Research Progress on the Antibacterial Activity of Natural Flavonoids
Abstract
1. Introduction
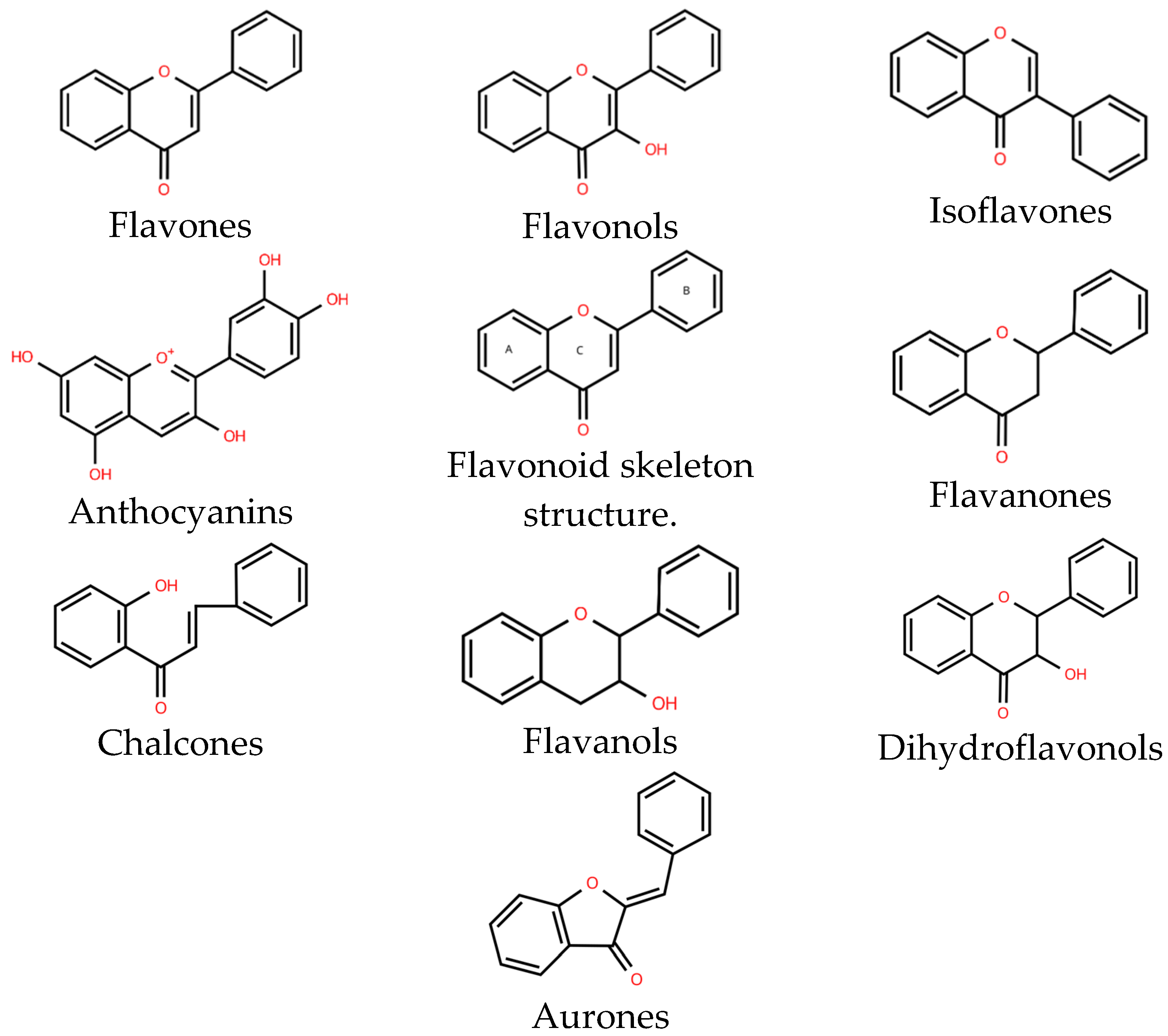
2. Antibacterial Activity of Natural Flavonoids
| Category | S.NO. | Flavonoids | Chemical Structure | Microorganism | MIC (μg/mL) | Action Mechanism | References |
|---|---|---|---|---|---|---|---|
| Flavones | 1 | Apigenin | 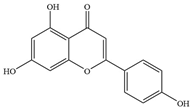 | Quinolone-resistant S. aureus Mu50 | 4 | Targeting the gyrA subunit with the Ser84Leu mutation | [26] |
| Meticillin-susceptible S. aureus FDA 209P | >128 | Targeting the gyrA subunit with the Ser84Leu mutation | [26] | ||||
| P. aeruginosa PAO1 | 64 | - | [27] | ||||
| Klebsiella pneumoniae ATCC 9997 | 64 | - | [27] | ||||
| 2 | Luteolin |  | MDR Trueperella pyogenes | - | Downregulating the expression of MATE-encoded efflux pump | [28] | |
| Clinical isolation MRSA | 500 | Damage to cell wall and membrane integrity | [29] | ||||
| P. aeruginosa PAO1 | 64 | - | [27] | ||||
| Klebsiella pneumoniae ATCC 9997 | 64 | - | [27] | ||||
| 3 | Patuletin | 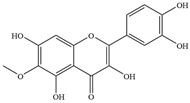 | S. aureus ATCC 27853 and clinical strains | 2000 | Inhibiting biofilm formation and production of virulence factor glucanthin | [30] | |
| 4 | Chrysin |  | S. aureus | 2–16 | Inhibition of α-hemolysin expression | [31] | |
| 5 | Diosmetin |  | S. aureus | >128 | - | [32] | |
| 6 | Tangeritin |  | E. coli | 137 | Inhibition of DNA gyrase | [33] | |
| 7 | Nobiletin | 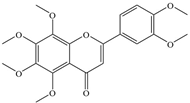 | E. coli | 177 | Inhibition of DNA gyrase | [33] | |
| 8 | Acacetin | 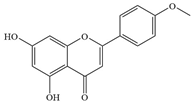 | Streptococcus pneumoniae (S. pneumoniae) | >32 | Inhibiting the formation of oligomers of pneumolysin (PLY) to attenuate its biological activity | [34] | |
| 9 | Wogonin | 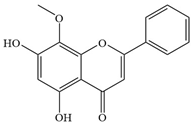 | S. aureus | >128 | - | [35] | |
| 10 | Scutellarein | 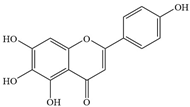 | A. baumannii | 1024 | Inhibiting polyphosphate kinase 1 (PPK1) | [36] | |
| 11 | Entadanin | 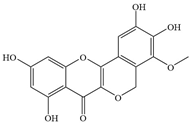 | S. typhi | 1.56 | - | [37] | |
| Flavonols | 1 | 3′,4′, 7-trihydroxyflavone | 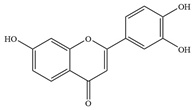 | E. coli ATCC8739 | 32 | Inhibiting efflux pump activity and enhancing the action of antibiotics | [38] |
| Klebsiella pneumoniae ATCC 11296 | 32 | Inhibiting efflux pump activity and enhancing the action of antibiotics | [38] | ||||
| P.aeruginosa PAO1 | 64 | Inhibiting efflux pump activity and enhancing the action of antibiotics | [38] | ||||
| 2 | Rhamnetin 3-O--β-D-glucopyranoside |  | S.aureus USA300 | - | Regulating carbon or glutamine/glutamate metabolism and reducing precursors of biofilm formation, thereby inhibiting biofilm formation | [39] | |
| 3 | Kaempferol | 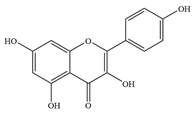 | S. aureus ATCC 29213 | >1024 | Inhibiting the activity of sorting enzyme A to affect the formation of biofilm | [40] | |
| S. mutans ATCC 25175 | 1000 | Inhibiting the enzyme activity of F-ATPase, destroying the ΔpH on the cell membrane, affecting the acidity of Streptococcus mutans | [41] | ||||
| Pneumococcus NCTC 7466 | - | Decreasing the biological activity of sorting enzyme SrtA, inhibiting the formation of biofilm, and inhibiting the hemolytic activity of pulmonolysin | [42] | ||||
| S. epidermidis DMST 5038 | >1024 | - | [43] | ||||
| 4 | Kaempferol-3-O-α-l-rhamnoside | 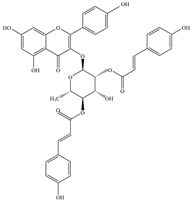 | S. aureus 1199B | 0.78 | Inhibition of NorA transporter | [44] | |
| 5 | Quercetin |  | S. epidermidis DMST 5038 | 256 | Damaging the cell membrane and increasing its permeability | [45] | |
| MRSA_A MRSE_178 | - | Inhibiting the activity of ATP synthase and reducing the level of ATP in cells; virulence-inhibiting factor coagulase | [43,45] | ||||
| MRSA ATCC 33591 | - | Stably binding to the formation site of SarA dimer, inhibiting biofilm formation, and reducing extracellular polymer production and eDNA concentration | [46] | ||||
| P. aeruginosa NCIM 20136 | 20 | - | [47] | ||||
| E. coli NCIM 2065 | 400 | - | [47] | ||||
| Serratia marcescens ATCC 14756 | 175 | Inhibition of EPS production and biofilm formation | [48] | ||||
| S. pyogenes DMST 30653 | 128 | Damaging the cell membrane and increasing its permeability | [49] | ||||
| 6 | Morin | 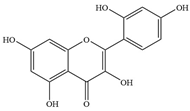 | S. pyogenes MGAS 6180 | - | Inhibition of biofilm formation | [50] | |
| S. enteritidis #1 | 150 | Inhibition of DNA synthesis | [51] | ||||
| Bacillus cereus ATCC11778 | 300 | Inhibition of DNA synthesis | [51] | ||||
| 7 | Myricetin | 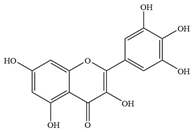 | E. coli ATCC 25922 | - | Inhibition of DnaB helicase | [52] | |
| S. aureus NCTC 8325-4 | >1024 | Inhibiting the production of virulence factor Hla and neutralizing Hla activity | [53] | ||||
| S. aureus ATCC 6538p | >1024 | Inhibiting sorting enzymes A (SrtA) and B (SrtB) activity | [54] | ||||
| 8 | Fisetin | 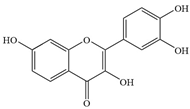 | NDM-1-positive E. coli | >1024 | Inhibiting New Delhi metallo-β-lactamase-1 (NDM-1) activity | [55] | |
