Colibacillosis in Kagoshima Prefecture, Japan. J. Glob. Antimicrob. Resist. 2023, 35, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Solà-Ginés, M.; Cameron-Veas, K.; Badiola, I.; Dolz, R.; Majó, N.; Dahbi, G.; Viso, S.; Mora, A.; Blanco, J.; Piedra-Carrasco, N.; et al. Diversity of Multi-Drug Resistant Avian Pathogenic Escherichia coli (APEC) Causing Outbreaks of Colibacillosis in Broilers during 2012 in Spain. PLoS ONE 2015, 10, e0143191. [Google Scholar] [CrossRef]
- da Silva, F.B.; Ferreira, M.R.A.; Sobrinho, I.d.S.J.; Dias, M.; Rodrigues, R.R.; Moreira, C.N. Occurrence of ESBL-Producing Avian Pathogenic Escherichia coli (APEC) Isolates in Spiced Chicken Meat in Goias, Brazil. Lett. Appl. Microbiol. 2023, 76, ovac070. [Google Scholar] [CrossRef]
- Seo, K.W.; Shim, J.B.; Kim, Y.B.; Son, S.H.; Bi Noh, E.; Yoon, S.; Lim, S.-K.; Ju Lee, Y. Impacts and Characteristics of Antimicrobial Resistance of Escherichia coli Isolates by Administration of Third-Generation Cephalosporins in Layer Hatcheries. Vet. Microbiol. 2020, 243, 108643. [Google Scholar] [CrossRef]
- Baron, S.; Jouy, E.; Larvor, E.; Eono, F.; Bougeard, S.; Kempf, I. Impact of Third-Generation-Cephalosporin Administration in Hatcheries on Fecal Escherichia coli Antimicrobial Resistance in Broilers and Layers. Antimicrob. Agents Chemother. 2014, 58, 5428–5434. [Google Scholar] [CrossRef]
- Saraiva, M.M.S.; Moreira Filho, A.L.B.; Freitas Neto, O.C.; Silva, N.M.V.; Givisiez, P.E.N.; Gebreyes, W.A.; Oliveira, C.J.B. Off-Label Use of Ceftiofur in One-Day Chicks Triggers a Short-Term Increase of ESBL-Producing E. coli in the Gut. PLoS ONE 2018, 13, e0203158. [Google Scholar] [CrossRef]
- Heinrich, K.; Chan, D.; Fussell, R.J.; Kay, J.F.; Sharman, M. Can the Unauthorised Use of Ceftiofur Be Detected in Poultry? Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1733–1738. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, A.; Zhao, R.; Yin, L.; Yin, D.; Dai, Y.; Hou, H.; Wang, J.; Hu, X.; Pan, X.; et al. Effects of Adding Antibiotics to an Inactivated Oil-Adjuvant Avian Influenza Vaccine on Vaccine Characteristics and Chick Health. Poult. Sci. 2024, 103, 104135. [Google Scholar] [CrossRef] [PubMed]
- Hassen, B.; Abbassi, M.S.; Ruiz-Ripa, L.; Mama, O.M.; Hassen, A.; Torres, C.; Hammami, S. High Prevalence of Mcr-1 Encoding Colistin Resistance and First Identification of Bla(CTX-M-55) in ESBL/CMY-2-Producing Escherichia coli Isolated from Chicken Faeces and Retail Meat in Tunisia. Int. J. Food Microbiol. 2020, 318, 108478. [Google Scholar] [CrossRef]
