Unfavorable Outcomes and Their Risk Factors in Hospitalized Patients with Staphylococcus aureus Bacteremia in the US: A Multicenter Retrospective Cohort Study, 2020–2022
Abstract
1. Introduction
2. Methods
2.1. Ethics Statement
2.2. Study Design and Patient Population
2.3. Data Source
2.4. Baseline Measures
2.5. Pre-Infection Onset Hospital and Process-of-Care Variables
2.6. Infection and Treatment Characteristics
2.7. Outcome Variables
- Death during index hospitalization (hospital mortality).
- Worsening of disease:
- Antimicrobial escalation:
- Switch from vancomycin to daptomycin;
- Switch from vancomycin to ceftaroline;
- Switch from daptomycin to ceftaroline;
- Switch from vancomycin to daptomycin + ceftaroline;
- Addition of ceftaroline to daptomycin;
- Addition of an aminoglycoside or a fluoroquinolone to vancomycin;
- Addition of an aminoglycoside or a fluoroquinolone to daptomycin.
- ICU following SAB onset;
- MV following SAB onset;
- Vasopressors following SAB onset;
- Dialysis following SAB onset.
- 3.
- Failure to eradicate S. aureus (SA, defined as blood cultures on day 8 or later following index culture continuing to grow index organism).
- 4.
- Prolonged post-infection onset length of stay (LOS, defined as longer than the group median LOS).
- 5.
- Readmission for any reason within 30 days of discharge among survivors.
- Post-infection onset hospital LOS;
- Post-infection onset ICU LOS (days);
- Duration of post-infection onset MV;
- Post-infection onset hospital costs ($).
2.8. Follow-Up
2.9. Statistical Analyses
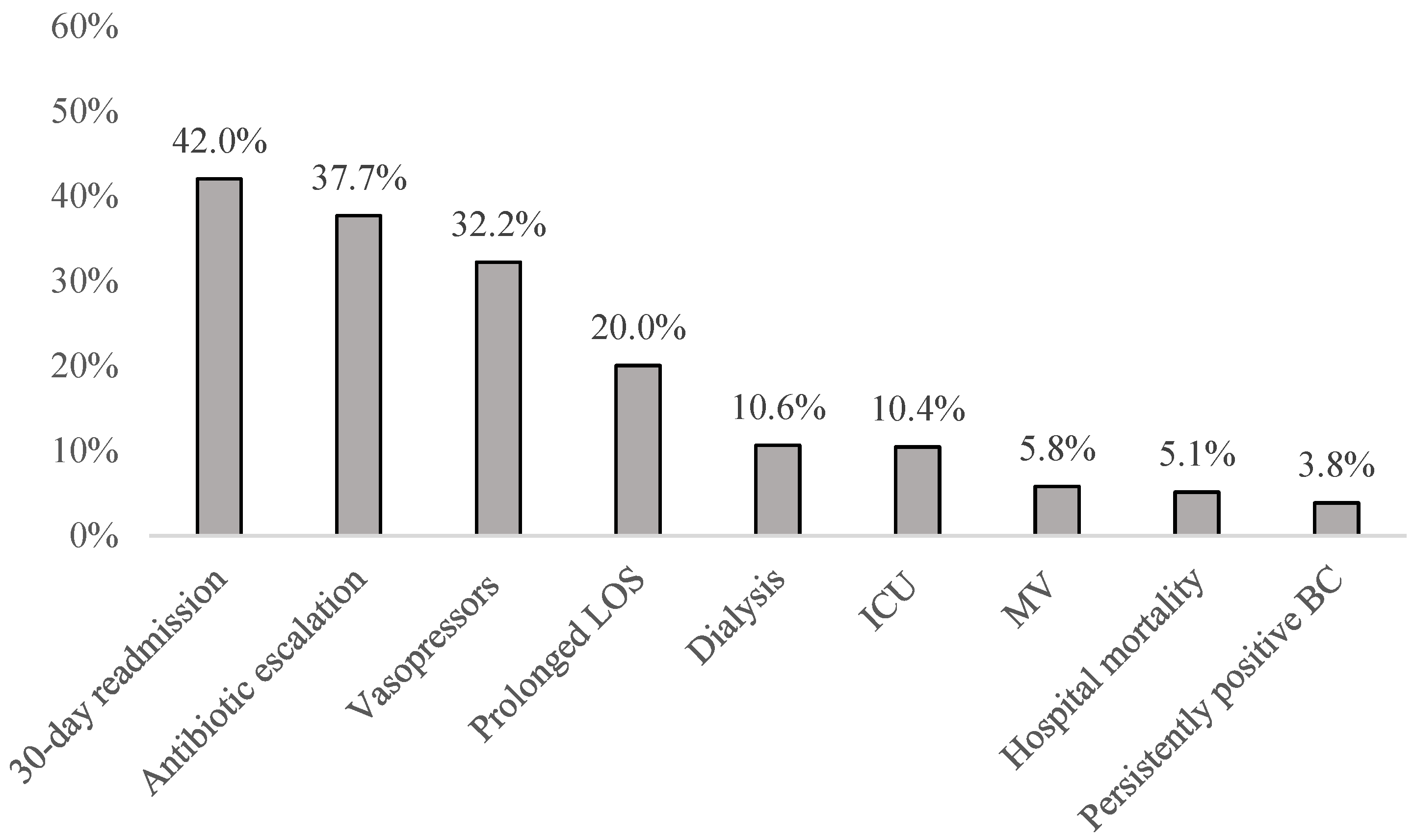
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAB | Staphylococcus aureus bacteremia |
| MRSA | methicillin-resistant S. aureus |
| MSSA | methicillin-sensitive S. aureus |
| MV | mechanical ventilation |
| IET | inappropriate empiric treatment |
| UO | unfavorable outcome |
| FO | favorable outcome |
| LOS | length of stay |
| BSI | bloodstream infection |
References
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive methi-cillin-resistant Staphylococcus aureus infections in the United States. JAMA 2007, 298, 1763–1771. [Google Scholar] [CrossRef]
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital Signs:Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-SusceptibleStaphylococcus aureusBloodstream Infections—United States. Morb. Mortal. Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- Kallen, A.J.; Mu, Y.; Bulens, S.; Reingold, A.; Petit, S.; Gershman, K.; Ray, S.M.