| 9 | Galangin |  | Penicillin-resistant S. aureus | 200–300 | Inhibiting the activity of penicillinase and β-lactamase | [56] | |
| 10 | Astragalin | 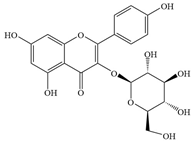 | H. pylori | 0.49–1.25 | Interacts with proteins | [57] | |
| 11 | Rutin | 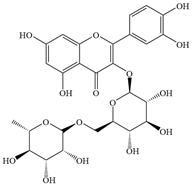 | P. aeruginosa, MRSA | 500–1000 | Inhibiting biofilm formation, downregulating gene expression, destruction of cell membrane | [58] | |
| Flavanones | 1 | Pinocembrin |  | Neisseria gonorrhoeae GC1–182 | 64 | - | [59] |
| Listeria monocytogenes ATCC19113 | 68 | - | [60] | ||||
| 2 | Hesperidin | 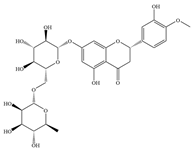 | E. coli ATCC 25922 | - | Inhibition of biofilm formation | [61] | |
| S.aureus ATCC 25923 | 512 | - | [62] | ||||
| P. aeruginosa IBRS P001 | 500 | - | [58] | ||||
| 3 | Hesperetin | 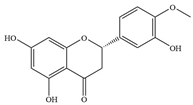 | Helicobacter pylori ATCC 49503 | 30.23 | Reducing the expression level of bacterial replication and transcription genes and inhibiting bacterial movement | [63] | |
| MRSA IBRS MRSA 011 | 500 | - | [58] | ||||
| P.aeruginosa IBRS P001 | 500 | - | [58] | ||||
| 4 | Naringin | 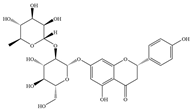 | Clinical isolation of Pseudomonas species | 128–512 | Inhibition of EPS production and biofilm formation | [64] | |
| S.aureus ATCC 25923 | 512 | - | [62] | ||||
| P. aeruginosa IBRS P001 | 250 | - | [58] | ||||
| E. coli IBRS E003 | 500 | - | [58] | ||||
| 5 | Naringenin | 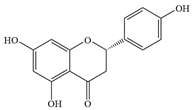 | S. mutans | 100–200 | Inhibition of biofilm formation | [65] | |
| 6 | Eriodictyol |  | S.aureus | 512 | Inhibiting alpha-hemolysin expression | [66] | |
| 7 | Liquiritigenin | 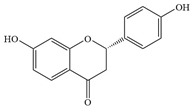 | S.aureus | 50–100 | Inhibiting alpha-hemolysin expression and may inhibit β-lactamase activity | [67,68] | |
| 8 | 7-Hydroxyflavanone |  | S. pneumoniae | 1000 | - | [69] | |
| 9 | Lupirifolin | 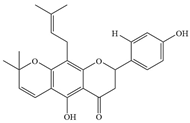 | S. aureus and Enterococcus faecalis (E. faecalis) | 0.5–2 | Binds to phosphatidylglycerol (PG) and cardiolipin (CL) in bacterial cell membranes, thereby disrupting the integrity of the cell membrane | [70] | |
| 10 | Ochnaflavone | 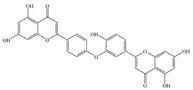 | P. aeruginosa | 31.3 | - | [71] | |
| 11 | Sophoraflavanone G |  | MRSA | 3.9 | Damages cell membrane, inhibits cell wall synthesis, interferes with energy metabolism | [72] | |
| 12 | Mimulone |  | MRSA | 2000–16,000 | Inhibiting the synthesis of peptidoglycan layer in bacterial cell wall and inhibition of β-lactamase activity | [73] | |
| 13 | Sepicanin A |  | MRSA | 998.7 | - | [74] | |
| Flavanols | 1 | Epigallocatechin gallate | 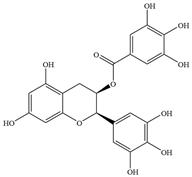 | S.aureus ATCC 25923 | 100 | Directly binding to peptidoglycan to interfere with the integrity of bacterial cell walls and biosynthesis and inhibiting penicillinase activity | [75] |
| Porphyromonas gingivalis ATCC 33277 | 250 | - | [76] | ||||
| Vibrio parahaemolyticus ATCC 17802 | 128 | Damaging cell membrane integrity and inhibiting biofilm formation | [77] | ||||
| 2 | Epigallocatechin | 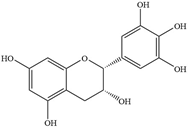 | Porphyromonas gingivalis 381 | 1000 | - | [76] | |
| Porphyromonas gingivalis ATCC 33277 | 1000 | - | [76] | ||||
| 3 | Catechin |  | E. coli | 1000–2000 | Downregulating the acrA gene, thereby inhibiting biofilm formation | [78] | |
| 4 | (-)-Epicatechin | 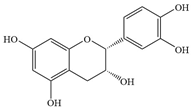 | S. Typhimurium | >1024 | Inhibition with ATP | [79] | |
| 5 | (-)-Epicatechin gallate | 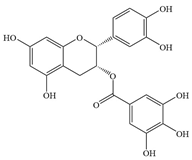 | S. Typhimurium | >512 | Inhibiting biofilm formation | [80] | |
| 6 | (-)-Catechin gallate |  | MRSA | 256–512 | Inhibiting biofilm formation and disrupting the secretion of virulence-related proteins | [81] | |
| Isoflavones | 1 | Genistein | 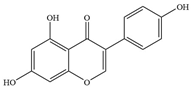 | MRSA Newman 67-0 | - | Inhibition of topoisomerase II and protein tyrosine kinase | [82] |
| E. coli ATCC 25922 | 5 | Inducing NO changes DNA and induces apoptosis | [83] | ||||
| 2 | Glabridin |  | MDRSA 4627 | - | Increasing oxidative stress, altering the integrity of DNA and proteins, and disrupting cell morphology | [84] | |
| 3 | Formononetin | 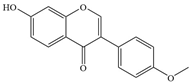 | S. aureus | 200 | - | [85] | |
| E. faecalis | 6.6–18.3 | - | [86] | ||||
| 4 | Lupalbigenin | 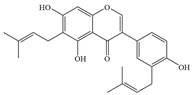 | S. aureus, E. faecalis, and Streptococcus pyogenes (S. pyogenes) | 4–8 | Inhibiting α-hemolysin and biofilm formation, and damaging bacterial cell membranes | [87,88] | |
| 5 | Gancaonin M |  | S. aureus, E. faecalis, and S. pyogenes | 2–8 | - | [87] | |
| 6 | Warangalone | 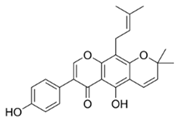 | S. aureus, E. faecalis, and S. pyogenes | 2–8 | - | [87] | |
| 7 | Auriculatin |  | S. aureus, E. faecalis, and S. pyogenes | 2–4 | - | [87] | |
| 8 | Millexatin F |  | S. aureus, E. faecalis, and S. pyogenes | 1–4 | - | [87] | |