- Irrgang, A.; Hammerl, J.A.; Falgenhauer, L.; Guiral, E.; Schmoger, S.; Imirzalioglu, C.; Fischer, J.; Guerra, B.; Chakraborty, T.; Käsbohrer, A. Diversity of CTX-M-1-Producing E. coli from German Food Samples and Genetic Diversity of the Bla(CTX-M-1) Region on IncI1 ST3 Plasmids. Vet. Microbiol. 2018, 221, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Girlich, D.; Poirel, L.; Carattoli, A.; Kempf, I.; Lartigue, M.-F.; Bertini, A.; Nordmann, P. Extended-Spectrum Beta-Lactamase CTX-M-1 in Escherichia coli Isolates from Healthy Poultry in France. Appl. Environ. Microbiol. 2007, 73, 4681–4685. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Stegger, M.; Aziz, M.; Johnson, T.J.; Waits, K.; Nordstrom, L.; Gauld, L.; Weaver, B.; Rolland, D.; Statham, S.; et al. Escherichia coli ST131-H22 as a Foodborne Uropathogen. mBio 2018, 9, e00470-18. [Google Scholar] [CrossRef]
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012, 3, 110. [Google Scholar] [CrossRef]
- Chiaretto, G.; Zavagnin, P.; Bettini, F.; Mancin, M.; Minorello, C.; Saccardin, C.; Ricci, A. Extended Spectrum Beta-Lactamase SHV-12-Producing Salmonella from Poultry. Vet. Microbiol. 2008, 128, 406–413. [Google Scholar] [CrossRef]
- Briñas, L.; Moreno, M.A.; Zarazaga, M.; Porrero, C.; Sáenz, Y.; García, M.; Dominguez, L.; Torres, C. Detection of CMY-2, CTX-M-14, and SHV-12 Beta-Lactamases in Escherichia coli Fecal-Sample Isolates from Healthy Chickens. Antimicrob. Agents Chemother. 2003, 47, 2056–2058. [Google Scholar] [CrossRef]
- Liakopoulos, A.; Mevius, D.; Ceccarelli, D. A Review of SHV Extended-Spectrum β-Lactamases: Neglected Yet Ubiquitous. Front. Microbiol. 2016, 7, 1374. [Google Scholar] [CrossRef]
- Touchon, M.; Hoede, C.; Tenaillon, O.; Barbe, V.; Baeriswyl, S.; Bidet, P.; Bingen, E.; Bonacorsi, S.; Bouchier, C.; Bouvet, O.; et al. Organised Genome Dynamics in the Escherichia coli Species Results in Highly Diverse Adaptive Paths. PLoS Genet. 2009, 5, e1000344. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Upadhyay, V.; Upadhyay, A.K.; Singh, S.M.; Panda, A.K. Protein Recovery from Inclusion Bodies of Escherichia coli Using Mild Solubilization Process. Microb. Cell Factories 2015, 14, 41. [Google Scholar] [CrossRef]
- Lagerstrom, K.M.; Hadly, E.A. Under-Appreciated Phylogroup Diversity of Escherichia coli within and between Animals at the Urban-Wildland Interface. Appl. Environ. Microbiol. 2023, 89, e0014223. [Google Scholar] [CrossRef]
- Blaak, H.; Hamidjaja, R.A.; van Hoek, A.H.A.M.; de Heer, L.; de Roda Husman, A.M.; Schets, F.M. Detection of Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli on Flies at Poultry Farms. Appl. Environ. Microbiol. 2014, 80, 239–246. [Google Scholar] [CrossRef]
- Zahoor, M.A.; Nawaz, Z.; Jamil, A.; Yasmin, A.; Alagawany, M.; Othman, S.I.; Allam, A.A.; El-Shall, N.A. Determining the Prevalence and Genetic Diversity of Plasmid-Mediated Sulfonamide Resistance in Escherichia coli from Commercial Broiler Samples. Poult. Sci. 2024, 103, 103258. [Google Scholar] [CrossRef]