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; et al. Healthcare-associated Invasive MRSA Infections, 2005–2008. JAMA 2010, 304, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Swets, M.C.; Bakk, Z.; Westgeest, A.C.; Berry, K.; Cooper, G.; Sim, W.; Lee, R.S.; Gan, T.Y.; Donlon, W.; Besu, A.; et al. Clinical Subphenotypes of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2024, 79, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services Office for Human Research Protections. Human Subject Regulations Decision Charts. Available online: https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts/index.html (accessed on 3 February 2024).
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Fan, W.; Shorr, A.F. A novel algorithm to analyze epidemiology and outcomes of carbapenem resistance among patients with hospital-acquired and ventilator-associated pneumonia: A retrospective cohort study. Chest 2019, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Fan, W.; Shorr, A.F. Development and validation of a bedside instrument to predict carbapenem resistance among gram-negative pathogens in complicated urinary tract infections. Infect. Control Hosp. Epidemiol. 2018, 39, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Ditch, K.; Lawrence, K.; Olesky, M.; Shorr, A.F. Carbapenem Treatment and Outcomes Among Patients with Culture-Positive Complicated Intra-abdominal Infections in US Hospitals: A Retrospective Cohort Study. Open Forum Infect. Dis. 2019, 6, ofz504. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, M.B.; Pekow, P.S.; Priya, A.; Zilberberg, M.D.; Belforti, R.; Skiest, D.; Lagu, T.; Higgins, T.L.; Lindenauer, P.K. Using Highly Detailed Administrative Data to Predict Pneumonia Mortality. PLoS ONE 2014, 9, e87382. [Google Scholar] [CrossRef] [PubMed]
- Rothberg, M.B.; Haessler, S.; Lagu, T.; Lindenauer, P.K.; Pekow, P.S.; Priya, A.; Skiest, D.; Zilberberg, M.D. Outcomes of patients with healthcare-associated pneumonia: Worse disease or sicker patients? Infect. Control Hosp. Epidemiol. 2014, 35 (Suppl. S3), S107–S115. [Google Scholar] [CrossRef] [PubMed]
- Reitz, K.M.; Kennedy, J.; Li, S.R.; Handzel, R.; Tonetti, D.A.; Neal, M.D.; Zuckerbraun, B.S.; Hall, D.E.; Sperry, J.L.; Angus, D.C.; et al. Association Between Time to Source Control in Sepsis and 90-Day Mortality. JAMA Surg. 2022, 157, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.J.; Hensche, M.K. Characteristics of 30-Day All-Cause Hospital Readmissions, 2016–2020. HCUP Statistical Brief #304; Agency for Healthcare Research and Quality: Rock-ville, MD, USA, September 2023. Available online: www.hcup-us.ahrq.gov/reports/statbriefs/sb304-readmissions-2016-2020.pdf (accessed on 19 August 2024).