| 9 | Millexatin A |  | S. aureus, S. epidermidis, and B. subtilis | 2–128 | - | [89] | |
| 10 | Isolupalbigenin |  | MRSA | 1.56–3.13 | - | [90] | |
| 11 | Erythrinin B |  | MRSA | 6.25–12.5 | - | [90] | |
| 12 | Laburnetin |  | MRSA | >25 | - | [90] | |
| Anthocyanins | 1 | Cyanidin-3-O-glucoside |  | S.aureus ATCC 25923, SJS001, SJS008, SJS009, SJS010 | 312.5 | Damaging cell membrane integrity | [84] |
| E. coli ATCC 25922 | 5000 | Damaging cell membrane integrity | [84] | ||||
| 2 | Delphinidin-3-glucoside | 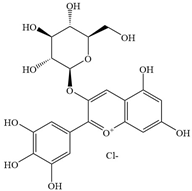 | E. coli, S.aureus | 1660–7110 | - | [91] | |
| 3 | Delphinidin-3-sambubioside |  | E. coli, S.aureus | 1660–7110 | - | [91] | |
| 4 | Pelargonidin-3-glucoside | 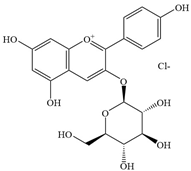 | E. coli | - | Inhibition of microbial ATP synthase | [92] | |
| 5 | Malvidin-3-glucoside | 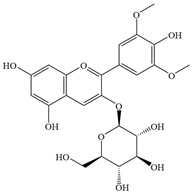 | Staphylococcus | 250 | Inhibiting biofilm formation | [93] | |
| 6 | Cyanidin-3-rutinoside |  | P. aeruginosa, K. pneumoniae | 400–9500 | - | [94] |
2.1. The Mixture of Flavonoids Extracted from Plants
2.2. Single Flavonoids
2.2.1. Flavones
2.2.2. Flavonols
2.2.3. Flavanones
2.2.4. Flavanols
2.2.5. Anthocyanins
2.2.6. Isoflavones
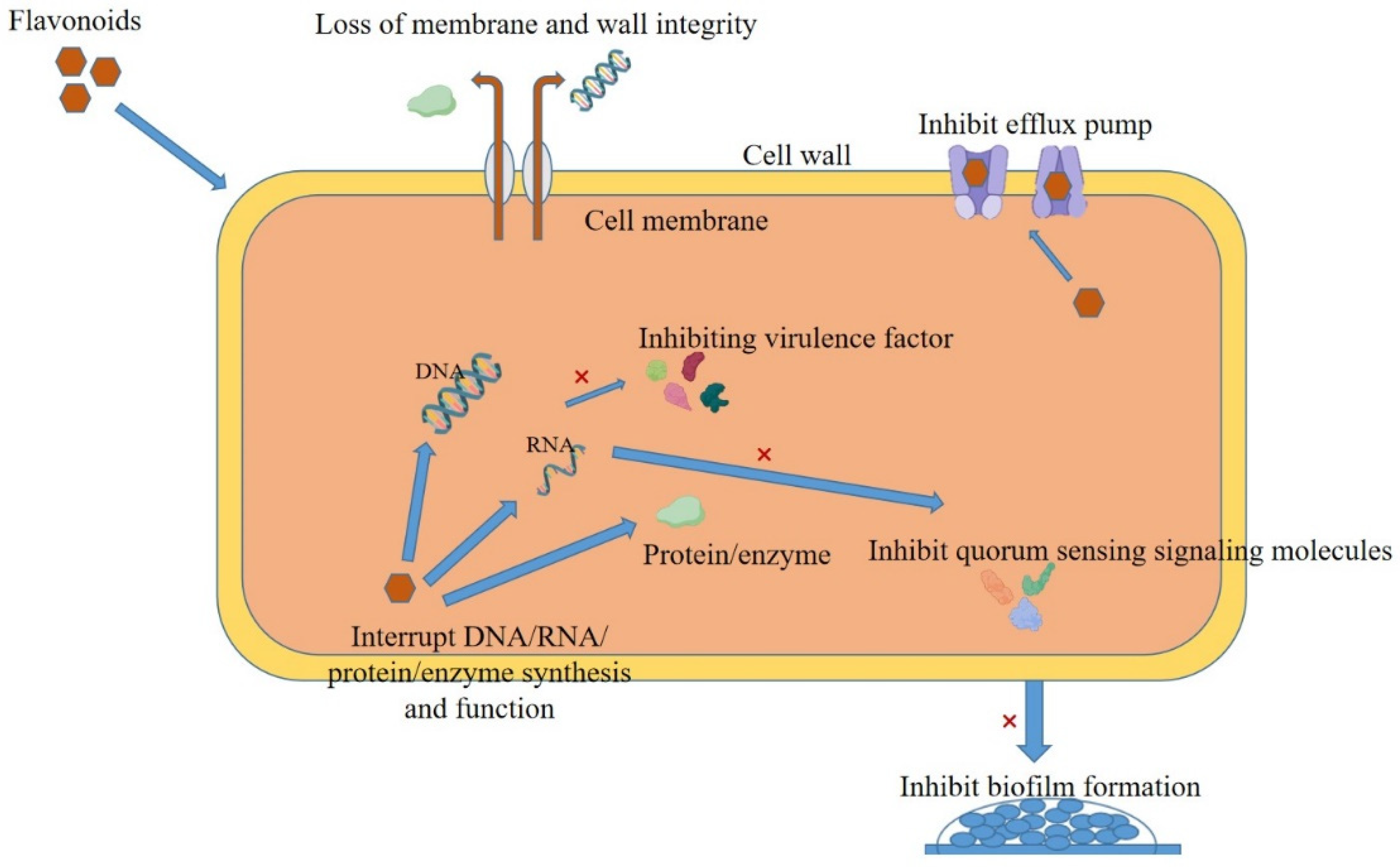
3. Antibacterial Mechanism of Flavonoids
3.1. Bacterial Cell Membrane Disruption
3.2. Bacterial Cell Wall Disruption
3.3. Impact on Bacterial Nucleic Acid
3.4. Proteins Affecting Bacteria
3.5. Inhibition of Bacterial Biofilms
3.6. Inhibition of Bacteria-Related Virulence Factors
3.7. Effects on Bacterial Signaling Pathways
3.8. Inhibition of Bacterial Efflux Pump
4. Antibacterial Activity of Flavonoids Combined with Antibiotics
| MICs (μg/mL) | |||||
|---|---|---|---|---|---|
| Strains | Agents | Alone | Combination | FICI | References |
| S. aureus 1199B | Chrysosplenetin | >256 | 32 | <0.375 | [141] |
| Norfloxacin | 32 | 8 | |||
| Penduletin | >128 | 2 | <0.079 | ||
| Norfloxacin | 32 | 2 | |||
| Chrysoeriol | >128 | 2 | <0.266 | ||
| Norfloxacin | 32 | 8 | |||
| E.coli C7F3 | Naringenin | 2000 | 250 | 0.1875 | [146] |
| Amikacin | 64 | 4 | |||
| S. aureus ATCC 700699 | Flavone | 1600 | 100 | 0.094 | [147] |
| Vancomycin | 8 | 0.25 | |||
| Flavone | 1600 | 100 | 0.126 | ||
| Oxacillin | 800 | 50 | |||
| E. coli HZ−46 | Baicalin | >2048 | 256 | 0.25 | [148] |
| Colistin | 2 | 0.25 | |||
| Aeromonas hydrophila (A. hydrophila) | Quercetin | 360 | 90 | 0.28 | [9] |
| Florfenicol | 2.5 | 0.078 | |||
| P. aeruginosa O1 | Quercetin | 500 | 62.5 | 0.25 | [150] |
| Tobramycin | 4 | 0.5 | |||
| Quercetin | 500 | 62.5 | 0.25 | ||
| Amikacin | 8 | 1 | |||
| Quercetin | 500 | 125 | 0.375 | ||
| Ceftriaxone | 8 | 1 | |||
| Quercetin | 500 | 125 | 0.375 | ||
| Gentamycin | 4 | 0.5 | |||
| Quercetin | 500 | 125 | 0.375 | ||
| Levofloxacin | 2 | 0.25 | |||
| Acinetobacter baumannii (A. baumannii) ColR-Ab4 | Quercetin | 256 | 16 | 0.1875 | [152] |
| Colistin | 32 | 4 | |||
| S. aureus ATCC33591 | Quercetin | >1024 | 4 | 0.5 | [154] |
| Tetracycline | 32 | 16 | |||
| Quercetin | >1024 | 4 | 0.25 | ||
| Doxycycline | 32 | 8 | |||
| MRSA | Apigenin | 32.5–62.5 | / | 0.18–0.47 | [157] |
| Ampicillin | 800 | 107 | |||
| Apigenin | 32.5–62.5 | / | 0.18–0.47 | ||
| Ceftriaxone | 58 | 2.6 | |||
| A. hydrophila MTCC 646 | Rutin | 1100 | 275 | 0.5 | [155] |
| Florfenicol | 16 | 4 | |||
| S. aureus ATCC 25923 | Luteolin | 62.5 | 3.9 | 0.125 | [159] |
| Ampicillin | 15.63 | 0.97 | |||
| E.coli DMST 20661 | Luteolin | 200 | 80 | <0.47 | |
| Amoxicillin | >1000 | 70 | |||
| A. baumannii | Chrysin | >128 | 1–8 | 0.047–0.256 | [163] |
| Colistin | 0.125–16 | 0.031–0.125 | |||
| P. aeruginosa, A. baumannii | Kaempferol | ≥512 | 4–16 | 0.031–0.266 | [164] |
| Colistin | 32–128 | 1–16 | |||
| Vibrio cholerae (V. cholerae) N16961 Vibrio cholerae | EGCG | 125 | 0.97 | 0.009 | [165] |
| Tetracycline | 3.91 | 0.004 | |||
| MDR E.coli, S. aureus | EGCG | 625–1250 | 78.125–156.25 | 0.325 | [166] |
| Gentamicin | 32 | 6.4 | |||
| Extended-Spectrum Beta-Lactamase E.coli | EGCG | 1500 | 50 | 0.1 | [167] |
| Cefotaxime | 128 | 8 | |||
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, e2100749. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, W.A. The treasure called antibiotics. Ann. Ib. Postgrad. Med. 2016, 14, 56–57. [Google Scholar]
- Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Sessa, R. Editorial for the Special Issue “Antibacterial Activity of Drug-Resistant Strains”. Int. J. Mol. Sci. 2024, 25, 1878. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: The Global Threats of Increasing Antimicrobial Resistance Require New Approaches to Drug Development, Including Molecular Antimicrobial Adjuvants. Med. Sci. Monit. 2024, 30, e945583. [Google Scholar] [CrossRef]
- Baquero, F. Threats of Antibiotic Resistance: An Obliged Reappraisal. Int. Microbiol. 2021, 24, 499–506. [Google Scholar] [CrossRef]
- Natural Products to Drugs: Natural Product Derived Compounds in Clinical Trials—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15806196/ (accessed on 2 September 2024).