- Zurfluh, K.; Wang, J.; Klumpp, J.; Nüesch-Inderbinen, M.; Fanning, S.; Stephan, R. Vertical Transmission of Highly Similar Bla CTX-M-1-Harboring IncI1 Plasmids in Escherichia coli with Different MLST Types in the Poultry Production Pyramid. Front. Microbiol. 2014, 5, 519. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Gallo, I.F.L.; Stehling, E.G. Genomic Characterization of Multidrug-Resistant Extraintestinal Pathogenic Escherichia coli Isolated from Grain Culture Soils. Pedosphere 2022, 32, 495–502. [Google Scholar] [CrossRef]
- Lübcke, P.; Heiden, S.E.; Homeier-Bachmann, T.; Bohnert, J.A.; Schulze, C.; Eger, E.; Schwabe, M.; Guenther, S.; Schaufler, K. Multidrug-Resistant High-Risk Clonal Escherichia coli Lineages Occur along an Antibiotic Residue Gradient in the Baltic Sea. Npj Clean Water 2024, 7, 94. [Google Scholar]
- Soncini, J.G.M.; Cerdeira, L.; Sano, E.; Koga, V.L.; Tizura, A.T.; Tano, Z.N.; Nakazato, G.; Kobayashi, R.K.T.; Aires, C.A.M.; Lincopan, N.; et al. Genomic Insights of High-Risk Clones of ESBL-Producing Escherichia coli Isolated from Community Infections and Commercial Meat in Southern Brazil. Sci. Rep. 2022, 12, 9354. [Google Scholar] [CrossRef]
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The Population Genetics of Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Ronco, T.; Stegger, M.; Olsen, R.H.; Sekse, C.; Nordstoga, A.B.; Pohjanvirta, T.; Lilje, B.; Lyhs, U.; Andersen, P.S.; Pedersen, K. Spread of Avian Pathogenic Escherichia coli ST117 O78:H4 in Nordic Broiler Production. BMC Genom. 2017, 18, 13. [Google Scholar] [CrossRef]
- Clermont, O.; Dixit, O.V.A.; Vangchhia, B.; Condamine, B.; Dion, S.; Bridier-Nahmias, A.; Denamur, E.; Gordon, D. Characterization and Rapid Identification of Phylogroup G in Escherichia coli, a Lineage with High Virulence and Antibiotic Resistance Potential. Environ. Microbiol. 2019, 21, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-W.; Karns, J.S.; Van Kessel, J.A.S.; Haley, B.J. Genome Sequences of Five Multidrug-Resistant Escherichia coli Sequence Type 117 Isolates Recovered from Dairy Calves. Genome Announc. 2017, 5, e00732-17. [Google Scholar] [CrossRef]
- Majó, N.; Dolz, R. Autopsie Des Volailles: Diagnostic Macroscopique et Méthodes de Prélèvements; Les éditions du Point Vétérinaire: Evreux à l’Eure, France, 2012; 82p. [Google Scholar]
- Ørskov, F.; Ørskov, I. The Serology of Capsular Antigens. Curr. Top. Microbiol. Immunol. 1990, 150, 43–63. [Google Scholar] [PubMed]
- Wayne, P. Clinical and Laboratory Standards Institute. M100: Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022; ISBN 9781684400331. [Google Scholar]
- Jarlier, V.; Nicolas, M.H.; Fournier, G.; Philippon, A. Extended Broad-Spectrum Beta-Lactamases Conferring Transferable Resistance to Newer Beta-Lactam Agents in Enterobacteriaceae: Hospital Prevalence and Susceptibility Patterns. Rev. Infect. Dis. 1988, 10, 867–878. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 12.0. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 25 June 2024).