- Inagaki, K.; Lucar, J.; Blackshear, C.; Hobbs, C.V. Methicillin-susceptible and Methicillin-resistant Staphylococcus aureus Bacteremia: Nationwide Estimates of 30-Day Readmission, In-hospital Mortality, Length of Stay, and Cost in the United States. Clin. Infect. Dis. 2019, 69, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Higgins, T.L.; Deshpande, A.; Zilberberg, M.D.; Lindenauer, P.K.; Imrey, P.B.; Yu, P.-C.; Haessler, S.D.; Richter, S.S.; Rothberg, M.B. Assessment of the Accuracy of Using ICD-9 Diagnosis Codes to Identify Pneumonia Etiology in Patients Hospitalized with Pneumonia. JAMA Netw. Open 2020, 3, e207750. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.D.; Lo, C.K.; Komorowski, A.S.; Suresh, M.; Guo, K.; Garg, A.; Tandon, P.; Senecal, J.; Del Corpo, O.; Stefanova, I.; et al. Staphylococcus aureus bacteraemia mortality: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2022, 28, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- van Hal, S.J.; Jensen, S.O.; Vaska, V.L.; Espedido, B.A.; Paterson, D.L.; Gosbell, I.B. Predictors of Mortality in Staphylococcus aureus Bacteremia. Clin. Microbiol. Rev. 2012, 25, 362–386. [Google Scholar] [CrossRef] [PubMed]
- Westgeest, A.C.; Buis, D.T.P.; E Sigaloff, K.C.; Ruffin, F.; Visser, L.G.; Yu, Y.; Schippers, E.F.; Lambregts, M.M.C.; Tong, S.Y.C.; de Boer, M.G.J.; et al. Global Differences in the Management of Staphylococcus aureus Bacteremia: No International Standard of Care. Clin. Infect. Dis. 2023, 77, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Falces-Romero, I.; Bloise, I.; García-Rodríguez, J.; Cendejas-Bueno, E.; SARS-CoV-2 Working Group. Staphylococcus aureus bacteremia in patients with SARS-CoV-2 infection. Med. Clin. Engl. Ed. 2023, 160, 495–498. [Google Scholar] [PubMed]
- A Cusumano, J.; Dupper, A.C.; Malik, Y.; Gavioli, E.M.; Banga, J.; Caban, A.B.; Nadkarni, D.; Obla, A.; Vasa, C.V.; Mazo, D.; et al. Staphylococcus aureus Bacteremia in Patients Infected with COVID-19: A Case Series. Open Forum Infect. Dis. 2020, 7, ofaa518. [Google Scholar] [CrossRef] [PubMed]
- Böing, C.W.; Froböse, N.J.; Schaumburg, F.; Kampmeier, S. Impact of the COVID-19 Pandemic on the Management of Staphylococcus aureus Bloodstream Infections in a Tertiary Care Hospital. Pathogens 2023, 12, 611. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.; Schmitz, R.P.; Hagel, S.; Pletz, M.W.; Gagelmann, N.; Scherag, A.; Schlattmann, P.; Brunkhorst, F.M. Infectious disease consultation for Staphylococcus aureus bacteremia—A systematic review and meta-analysis. J. Infect. 2015, 72, 19–28. [Google Scholar] [CrossRef] [PubMed]
| Favorable Outcomes | % | Unfavorable Outcomes | % | p-Value | |
|---|---|---|---|---|---|
| N = 1653 | N = 2427 | ||||
| Admission year | |||||
| 2020 | 616 | 37.27% | 840 | 34.61% | 0.179 |