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, X.; Yang, Y.; Zhu, L.; Li, L.; Kong, X. Synergistic Effect of Quercetin on Antibacterial Activity of Florfenicol Against Aeromonas Hydrophila In Vitro and In Vivo. Antibiotics 2022, 11, 929. [Google Scholar] [CrossRef]
- Park, M.-Y.; Kang, D.-H. Antibacterial Activity of Caffeic Acid Combined with UV-A Light against Escherichia coli O157:H7, Salmonella enterica Serovar Typhimurium, and Listeria monocytogenes. Appl. Environ. Microbiol. 2021, 87, e0063121. [Google Scholar] [CrossRef]
- Li, X.; Sun, S.; Feng, X.; Chen, Y.; Chen, S.; Ma, J.; Zhou, F. Tannic Acid-Crosslinked O-Carboxymethyl Chitosan Hydrogels for Enhanced Antibacterial Activity and Rapid Hemostasis. J. Biomater. Sci. Polym. Ed. 2023, 34, 184–199. [Google Scholar] [CrossRef]
- Li, K.; Zhong, W.; Li, P.; Ren, J.; Jiang, K.; Wu, W. Antibacterial Mechanism of Lignin and Lignin-Based Antimicrobial Materials in Different Fields. Int. J. Biol. Macromol. 2023, 252, 126281. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Cobo, A.; Alejo-Armijo, A.; Altarejos, J.; Salido, S.; Ortega-Morente, E. Evaluation of Antibacterial and Antibiofilm Properties of Phenolics with Coumarin, Naphthoquinone and Pyranone Moieties Against Foodborne Microorganisms. Molecules 2025, 30, 944. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Kusuda, M.; Inada, K.; Ogawa, T.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Effects of Tannins and Related Polyphenols on Methicillin-Resistant Staphylococcus aureus. Phytochemistry 2005, 66, 2047–2055. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, Inflammation, and Cardiovascular Disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial Activity of Flavonoids and Their Structure-Activity Relationship: An Update Review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Rengasamy, K.R.; Khan, H.; Gowrishankar, S.; Lagoa, R.J.; Mahomoodally, F.M.; Khan, Z.; Suroowan, S.; Tewari, D.; Zengin, G.; Hassan, S.T.; et al. The Role of Flavonoids in Autoimmune Diseases: Therapeutic Updates. Pharmacol. Ther. 2019, 194, 107–131. [Google Scholar] [CrossRef]
- Cai, J.; Tan, X.; Hu, Q.; Pan, H.; Zhao, M.; Guo, C.; Zeng, J.; Ma, X.; Zhao, Y. Flavonoids and Gastric Cancer Therapy: From Signaling Pathway to Therapeutic Significance. Drug Des. Dev. Ther. 2024, 18, 3233–3253. [Google Scholar] [CrossRef]
- Szala-Rycaj, J.; Zagaja, M.; Szewczyk, A.; Andres-Mach, M. Selected Flavonoids and Their Role in the Treatment of Epilepsy—A Review of the Latest Reports from Experimental Studies. Acta Neurobiol. Exp. 2021, 81, 151–160. [Google Scholar]
- Nicolucci, C.; Padovani, M.; Rodrigues, F.d.C.; Fritsch, L.N.; Santos, A.C.; Priolli, D.G.; Sciani, J.M. Flavonoids: The Use in Mental Health and Related Diseases. Nat. Prod. Res. 2024, 38, 4223–4233. [Google Scholar] [CrossRef]
- Liang, M.; Ge, X.; Xua, H.; Ma, K.; Zhang, W.; Zan, Y.; Efferth, T.; Xue, Z.; Hua, X. Phytochemicals with Activity against Methicillin-Resistant Staphylococcus aureus. Phytomedicine 2022, 100, 154073. [Google Scholar] [CrossRef]
- Eumkeb, G.; Siriwong, S.; Phitaktim, S.; Rojtinnakorn, N.; Sakdarat, S. Synergistic Activity and Mode of Action of Flavonoids Isolated from Smaller Galangal and Amoxicillin Combinations against Amoxicillin-Resistant Escherichia coli. J. Appl. Microbiol. 2012, 112, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial Flavonoids as a Potential Substitute for Overcoming Antimicrobial Resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Ali Shah, S.A.; Khatib, A.; Mukhtar, S.; Alsharif, M.A.; Parveen, H.; Zakaria, Z.A. Antibacterial Effects of Flavonoids and Their Structure-Activity Relationship Study: A Comparative Interpretation. Molecules 2022, 27, 1149. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Baba, T.; Sasaki, T.; Hiramatsu, K. Apigenin as an Anti-Quinolone-Resistance Antibiotic. Int. J. Antimicrob. Agents 2015, 46, 666–673. [Google Scholar] [CrossRef]
- Morimoto, Y.; Aiba, Y.; Miyanaga, K.; Hishinuma, T.; Cui, L.; Baba, T.; Hiramatsu, K. CID12261165, a Flavonoid Compound as Antibacterial Agents against Quinolone-Resistant Staphylococcus aureus. Sci. Rep. 2023, 13, 1725. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, X.; Song, X.; Zhou, W.; Hong, W.; Tian, C.; Liu, Y.; Liu, M. Luteolin Showed a Resistance Elimination Effect on Gentamicin by Decreasing MATE mRNA Expression in Trueperella Pyogenes. Microb. Drug Resist. 2019, 25, 619–626. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Zhou, Q.; Liu, B.; Wang, Y.; Li, P.; Liao, K.; Su, W. Pithecellobium Clypearia Extract Enriched in Gallic Acid and Luteolin Has Antibacterial Activity against MRSA and Reduces Resistance to Erythromycin, Ceftriaxone Sodium and Levofloxacin. J. Appl. Microbiol. 2020, 129, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Metwaly, A.M.; Saleh, M.M.; Alsfouk, B.A.; Ibrahim, I.M.; Abd-Elraouf, M.; Elkaeed, E.B.; Eissa, I.H. Anti-Virulence Potential of Patuletin, a Natural Flavone, against Staphylococcus aureus: In Vitro and In Silico Investigations. Heliyon 2024, 10, e24075. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J.; Dong, J.; Li, H.; Luo, M.; Dai, X.; Zhang, Y.; Leng, B.; Niu, X.; Zhao, S.; et al. Chrysin Protects Mice from Staphylococcus aureus Pneumonia. J. Appl. Microbiol. 2011, 111, 1551–1558. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Sun, Z.-L.; Liu, T.; Gibbons, S.; Zhang, W.-J.; Qing, M. Flavonoids from Sophora moorcroftiana and Their Synergistic Antibacterial Effects on MRSA. Phytother. Res. 2014, 28, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zang, X.; He, M.; Pan, S.; Xu, X. Structure-Activity Relationship of Flavonoids on Their Anti-Escherichia coli Activity and Inhibition of DNA Gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lv, Q.; Sun, X.; Tang, T.; Deng, X.; Yin, Y.; Li, L. Acacetin Inhibits Streptococcus pneumoniae Virulence by Targeting Pneumolysin. J. Pharm. Pharmacol. 2020, 72, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-X.; Schrader, K.K.; Khan, I.A.; Rimando, A.M. Activities of Wogonin Analogs and Other Flavones against Flavobacterium Columnare. Chem. Biodivers. 2015, 12, 259–272. [Google Scholar] [CrossRef]
- Song, Y.; Lv, H.; Xu, L.; Liu, Z.; Wang, J.; Fang, T.; Deng, X.; Zhou, Y.; Li, D. In Vitro and in Vivo Activities of Scutellarein, a Novel Polyphosphate Kinase 1 Inhibitor against Acinetobacter baumannii Infection. Microb. Cell Fact. 2024, 23, 269. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Melong, R.; Tsamo, A.T.; Tchinda, A.T.; Kapche, D.G.W.F.; Ngadjui, B.T.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, Antimicrobial and Antioxidant Activity of Eight Compounds Isolated from Entada abyssinica (Fabaceae). BMC Res. Notes 2017, 10, 118. [Google Scholar] [CrossRef]
- Dzotam, J.K.; Simo, I.K.; Bitchagno, G.; Celik, I.; Sandjo, L.P.; Tane, P.; Kuete, V. In Vitro Antibacterial and Antibiotic Modifying Activity of Crude Extract, Fractions and 3′,4′,7-Trihydroxyflavone from Myristica Fragrans Houtt against MDR Gram-Negative Enteric Bacteria. BMC Complement. Altern. Med. 2018, 18, 15. [Google Scholar] [CrossRef]
- Yu, J.S.; Kim, J.-H.; Rashan, L.; Kim, I.; Lee, W.; Kim, K.H. Potential Antimicrobial Activity of Galloyl-Flavonoid Glycosides From Woodfordia uniflora Against Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2021, 12, 784504. [Google Scholar] [CrossRef]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Guan, X.; Zhou, Y.; Liang, X.; Xiao, J.; He, L.; Li, J. Effects of Compounds Found in Nidus vespae on the Growth and Cariogenic Virulence Factors of Streptococcus mutans. Microbiol. Res. 2012, 167, 61–68. [Google Scholar] [CrossRef]