- Holmes, D.S.; Quigley, M. A Rapid Boiling Method for the Preparation of Bacterial Plasmids. Anal. Biochem. 1981, 114, 193–197. [Google Scholar] [CrossRef]
- Carvalho, I.; Safia Chenouf, N.; Cunha, R.; Martins, C.; Pimenta, P.; Pereira, A.R.; Martínez-Álvarez, S.; Ramos, S.; Silva, V.; Igrejas, G.; et al. Antimicrobial Resistance Genes and Diversity of Clones among ESBL- and Acquired AmpC-Producing Escherichia coli Isolated from Fecal Samples of Healthy and Sick Cats in Portugal. Antibiotics 2021, 10, 262. [Google Scholar] [CrossRef]
- Carattoli, A.; Bertini, A.; Villa, L.; Falbo, V.; Hopkins, K.L.; Threlfall, E.J. Identification of Plasmids by PCR-Based Replicon Typing. J. Microbiol. Methods 2005, 63, 219–228. [Google Scholar] [CrossRef]
| Serogroup | Broiler Isolates (n = 185) | Turkey Isolates (n = 26) | Total Isolates (n = 211) | |||
|---|---|---|---|---|---|---|
| O1 | 50 (27%) | 143 (77.3%) | 16 (61.5%) | 21 (80.8%) | 66 (31.3%) | 164 (77.8%) |
| O2 | 66 (35.7%) | 4 (15.4%) | 70 (33.2%) | |||
| O78 | 27 (14.6%) | 1 (3.8%) | 28 (13.3%) | |||
| Non-Typable isolates | 42 (22.7%) | 5 (19.2%) | 47 (22.2%) | |||
| Antibiotic Disks Used | Broiler APEC Isolates (n = 185) | Turkey APEC Isolates (n = 26) | Total APEC Isolates n = 211 |
|---|---|---|---|
| Ampicillin | 150 (81.1%) | 26 (100%) | 176 (83.4%) |
| Amoxicillin/clavulanic acid | 140 (75.7%) | 18 (69.2%) | 158 (74.9%) |
| Cefotaxime | 12 (6.5%) | 5 (19.2%) | 17 (8%) |
| Ceftazidime | 2 (1.1%) | 1 (3.8%) | 3 (1.4%) |
| Imipenem | 0 (0%) | 0 (0%) | 0 (0%) |
| Nalidixic acid | 175 (94.6%) | 25 (96.1%) | 200 (94.8%) |
| Ciprofloxacin | 121 (65.4%) | 19 (73%) | 140 (66.3%) |
| Neomycin | 63 (34.1%) | 8 (30.8%) | 71 (33.6%) |
| Gentamicin | 11 (5.9%) | 3 (11.5%) | 14 (6.6%) |
| Trimethoprim/sulfamethoxazole | 140 (75.7%) | 23 (88.5%) | 163 (77.2%) |
| Chloramphenicol | 36 (19.5%) | 11 (42.3%) | 47 (22.3%) |
| Tetracycline | 181 (97.8%) | 26 (100%) | 207 (98.1%) |
| Nitrofurantoin | 65 (35.1%) | 10 (38.5%) | 75 (35.5%) |
| Antimicrobial Classes with Resistance | Broiler APEC Isolates (n = 185) | Turkey APEC Isolates (n = 26) | ||
|---|---|---|---|---|
| Number of Isolates | Prevalence (%) | Number of Isolates | Prevalence (%) | |
| 0 | 2 | 1.1 | 0 | 0 |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 9 | 4.9 | 0 | 0 |
| 3 * | 37 | 20 | 3 | 11.5 |
| 4 * | 51 | 27.6 | 8 | 30.8 |
| 5 * | 42 | 22.7 | 9 | 34.6 |
| 6 * | 30 | 16.2 | 5 | 19.2 |
| 7 * | 14 | 7.6 | 1 | 3.8 |
| Total | 185 | 100 | 26 | 100% |
| ESBL Isolate Code | Poultry House Location | Sampling Point (Year) | Breed Type (Age) | Serogroup | Phylogroup/ST | Antimicrobial Resistance Phenotype | MIC of COL (µg/µL) | Antimicrobial Resistance Genotype |
|---|---|---|---|---|---|---|---|---|
| C8195 | Djemila (Setif) | Slaughterhouse (2016) | Broiler (7–8 weeks) | O2 | B1/ST5087 | AMP-AMC-CTX-SXT-FUR-CLR-NAL-CIP-TET | ≤1 | blaCTX-M-1, tet(A), Intl1, Cat, sul2 |
| C8196 | Hamma Bouziane (Constantine) | Slaughterhouse (2016) | Broiler (7–8 weeks) | O2 | B1/ST48 | AMP-CTX-SXT-TET | ≤1 | blaCTX-M-1, tet(A), Intl1, sul2 |
| X800 | Ain Arnat (Setif) | Slaughterhouse (2016) | Broiler (7–8 weeks) | O1 | D/ST38 | AMP-AMC-CTX-SXT-NAL-CIP-TET | ≤1 | blaCTX-M-1, tet(A), Intl1, sul2 |
| X801 X802 X803 | Bejaia | Slaughterhouse (2016) | Broilers (7–8 weeks) | O78 O78 O1 | B1/ST23 B1/ST23 B1/ST23 | AMP-AMC-CTX-SXT-NAL-CIP-TET AMP-AMC-CTX-NAL-TET AMP-AMC-CTX-NAL-TET | ≤1 | blaCTX-M-1, tet(A), sul1 blaCTX-M-1, tet(A) blaCTX-M-1, tet(A) |
| X804 | Remada (Setif) | Veterinary clinic (2018) | Turkey (12 weeks) | O2 | B2/ST131 | AMP-CTX-GEN-SXT-CLR-NAL-CIP-TET | ≤1 | blaCTX-M-1, tet(A), Intl1, cat, sul1, aac(3)-II |
| X805 X806 | Bir Hadada (Setif) | Veterinary clinic (2018) | Broilers (3 weeks) | O1 O1 | D/ST117 D/ND | AMP-CTX-SXT-NAL-CIP-TET AMP-CTX-SXT-NAL-CIP-TET | ≤1 | blaCTX-M-1, blaTEM-1, tet(A), tet(B), Intl1, sul1 blaCTX-M-1, blaTEM-1, tet(A), tet(B), Intl1, sul2 |
| X807 X808 | Bellaa (Setif) | Veterinary clinic (2018) | Turkeys (4 weeks) | O1 O1 | D/ST117 D/ST117 | AMP-CTX-SXT-NAL-TET AMP-CTX-SXT-NAL-CIP-TET | ≤1 | blaCTX-M-1, blaTEM-1, tet(A), tet(B), Intl1, sul2 blaCTX-M-1, blaTEM-1, tet(A), Intl1, sul2 |
| X809 X810 | Bir Hadada (Setif) | Veterinary clinic (2018) | Broilers (5 weeks) | O1 O1 | D/ST117 D/ND | AMP-CTX-NAL-TET AMP-CTX-NAL-TET | ≤1 | blaCTX-M-1, tet(A), blaCTX-M-1, tet(A) |
| X811 | Bir Hadada (Setif) | Veterinary clinic (2018) | Turkey (7 weeks) | O1 | D/ST117 | AMP-CTX-NAL-TET | ≤1 | blaCTX-M-1, tet(A) |
| X1241 | Beida Bordj (Setif) | Slaughterhouse (2018) | Turkey (7–8 weeks) | NT | B1/ST1146 | AMP-CTX-CAZ-NEO-SXT-TET | ≤1 | blaSHV-12, blaTEM-1, tet(A), sul3 |
| BBA001 | (Bordj Bou Arréridj) | Slaughterhouse (2021) | Broiler (7–8 weeks) | NT | D/ND | AMP-CTX-CAZ-TET-SXT | ≤1 | blaCTX-M-15, tet(A), sul1 |
| BBA002 | (Bordj Bou Arréridj) | Slaughterhouse (2021) | Broiler (7–8 weeks) | NT | D/ND | AMP-CTX-CAZ-TET-SXT | ≤1 | blaCTX-M-15, tet(A), sul1 |
| ESBL Isolate (Donor) | Conjugation/Plasmids in Donors | Transconjugants | Antimicrobial Resistance Phenotype in TC | Plasmids/Antimicrobial Resistance Genotype in TC |
|---|---|---|---|---|
| C8195 | +/IncI1, FIB, FIC, IncK | X1223 | ESBL, RIF R | IncK/blaCTX-M-1 |