- Xu, L.; Fang, J.; Ou, D.; Xu, J.; Deng, X.; Chi, G.; Feng, H.; Wang, J. Therapeutic Potential of Kaempferol on Streptococcus pneumoniae Infection. Microbes Infect. 2023, 25, 105058. [Google Scholar] [CrossRef] [PubMed]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The Synergy and Mode of Action of Quercetin plus Amoxicillin against Amoxicillin-Resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Holler, J.G.; Christensen, S.B.; Slotved, H.-C.; Rasmussen, H.B.; Guzman, A.; Olsen, C.-E.; Petersen, B.; Molgaard, P. Novel Inhibitory Activity of the Staphylococcus aureus NorA Efflux Pump by a Kaempferol Rhamnoside Isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, B.; Si, X.; Liu, X.; Deng, X.; Niu, X.; Jin, Y.; Wang, D.; Wang, J. Quercetin Protects Rats from Catheter-Related Staphylococcus aureus Infections by Inhibiting Coagulase Activity. J. Cell Mol. Med. 2019, 23, 4808–4818. [Google Scholar] [CrossRef]
- Liu, P.; Kang, X.; Chen, X.; Luo, X.; Li, C.; Wang, G. Quercetin Targets SarA of Methicillin-Resistant Staphylococcus aureus to Mitigate Biofilm Formation. Microbiol. Spectr. 2024, 12, e02722-23. [Google Scholar] [CrossRef]
- Jaisinghani, R.N. Antibacterial Properties of Quercetin. Microbiol. Res. 2017, 8, 13–14. [Google Scholar] [CrossRef]
- Kannan, S.; Balakrishnan, J.; Govindasamy, A.; Arunagiri, R. New Insights into the Antibacterial Mode of Action of Quercetin against Uropathogen Serratia Marcescens In-Vivo and in-Vitro. Sci. Rep. 2022, 12, 21912. [Google Scholar] [CrossRef]
- Siriwong, S.; Thumanu, K.; Hengpratom, T.; Eumkeb, G. Synergy and Mode of Action of Ceftazidime plus Quercetin or Luteolin on Streptococcus pyogenes. Evid.-Based Complement. Altern. Med. 2015, 2015, 759459. [Google Scholar] [CrossRef]
- Green, A.E.; Rowlands, R.S.; Cooper, R.A.; Maddocks, S.E. The Effect of the Flavonol Morin on Adhesion and Aggregation of Streptococcus pyogenes. FEMS Microbiol. Lett. 2012, 333, 54–58. [Google Scholar] [CrossRef]
- Arima, H.; Ashida, H.; Danno, G. Rutin-Enhanced Antibacterial Activities of Flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Griep, M.A.; Blood, S.; Larson, M.A.; Koepsell, S.A.; Hinrichs, S.H. Myricetin Inhibits Escherichia coli DnaB Helicase but Not Primase. Bioorg. Med. Chem. 2007, 15, 7203–7208. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, P.; Lv, H.; Deng, X.; Wang, J. A Natural Dietary Flavone Myricetin as an α-Hemolysin Inhibitor for Controlling Staphylococcus aureus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 330. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, J.-G.; Lee, T.-H.; Oh, K.-B. Flavonols Inhibit Sortases and Sortase-Mediated Staphylococcus aureus Clumping to Fibrinogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, Y.; Xu, X.; Li, L.; Zhou, Y.; Jia, G.; Wei, L.; Yu, Q.; Wang, J. Metallo-β-Lactamases Inhibitor Fisetin Attenuates Meropenem Resistance in NDM-1-Producing Escherichia coli. Eur. J. Med. Chem. 2022, 231, 114108. [Google Scholar] [CrossRef]
- Eumkeb, G.; Sakdarat, S.; Siriwong, S. Reversing β-Lactam Antibiotic Resistance of Staphylococcus aureus with Galangin from Alpinia Officinarum Hance and Synergism with Ceftazidime. Phytomedicine 2010, 18, 40–45. [Google Scholar] [CrossRef]
- Tan, A.; Scortecci, K.C.; Cabral De Medeiros, N.M.; Kukula-Koch, W.; Butler, T.J.; Smith, S.M.; Boylan, F. Plukenetia Volubilis Leaves as Source of Anti-Helicobacter pylori Agents. Front. Pharmacol. 2024, 15, 1461447. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria-Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Ruddock, P.S.; Charland, M.; Ramirez, S.; Lopez, A.; Towers, G.H.N.; Arnason, J.T.; Liao, M.; Dillon, J.-A.R. Antimicrobial Activity of Flavonoids From Piper lanceaefolium and Other Colombian Medicinal Plants Against Antibiotic Susceptible and Resistant Strains of Neisseria gonorrhoeae. Sex. Transm. Dis. 2011, 38, 82–88. [Google Scholar] [CrossRef]
- Gress-Antonio, C.D.; Rivero-Perez, N.; Marquina-Bahena, S.; Alvarez, L.; Zaragoza-Bastida, A.; Martinez-Juarez, V.M.; Sosa-Gutierrez, C.G.; Ocampo-Lopez, J.; Zepeda-Bastida, A.; Ojeda-Ramirez, D. Litsea Glaucescens Kuth Possesses Bactericidal Activity against Listeria monocytogenes. PeerJ 2023, 11, e16522. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Nakonechny, F.; Budovsky, A.; Zeigerman, H.; Khalfin, B.; Sharon, E.; Yarmolinsky, L.; Ben-Shabat, S.; Nisnevitch, M. Antimicrobial and Antiviral Compounds of Phlomis viscosa Poiret. Biomedicines 2023, 11, 441. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, Y.; Zhu, J.; Xia, X.; Yuan, G.; Li, S.; Deng, B.; Luo, X. Quinone Pool, a Key Target of Plant Flavonoids Inhibiting Gram-Positive Bacteria. Molecules 2023, 28, 4972. [Google Scholar] [CrossRef]
- Kim, H.W.; Woo, H.J.; Yang, J.Y.; Kim, J.-B.; Kim, S.-H. Hesperetin Inhibits Expression of Virulence Factors and Growth of Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 10035. [Google Scholar] [CrossRef]
- Husain, F.M.; Perveen, K.; Abul Qais, F.; Ahmad, I.; Alfarhan, A.H.; El-Sheikh, M.A. Naringin Inhibits the Biofilms of Metallo-β-Lactamases (MβLs) Producing Pseudomonas Species Isolated from Camel Meat. Saudi J. Biol. Sci. 2021, 28, 333–341. [Google Scholar] [CrossRef]
- Yue, J.; Yang, H.; Liu, S.; Song, F.; Guo, J.; Huang, C. Influence of Naringenin on the Biofilm Formation of Streptococcus mutans. J. Dent. 2018, 76, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Xuewen, H.; Ping, O.; Zhongwei, Y.; Zhongqiong, Y.; Hualin, F.; Juchun, L.; Changliang, H.; Gang, S.; Zhixiang, Y.; Xu, S.; et al. Eriodictyol Protects against Staphylococcus aureus-Induced Lung Cell Injury by Inhibiting Alpha-Hemolysin Expression. World J. Microbiol. Biotechnol. 2018, 34, 64. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.-H.; Li, H.-E.; Lu, C.-J.; Wang, J.-F.; Dong, J.; Wei, J.-Y.; Zhang, Y.; Wang, X.; Tan, W.; Deng, X.-M.; et al. Liquiritigenin Prevents Staphylococcus aureus-Mediated Lung Cell Injury via Inhibiting the Production of α-Hemolysin. J. Asian Nat. Prod. Res. 2013, 15, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Gupta, V.K.; Singh, P.; Pal, A.; Darokar, M.P.; Bhakuni, R.S. Drug Resistance Reversal Potential of Isoliquiritigenin and Liquiritigenin Isolated from Glycyrrhiza Glabra Against Methicillin-Resistant Staphylococcus aureus (MRSA). Phytother. Res. 2016, 30, 1708–1715. [Google Scholar] [CrossRef]
- Zampini, I.C.; Villena, J.; Salva, S.; Herrera, M.; Isla, M.I.; Alvarez, S. Potentiality of Standardized Extract and Isolated Flavonoids from Zuccagnia Punctata for the Treatment of Respiratory Infections by Streptococcus pneumoniae: In Vitro and in Vivo Studies. J. Ethnopharmacol. 2012, 140, 287–292. [Google Scholar] [CrossRef]
- Dong, H.; Liao, L.; Long, B.; Che, Y.; Peng, T.; He, Y.; Mei, L.; Xu, B. Total Synthesis and Antibacterial Evaluation of Lupinifolin and Its Natural Analogues. J. Nat. Prod. 2024, 87, 1044–1058. [Google Scholar] [CrossRef]
- Makhafola, T.J.; Samuel, B.B.; Elgorashi, E.E.; Eloff, J.N. Ochnaflavone and Ochnaflavone 7-O-Methyl Ether Two Antibacterial Biflavonoids from Ochna pretoriensis (Ochnaceae). Nat. Prod. Commun. 2012, 7, 1601–1604. [Google Scholar] [CrossRef]
- Weng, Z.; Zeng, F.; Wang, M.; Guo, S.; Tang, Z.; Itagaki, K.; Lin, Y.; Shen, X.; Cao, Y.; Duan, J.-A.; et al. Antimicrobial Activities of Lavandulylated Flavonoids in Sophora flavences against Methicillin-Resistant Staphylococcus aureus via Membrane Disruption. J. Adv. Res. 2024, 57, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, A.; Nešuta, O.; Vančatová, I.; Čížek, A.; Varela-M, R.E.; López-Abán, J.; Villa-Pulgarin, J.A.; Mollinedo, F.; Muro, A.; Žemličková, H.; et al. C-Geranylated Flavonoids from Paulownia Tomentosa Fruits with Antimicrobial Potential and Synergistic Activity with Antibiotics. Pharm. Biol. 2016, 54, 1398–1407. [Google Scholar] [CrossRef]
- Radwan, M.M.; Rodriguez-Guzman, R.; Manly, S.P.; Jacob, M.; Ross, S.A. Sepicanin A—A New Geranyl Flavanone from Artocarpus Sepicanus with Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Phytochem. Lett. 2009, 2, 141–143. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.; Aizawa, M.; Kim, M.; Yamamoto, T. Inhibitory Effects of Green Tea Polyphenols on Growth and Cellular Adherence of an Oral Bacterium, Porphyromonas Gingivalis. Biosci. Biotechnol. Biochem. 1996, 60, 745–749. [Google Scholar] [CrossRef]
- Wang, H.; Zou, H.; Wang, Y.; Jin, J.; Zhou, M. Inhibition Effect of Epigallocatechin Gallate on the Growth and Biofilm Formation of Vibrio parahaemolyticus. Lett. Appl. Microbiol. 2022, 75, 81–88. [Google Scholar] [CrossRef]
- Jubair, N.; R, M.; Fatima, A.; Mahdi, Y.K.; Abdullah, N.H. Evaluation of Catechin Synergistic and Antibacterial Efficacy on Biofilm Formation and acrA Gene Expression of Uropathogenic E. coli Clinical Isolates. Antibiotics 2022, 11, 1223. [Google Scholar] [CrossRef] [PubMed]
- Rambaher, M.H.; Zdovc, I.; Glavač, N.K.; Gobec, S.; Frlan, R. Mur Ligase F as a New Target for the Flavonoids Quercitrin, Myricetin, and (-)-Epicatechin. J. Comput. Aided Mol. Des. 2023, 37, 721–733. [Google Scholar] [CrossRef]
- Hossain, M.A.; Park, H.-C.; Lee, K.-J.; Park, S.-W.; Park, S.-C.; Kang, J. In Vitro Synergistic Potentials of Novel Antibacterial Combination Therapies against Salmonella enterica Serovar Typhimurium. BMC Microbiol. 2020, 20, 118. [Google Scholar] [CrossRef]
- Taylor, P.W. Interactions of Tea-Derived Catechin Gallates with Bacterial Pathogens. Molecules 2020, 25, 1986. [Google Scholar] [CrossRef]
- Verdrengh, M.; Collins, L.V.; Bergin, P.; Tarkowski, A. Phytoestrogen Genistein as an Anti-Staphylococcal Agent. Microbes Infect. 2004, 6, 86–92. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.G. Nitric Oxide-Inducing Genistein Elicits Apoptosis-like Death via an Intense SOS Response in Escherichia coli. Appl. Microbiol. Biotechnol. 2020, 104, 10711–10724. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Pal, A.; Darokar, M.P. Glabridin Synergy with Norfloxacin Induces ROS in Multidrug Resistant Staphylococcus aureus. J. Gen. Appl. Microbiol. 2021, 67, 269–272. [Google Scholar] [CrossRef] [PubMed]
- das Neves, M.V.M.; da Silva, T.M.S.; Lima, E.d.O.; da Cunha, E.V.L.; Oliveira, E.d.J. Isoflavone Formononetin from Red Propolis Acts as a Fungicide against Candida sp. Braz. J. Microbiol. 2016, 47, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Delfan, N.; Moghadam, M.D.; Shakib, P.; Sepahdar, A.; Naghibeiranvand, Z. Antimicrobial Effect of Formononetin Against the Periodental Pathogens Enterococcus faecalis and Candida albicans. Recent Pat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Polbuppha, I.; Suthiphasilp, V.; Maneerat, T.; Charoensup, R.; Limtharakul, T.; Cheenpracha, S.; Pyne, S.G.; Laphookhieo, S. Macluracochinones A-E, Antimicrobial Flavonoids from Maclura cochinchinensis (Lour.) Corner. Phytochemistry 2021, 187, 112773. [Google Scholar] [CrossRef]
- Liao, L.; Wang, N.; Mei, L.; Long, B.; Luo, T.; Wang, M.-Q.; Lu, L.; Dong, H.-B. Total Synthesis and Antibacterial Evaluation of Lupalbigenin and Isolupalbigenin. J. Asian Nat. Prod. Res. 2024, 27, 556–567. [Google Scholar] [CrossRef]
- Raksat, A.; Maneerat, W.; Andersen, R.J.; Pyne, S.G.; Laphookhieo, S. Antibacterial Prenylated Isoflavonoids from the Stems of Millettia Extensa. J. Nat. Prod. 2018, 81, 1835–1840. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Tani, N.; Nagayama, M.; Yamaguchi, R. Different Antibacterial Actions of Isoflavones Isolated from Erythrina Poeppigiana against Methicillin-Resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2006, 43, 243–248. [Google Scholar] [CrossRef]
- Chongwilaikasem, N.; Sithisarn, P.; Rojsanga, P.; Sithisarn, P. Green Extraction and Partial Purification of Roselle (Hibiscus sabdariffa L.) Extracts with High Amounts of Phytochemicals and in Vitro Antioxidant and Antibacterial Effects. J. Food Sci. 2024, 89, 8819–8835. [Google Scholar] [CrossRef]
- Lakhani, M.; Azim, S.; Akhtar, S.; Ahmad, Z. Inhibition of Escherichia coli ATP Synthase and Cell Growth by Dietary Pomegranate Phenolics. Int. J. Biol. Macromol. 2022, 213, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Costa, E.M.; Machado, M.; Morais, R.; Calhau, C.; Pintado, M. Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus. Metabolites 2022, 12, 1062. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, A.B.; Cătunescu, G.M.; González, M.M.-P.; Hornedo-Ortega, R.; Pop, C.R.; Rusu, C.C.; Chirilă, F.; Rotar, A.M.; Garcia-Parrilla, M.C.; Troncoso, A.M. Anthocyanins in Blueberries Grown in Hot Climate Exert Strong Antioxidant Activity and May Be Effective against Urinary Tract Bacteria. Antioxidants 2020, 9, 478. [Google Scholar] [CrossRef]
- Zhong, L.; Lin, Y.; Wang, C.; Niu, B.; Xu, Y.; Zhao, G.; Zhao, J. Chemical Profile, Antimicrobial and Antioxidant Activity Assessment of the Crude Extract and Its Main Flavonoids from Tartary Buckwheat Sprouts. Molecules 2022, 27, 374. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Z.; Liu, Y.; Wang, H.; Qiu, M.; Qu, Y.; Yuan, W. The Optimization of Extraction Process, Antioxidant, Whitening and Antibacterial Effects of Fengdan Peony Flavonoids. Molecules 2022, 27, 506. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Shao, X.; Wei, Y.; Xu, F.; Wang, H. Flavonoids from Sedum aizoon L. Inhibit Botrytis cinerea by Negatively Affecting Cell Membrane Lipid Metabolism. Appl. Microbiol. Biotechnol. 2022, 106, 7139–7151. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Singh, A.K.; Kumar, R.; Croley, C.R.; Pandey, A.K.; Coy-Barrera, E.; Kumar Patra, J.; Das, G.; Kerry, R.G.; Annunziata, G.; et al. Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders. Int. J. Mol. Sci. 2019, 20, 4957. [Google Scholar] [CrossRef] [PubMed]
- Mahamud, A.G.M.S.U.; Ashrafudoulla, M.; Nahar, S.; Chowdhury, M.A.H.; Park, S.H.; Ha, S.-D. Luteolin Exhibits Antimicrobial Actions against Salmonella Typhimurium and Escherichia coli: Impairment of Cell Adhesion, Membrane Integrity, and Energy Metabolism. Food Control 2024, 166, 110734. [Google Scholar] [CrossRef]
- Wang, S.; Yao, J.; Zhou, B.; Yang, J.; Chaudry, M.T.; Wang, M.; Xiao, F.; Li, Y.; Yin, W. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J. Food Prot. 2018, 81, 68–78. [Google Scholar] [CrossRef]
- Li, A.-P.; He, Y.-H.; Zhang, S.-Y.; Shi, Y.-P. Antibacterial Activity and Action Mechanism of Flavonoids against Phytopathogenic Bacteria. Pestic. Biochem. Physiol. 2022, 188, 105221. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Zhou, Z.; Yang, J.; Liu, W.; Zhang, Y.; Zhang, P.; Guo, T.; Li, G. Baicalein Resensitizes Multidrug-Resistant Gram-Negative Pathogens to Doxycycline. Microbiol. Spectr. 2023, 11, e0470222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Y.; Yuan, F.W.; Liang, T.; Liang, X.C.; Luo, Y.R.; Jiang, M.; Qing, S.Z.; Zhang, W.M. Baicalin Inhibits Escherichia coli Isolates in Bovine Mastitic Milk and Reduces Antimicrobial Resistance. J. Dairy Sci. 2018, 101, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as Anti-Inflammatory Agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef]
- Agus, S.; Achmadi, S.S.; Mubarik, N.R. Antibacterial Activity of Naringenin-Rich Fraction of Pigeon Pea Leaves toward Salmonella thypi. Asian Pac. J. Trop. Biomed. 2017, 7, 725–728. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef]
- Alkufeidy, R.M.; Ameer Altuwijri, L.; Aldosari, N.S.; Alsakabi, N.; Dawoud, T.M. Antimicrobial and Synergistic Properties of Green Tea Catechins against Microbial Pathogens. J. King Saud. Univ.—Sci. 2024, 36, 103277. [Google Scholar] [CrossRef]
- Noor Mohammadi, T.; Maung, A.T.; Sato, J.; Sonoda, T.; Masuda, Y.; Honjoh, K.; Miyamoto, T. Mechanism for Antibacterial Action of Epigallocatechin Gallate and Theaflavin-3,3′-Digallate on Clostridium Perfringens. J. Appl. Microbiol. 2019, 126, 633–640. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Ma, R.; Sun, W.; Ji, Z. Antibacterial Activity of Epigallocatechin Gallate (EGCG) against Shigella Flexneri. Int. J. Environ. Res. Public Health 2023, 20, 4676. [Google Scholar] [CrossRef]
- Li, L.; Zhou, P.; Wang, Y.; Pan, Y.; Chen, M.; Tian, Y.; Zhou, H.; Yang, B.; Meng, H.; Zheng, J. Antimicrobial activity of cyanidin-3-O-glucoside-lauric acid ester against Staphylococcus aureus and Escherichia coli. Food Chem. 2022, 383, 132410. [Google Scholar] [CrossRef]