| C8196 | +/IncI1, IncK | X1224 | ESBL, RIF R | IncK/blaCTX-M-1 |
| X800 | +/IncI1 | X1225 | ESBL, RIF R, TET R | IncI1/blaCTX-M-1, tet(A) |
| X801 | +/IncI1, FIB, IncK | X1468 | ESBL, RIF R | IncK/blaCTX-M-1 |
| X802 | +/IncI1, FIB, IncK | X1226 | ESBL, RIF R | IncK/blaCTX-M-1 |
| X803 | +/IncI1, FIB | X1469 | ESBL, RIF R | IncK/blaCTX-M-1 |
| X804 | +/IncI1, FIB | X1227 | ESBL, RIF R, GEN R | Non-typable/blaCTX-M-1, aac(3)-II |
| X805 | +/IncI1, FIB, FIC | X1228 | ESBL, RIF R | Non-typable/blaCTX-M-1 |
| X806 | +/IncI1, FIB | X1470 | ESBL, RIF R | Non-typable/blaCTX-M-1 |
| X807 | +/IncI1, FIB, FIC | X1229 | ESBL, RIF R | Non-typable/blaCTX-M-1 |
| X808 | +/IncI1, FIB | X1471 | ESBL, RIF R | Non-typable/blaCTX-M-1 |
| X809 | +/IncI1, FIB | X1472 | ESBL, RIF R | Non-typable/blaCTX-M-1 |
| X810 | +/IncI1, FIB | X1473 | ESBL, RIF R | Non-typable/blaCTX-M-1 |
| X811 | +/IncI1, FIB | X1474 | ESBL, RIF R | Non-typable/blaCTX-M-1 |
| X1241 | +/K, IncI1 | X1475 | ESBL, RIF R | Non-typable/blaSHV-12 |
| BBA001 | − | / | / | / |
| BBA002 | − | / | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chenouf, N.S.; Messaï, C.R.; Carvalho, I.; Álvarez-Gómez, T.; Silva, V.; Zitouni, A.; Hakem, A.; Poeta, P.; Torres, C. Serogrouping and Molecular Characterization of ESBL-Producing Avian Pathogenic Escherichia coli from Broilers and Turkeys with Colibacillosis in Algeria. Antibiotics 2025, 14, 356. https://doi.org/10.3390/antibiotics14040356
Chenouf NS, Messaï CR, Carvalho I, Álvarez-Gómez T, Silva V, Zitouni A, Hakem A, Poeta P, Torres C. Serogrouping and Molecular Characterization of ESBL-Producing Avian Pathogenic Escherichia coli from Broilers and Turkeys with Colibacillosis in Algeria. Antibiotics. 2025; 14(4):356. https://doi.org/10.3390/antibiotics14040356
Chicago/Turabian StyleChenouf, Nadia Safia, Chafik Redha Messaï, Isabel Carvalho, Tamara Álvarez-Gómez, Vanessa Silva, Abdelghani Zitouni, Ahcene Hakem, Patricia Poeta, and Carmen Torres. 2025. "Serogrouping and Molecular Characterization of ESBL-Producing Avian Pathogenic Escherichia coli from Broilers and Turkeys with Colibacillosis in Algeria" Antibiotics 14, no. 4: 356. https://doi.org/10.3390/antibiotics14040356
APA StyleChenouf, N. S., Messaï, C. R., Carvalho, I., Álvarez-Gómez, T., Silva, V., Zitouni, A., Hakem, A., Poeta, P., & Torres, C. (2025). Serogrouping and Molecular Characterization of ESBL-Producing Avian Pathogenic Escherichia coli from Broilers and Turkeys with Colibacillosis in Algeria. Antibiotics, 14(4), 356. https://doi.org/10.3390/antibiotics14040356