- Gong, S.; Fei, P.; Sun, Q.; Guo, L.; Jiang, L.; Duo, K.; Bi, X.; Yun, X. Action Mode of Cranberry Anthocyanin on Physiological and Morphological Properties of Staphylococcus aureus and Its Application in Cooked Meat. Food Microbiol. 2021, 94, 103632. [Google Scholar] [CrossRef]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial Effect of the Tunisian Nana Variety Punica granatum L. Extracts against Salmonella enterica (Serovars Kentucky and Enteritidis) Isolated from Chicken Meat and Phenolic Composition of Its Peel Extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef]
- Lacombe, A.; Wu, V.C.H.; White, J.; Tadepalli, S.; Andre, E.E. The Antimicrobial Properties of the Lowbush Blueberry (Vaccinium angustifolium) Fractional Components against Foodborne Pathogens and the Conservation of Probiotic Lactobacillus rhamnosus. Food Microbiol. 2012, 30, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary Phenolics: Chemistry, Bioavailability and Effects on Health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Hummelova, J.; Rondevaldova, J.; Balastikova, A.; Lapcik, O.; Kokoska, L. The Relationship between Structure and in Vitro Antibacterial Activity of Selected Isoflavones and Their Metabolites with Special Focus on Antistaphylococcal Effect of Demethyltexasin. Lett. Appl. Microbiol. 2015, 60, 242–247. [Google Scholar] [CrossRef]
- Lim, J.-W.; Jo, Y.H.; Choi, J.-S.; Lee, M.K.; Lee, K.Y.; Kang, S.Y. Antibacterial Activities of Prenylated Isoflavones from Maclura Tricuspidata against Fish Pathogenic Streptococcus: Their Structure-Activity Relationships and Extraction Optimization. Molecules 2021, 26, 7451. [Google Scholar] [CrossRef]
- Yuan, G.; Guan, Y.; Yi, H.; Lai, S.; Sun, Y.; Cao, S. Antibacterial Activity and Mechanism of Plant Flavonoids to Gram-Positive Bacteria Predicted from Their Lipophilicities. Sci. Rep. 2021, 11, 10471. [Google Scholar] [CrossRef]
- Yang, R.; Li, J.; Wang, J.; Wang, Y.; Ma, F.; Zhai, R.; Li, P. Kaempferol Inhibits the Growth of Helicobacter pylori in a Manner Distinct from Antibiotics. J. Food Biochem. 2022, 46, e14210. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Li, W.; Ma, H.; Guo, S.; Wang, D.; Niu, Y. Inhibitory Activity of Flavonoids Fraction from Astragalus Membranaceus Fisch. Ex Bunge Stems and Leaves on Bacillus Cereus and Its Separation and Purification. Front. Pharmacol. 2023, 14, 1183393. [Google Scholar] [CrossRef]
- Wang, J.; Chi, Z.; Zhao, K.; Wang, H.; Zhang, X.; Xu, F.; Shao, X.; Wei, Y. A Transcriptome Analysis of the Antibacterial Mechanism of Flavonoids from Sedum aizoon L. against Shewanella Putrefaciens. World J. Microbiol. Biotechnol. 2020, 36, 94. [Google Scholar] [CrossRef]
- Keller, M.R.; Dörr, T. Bacterial Metabolism and Susceptibility to Cell Wall-Active Antibiotics. Adv. Microb. Physiol. 2023, 83, 181–219. [Google Scholar] [CrossRef]
- Liang, H.; He, K.; Li, T.; Cui, S.; Tang, M.; Kang, S.; Ma, W.; Song, L. Mechanism and Antibacterial Activity of Vine Tea Extract and Dihydromyricetin against Staphylococcus aureus. Sci. Rep. 2020, 10, 21416. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Ouyang, K.; Zhang, Q.; Wang, W. Antibacterial Activity and Mechanism of Flavonoids from Chimonanthus salicifolius S. Y. Hu. and Its Transcriptome Analysis against Staphylococcus aureus. Front. Microbiol. 2022, 13, 1103476. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, H.; Xie, M. Antibacterial Mechanism of Soybean Isoflavone on Staphylococcus aureus. Arch. Microbiol. 2010, 192, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Geethalakshmi, R.; Sundaramurthi, J.C.; Sarada, D.V.L. Antibacterial Activity of Flavonoid Isolated from Trianthema Decandra against Pseudomonas aeruginosa and Molecular Docking Study of FabZ. Microb. Pathog. 2018, 121, 87–92. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Huang, C.-C.; Chen, C.-C.; Yang, K.-J.; Huang, C.-Y. Inhibition of Staphylococcus aureus PriA Helicase by Flavonol Kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Rock, C.O. Evaluation of Epigallocatechin Gallate and Related Plant Polyphenols as Inhibitors of the FabG and FabI Reductases of Bacterial Type II Fatty-Acid Synthase. J. Biol. Chem. 2004, 279, 30994–31001. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, Z.; Wang, Y. Biofilm Formation and Inhibition Mediated by Bacterial Quorum Sensing. Appl. Microbiol. Biotechnol. 2022, 106, 6365–6381. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, J.; Meng, J.; Qiu, T.; Wang, W.; Wang, R.; Liu, J. Baicalin Inhibits Biofilm Formation by Influencing Primary Adhesion and Aggregation Phases in Staphylococcus saprophyticus. Vet. Microbiol. 2021, 262, 109242. [Google Scholar] [CrossRef]
- Qayyum, S.; Sharma, D.; Bisht, D.; Khan, A.U. Identification of Factors Involved in Enterococcus faecalis Biofilm under Quercetin Stress. Microb. Pathog. 2019, 126, 205–211. [Google Scholar] [CrossRef]
- Leitão, J.H. Microbial Virulence Factors. Int. J. Mol. Sci. 2020, 21, 5320. [Google Scholar] [CrossRef]
- Yin, N.; Yang, X.; Wang, L.; Zhang, C.; Guan, J.; Tao, Y.; Guo, X.; Zhao, Y.; Song, W.; Wang, B.; et al. Kaempferol Inhibits the Expression of α-Hemolysin and Protects Mice from Methicillin-Resistant Staphylococcus aureus-Induced Lethal Pneumonia. Microb. Pathog. 2022, 162, 105336. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, W.-H.; Zhou, X.; Liu, Y.-H.; Li, Z.; Tang, Y.-S.; Kou, X.; Wang, S.-D.; Bao, M.; Qu, L.-D.; et al. Puerarin Protects against Staphylococcus aureus-Induced Injury of Human Alveolar Epithelial A549 Cells via Downregulating Alpha-Hemolysin Secretion. Microb. Drug Resist. 2014, 20, 357–363. [Google Scholar] [CrossRef]
- Peng, L.-Y.; Yuan, M.; Cui, Z.-Q.; Wu, Z.-M.; Yu, Z.-J.; Song, K.; Tang, B.; Fu, B.-D. Rutin Inhibits Quorum Sensing, Biofilm Formation and Virulence Genes in Avian Pathogenic Escherichia coli. Microb. Pathog. 2018, 119, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Dutta, B.; Mukherjee, I.; Ghosh, S.; Dey, A.; Banerjee, R.; Ray, R.R. Catechin as the Most Efficient Bioactive Compound from Azadirachta Indica with Antibiofilm and Anti-Quorum Sensing Activities Against Dental Biofilm: An In Vitro and In Silico Study. Appl. Biochem. Biotechnol. 2021, 193, 1617–1630. [Google Scholar] [CrossRef]
- Nain, Z.; Mansur, F.J.; Syed, S.B.; Islam, M.A.; Azakami, H.; Islam, M.R.; Karim, M.M. Inhibition of Biofilm Formation, Quorum Sensing and Other Virulence Factors in Pseudomonas aeruginosa by Polyphenols of Gynura Procumbens Leaves. J. Biomol. Struct. Dyn. 2022, 40, 5357–5371. [Google Scholar] [CrossRef]
- Zong, B.; Xiao, Y.; Wang, P.; Liu, W.; Ren, M.; Li, C.; Fu, S.; Zhang, Y.; Qiu, Y. Baicalin Weakens the Virulence of Porcine Extraintestinal Pathogenic Escherichia coli by Inhibiting the LuxS/AI-2 Quorum-Sensing System. Biomolecules 2024, 14, 452. [Google Scholar] [CrossRef]
- Chang, A.; He, Q.; Li, L.; Yu, X.; Sun, S.; Zhu, H. Exploring the Quorum Sensing Inhibition of Isolated Chrysin from Penicillium Chrysogenum DXY-1. Bioorg Chem. 2021, 111, 104894. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Q.; Wei, Z.; Yang, Q.; Guo, Z.; Shen, L.; Duan, K.; Chen, L. Baicalin Represses Type Three Secretion System of Pseudomonas aeruginosa through PQS System. Molecules 2021, 26, 1497. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Lv, Y.; Dou, X.; Wassy, S.L.; Jia, G.; Wei, L.; Yu, Q.; Deng, X.; Zhang, C.; Wang, J. Myricetin Inhibits the Type III Secretion System of Salmonella enterica Serovar Typhimurium by Downregulating the Salmonella Pathogenic Island I Gene Regulatory Pathway. Microb. Pathog. 2021, 150, 104695. [Google Scholar] [CrossRef]
- Lan, J.-E.; Li, X.-J.; Zhu, X.-F.; Sun, Z.-L.; He, J.-M.; Zloh, M.; Gibbons, S.; Mu, Q. Flavonoids from Artemisia Rupestris and Their Synergistic Antibacterial Effects on Drug-Resistant Staphylococcus aureus. Nat. Prod. Res. 2021, 35, 1881–1886. [Google Scholar] [CrossRef]
- Zou, D.; Xie, K.; Wang, H.; Chen, Y.; Xie, M. Inhibitory effects of biochanin A on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA). Wei Sheng Wu Xue Bao 2014, 54, 1204–1211. [Google Scholar] [PubMed]
- Wang, D.; Xie, K.; Zou, D.; Meng, M.; Xie, M. Inhibitory Effects of Silybin on the Efflux Pump of Methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2018, 18, 827–833. [Google Scholar] [CrossRef]
- Alves Borges Leal, A.L.; Teixeira da Silva, P.; Nunes da Rocha, M.; Marinho, E.M.; Marinho, E.S.; Marinho, M.M.; Bandeira, P.N.; Sampaio Nogueira, C.E.; Barreto, H.M.; Rodrigues Teixeira, A.M.; et al. Potentiating Activity of Norfloxacin by Synthetic Chalcones against NorA Overproducing Staphylococcus aureus. Microb. Pathog. 2021, 155, 104894. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.; Pratiwi, L.; Apridamayanti, P. The Highest Dosage Combination Activity Screening from the Leaf Fraction of Melastoma Malabathricum with Antibiotic Gentamicin and Ciprofloxacin. J. Pharmacopunct. 2022, 25, 101–105. [Google Scholar] [CrossRef]
- Yi, L.; Cao, M.; Chen, X.; Bai, Y.; Wang, W.; Wei, X.; Shi, Y.; Zhang, Y.; Ma, T.; Zhu, Z.; et al. In Vitro Antimicrobial Synergistic Activity and the Mechanism of the Combination of Naringenin and Amikacin Against Antibiotic-Resistant Escherichia coli. Microorganisms 2024, 12, 1871. [Google Scholar] [CrossRef]
- Bakar, N.S.; Zin, N.M.; Basri, D.F. Synergy of Flavone with Vancomycin and Oxacillin against Vancomycin-Intermediate Staphyloccus aureus. Pak. J. Pharm. Sci. 2012, 25, 633–638. [Google Scholar]
- Cheng, P.; Sun, Y.; Wang, B.; Liang, S.; Yang, Y.; Gui, S.; Zhang, K.; Qu, S.; Li, L. Mechanism of Synergistic Action of Colistin with Resveratrol and Baicalin against Mcr-1-Positive Escherichia coli. Biomed. Pharmacother. 2024, 180, 117487. [Google Scholar] [CrossRef]
- Zhong, Z.-X.; Zhou, S.; Liang, Y.-J.; Wei, Y.-Y.; Li, Y.; Long, T.-F.; He, Q.; Li, M.-Y.; Zhou, Y.-F.; Yu, Y.; et al. Natural Flavonoids Disrupt Bacterial Iron Homeostasis to Potentiate Colistin Efficacy. Sci. Adv. 2023, 9, eadg4205. [Google Scholar] [CrossRef]
- Vipin, C.; Saptami, K.; Fida, F.; Mujeeburahiman, M.; Rao, S.S.; Athmika; Arun, A.B.; Rekha, P.D. Potential Synergistic Activity of Quercetin with Antibiotics against Multidrug-Resistant Clinical Strains of Pseudomonas aeruginosa. PLoS ONE 2020, 15, e0241304. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Quercetin Potentiates Meropenem Activity among Pathogenic Carbapenem-Resistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Appl. Microbiol. 2019, 127, 1038–1047. [Google Scholar] [CrossRef]
- Odabaş Köse, E.; Koyuncu Özyurt, Ö.; Bilmen, S.; Er, H.; Kilit, C.; Aydemir, E. Quercetin: Synergistic Interaction with Antibiotics against Colistin-Resistant Acinetobacter baumannii. Antibiotics 2023, 12, 739. [Google Scholar] [CrossRef]
- Atta, S.; Waseem, D.; Fatima, H.; Naz, I.; Rasheed, F.; Kanwal, N. Antibacterial Potential and Synergistic Interaction between Natural Polyphenolic Extracts and Synthetic Antibiotic on Clinical Isolates. Saudi J. Biol. Sci. 2023, 30, 103576. [Google Scholar] [CrossRef]
- Ma, Y.; Kang, X.; Wang, G.; Luo, S.; Luo, X.; Wang, G. Inhibition of Staphylococcus aureus Biofilm by Quercetin Combined with Antibiotics. Biofouling 2024, 40, 996–1011. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Vijayakumar, T.S.; Sasirekha, R.; Vimala, R.T.V.; Sivasubramanian, S.; Arun, S.; Babu, M.D.; Thirumurugan, R. Antibacterial Synergy between Rutin and Florfenicol Enhances Therapeutic Spectrum against Drug Resistant Aeromonas Hydrophila. Microb. Pathog. 2019, 135, 103612. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.U.; Khurram, M.; Khattak, B.; Khan, J. Antibiotic Additive and Synergistic Action of Rutin, Morin and Quercetin against Methicillin Resistant Staphylococcus aureus. BMC Complement. Altern. Med. 2015, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Akilandeswari, K.; Ruckmani, K. Synergistic Antibacterial Effect of Apigenin with β-Lactam Antibiotics and Modulation of Bacterial Resistance by a Possible Membrane Effect against Methicillin Resistant Staphylococcus aureus. Cell. Mol. Biol. 2016, 62, 74–82. [Google Scholar] [CrossRef]
- Eumkeb, G.; Siriwong, S.; Thumanu, K. Synergistic Activity of Luteolin and Amoxicillin Combination against Amoxicillin-Resistant Escherichia coli and Mode of Action. J. Photochem. Photobiol. B 2012, 117, 247–253. [Google Scholar] [CrossRef]
- Joung, D.-K.; Kang, O.-H.; Seo, Y.-S.; Zhou, T.; Lee, Y.-S.; Han, S.-H.; Mun, S.-H.; Kong, R.; Song, H.-J.; Shin, D.-W.; et al. Luteolin Potentiates the Effects of Aminoglycoside and β-Lactam Antibiotics against Methicillin-Resistant Staphylococcus aureus in Vitro. Exp. Ther. Med. 2016, 11, 2597–2601. [Google Scholar] [CrossRef]
- Kim, S.; Woo, E.-R.; Lee, D.G. Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida Albicans. IUBMB Life 2019, 71, 283–292. [Google Scholar] [CrossRef]
- Fournier-Larente, J.; Morin, M.-P.; Grenier, D. Green Tea Catechins Potentiate the Effect of Antibiotics and Modulate Adherence and Gene Expression in Porphyromonas Gingivalis. Arch. Oral Biol. 2016, 65, 35–43. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic Antimicrobial Activities of Epigallocatechin Gallate, Myricetin, Daidzein, Gallic Acid, Epicatechin, 3-Hydroxy-6-Methoxyflavone and Genistein Combined with Antibiotics against ESKAPE Pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Feng, L.; Xu, M.; Wen, H.; Yao, Z.; Shi, S.; Wu, Q.; Zhou, C.; Cao, J.; et al. In Vitro and in Vivo Synergistic Effect of Chrysin in Combination with Colistin against Acinetobacter baumannii. Front. Microbiol. 2022, 13, 961498. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, M.; Guo, W.; Yao, Z.; Du, X.; Chen, L.; Sun, Y.; Shi, S.; Cao, J.; Zhou, T. The Antibacterial Activity of Kaempferol Combined with Colistin against Colistin-Resistant Gram-Negative Bacteria. Microbiol. Spectr. 2022, 10, e0226522. [Google Scholar] [CrossRef]
- Siriphap, A.; Kiddee, A.; Duangjai, A.; Yosboonruang, A.; Pook-In, G.; Saokaew, S.; Sutheinkul, O.; Rawangkan, A. Antimicrobial Activity of the Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) against Clinical Isolates of Multidrug-Resistant Vibrio cholerae. Antibiotics 2022, 11, 518. [Google Scholar] [CrossRef]
- Parvez, M.A.K.; Saha, K.; Rahman, J.; Munmun, R.A.; Rahman, M.A.; Dey, S.K.; Rahman, M.S.; Islam, S.; Shariare, M.H. Antibacterial Activities of Green Tea Crude Extracts and Synergistic Effects of Epigallocatechingallate (EGCG) with Gentamicin against MDR Pathogens. Heliyon 2019, 5, e02126. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kim, S.H.; Kim, H.; Yeom, J.; Ko, K.; Park, W.; Park, S. AFM Probing the Mechanism of Synergistic Effects of the Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) with Cefotaxime against Extended-Spectrum Beta-Lactamase (ESBL)-Producing Escherichia coli. PLoS ONE 2012, 7, e48880. [Google Scholar] [CrossRef]
- Denny, B.J.; West, P.W.J.; Mathew, T.C. Antagonistic Interactions between the Flavonoids Hesperetin and Naringenin and Beta-Lactam Antibiotics against Staphylococcus aureus. Br. J. Biomed. Sci. 2008, 65, 145–147. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Cao, M.; Shang, Z.; Xu, J.; Chen, X.; Zhu, Z.; Wang, W.; Wei, X.; Zhou, X.; Bai, Y.; et al. Research Progress on the Antibacterial Activity of Natural Flavonoids. Antibiotics 2025, 14, 334. https://doi.org/10.3390/antibiotics14040334
Zhang Z, Cao M, Shang Z, Xu J, Chen X, Zhu Z, Wang W, Wei X, Zhou X, Bai Y, et al. Research Progress on the Antibacterial Activity of Natural Flavonoids. Antibiotics. 2025; 14(4):334. https://doi.org/10.3390/antibiotics14040334
Chicago/Turabian StyleZhang, Zhijin, Mingze Cao, Zixuan Shang, Jing Xu, Xu Chen, Zhen Zhu, Weiwei Wang, Xiaojuan Wei, Xuzheng Zhou, Yubin Bai, and et al. 2025. "Research Progress on the Antibacterial Activity of Natural Flavonoids" Antibiotics 14, no. 4: 334. https://doi.org/10.3390/antibiotics14040334
APA StyleZhang, Z., Cao, M., Shang, Z., Xu, J., Chen, X., Zhu, Z., Wang, W., Wei, X., Zhou, X., Bai, Y., & Zhang, J. (2025). Research Progress on the Antibacterial Activity of Natural Flavonoids. Antibiotics, 14(4), 334. https://doi.org/10.3390/antibiotics14040334