| 2021 | 597 | 36.12% | 934 | 38.48% | |
| 2022 | 440 | 26.62% | 653 | 26.91% | |
| Age, years | |||||
| Mean (SD) | 60.8 (17.0) | 60.0 (16.3) | 0.098 | ||
| Median [IQR] | 62 [50, 74] | 61 [49, 72] | 0.067 | ||
| Gender: male | 1060 | 64.13% | 1536 | 63.29% | 0.585 |
| Race | |||||
| White | 1384 | 83.73% | 1896 | 78.12% | <0.001 |
| Black | 164 | 9.92% | 332 | 13.68% | |
| Asian | 16 | 0.97% | 30 | 1.24% | |
| Other | 72 | 4.36% | 131 | 5.40% | |
| Unknown | 17 | 1.03% | 38 | 1.57% | |
| Hispanic ethnicity | 101 | 6.11% | 195 | 8.03% | 0.020 |
| Admission source | |||||
| Non-healthcare facility (including from home) | 1505 | 91.05% | 2191 | 90.28% | 0.490 |
| Clinic | 64 | 3.87% | 83 | 3.42% | |
| Transfer from ECF or ICF | 47 | 2.84% | 90 | 3.71% | |
| Transfer from another non-acute care facility | 29 | 1.75% | 51 | 2.10% | |
| Other | 8 | 0.48% | 12 | 0.49% | |
| Admission type | |||||
| Emergency | 1508 | 91.23% | 2209 | 91.02% | |
| Urgent | 92 | 5.57% | 146 | 6.02% | |
| Elective | 39 | 2.36% | 54 | 2.22% | |
| Other/information not available | 14 | 0.85% | 18 | 0.74% | 0.905 |
| Do Not Resuscitate order | |||||
| Present on admission | 129 | 7.80% | 174 | 7.17% | 0.448 |
| Any time | 182 | 11.01% | 321 | 13.23% | 0.035 |
| Charlson comorbidity score | |||||
| 0 | 338 | 20.45% | 377 | 15.53% | <0.001 |
| 1 | 318 | 19.24% | 369 | 15.20% | |
| 2 | 290 | 17.54% | 384 | 15.82% | |
| 3 | 191 | 11.55% | 315 | 12.98% | |
| 4 | 174 | 10.53% | 311 | 12.81% | |
| 5+ | 342 | 20.69% | 671 | 27.65% | |
| Mean (SD) | 2.6 (2.3) | 3.1 (2.4) | <0.001 | ||
| Median [IQR] | 2 [1, 4] | 3 [1, 5] | <0.001 | ||
| Hospital characteristics | |||||
| Census region | |||||
| Midwest | 299 | 18.09% | 477 | 19.65% | 0.145 |
| Northeast | 353 | 21.36% | 486 | 20.02% | |
| South | 968 | 58.56% | 1433 | 59.04% | |
| West | 33 | 2.00% | 31 | 1.28% | |
| Number of beds | |||||
| <100 | 144 | 8.71% | 120 | 4.94% | <0.001 |
| 100 to 199 | 283 | 17.12% | 308 | 12.69% | |
| 200 to 299 | 262 | 15.85% | 318 | 13.10% | |
| 300 to 399 | 195 | 11.80% | 277 | 11.41% | |
| 400 to 499 | 206 | 12.46% | 362 | 14.92% | |
| 500+ | 563 | 34.06% | 1042 | 42.93% | |
| Teaching | 807 | 48.82% | 1414 | 58.26% | <0.001 |
| Urban | 1318 | 79.73% | 2049 | 84.43% | <0.001 |
| Favorable Outcomes | % | Unfavorable Outcomes | % | p-Value | |
|---|---|---|---|---|---|
| N = 1653 | N = 2427 | ||||
| ICU admission | 243 | 14.70% | 457 | 18.83% | 0.001 |
| Time from hospital admission to ICU admission, days | |||||
| Mean (SD) | 1.5 (1.6) | 1.6 (2.3) | 0.248 | ||
| Median [IQR] | 1 [1, 1] | 1 [1, 1] | 0.467 | ||
| ICU LOS, days | |||||
| Mean (SD) | 3.3 (4.9) | 3.4 (6.3) | 0.740 | ||
| Median [IQR] | 1 [1, 4] | 1 [1, 3] | 0.482 | ||
| MV | 75 | 4.54% | 143 | 5.89% | 0.059 |
| Time from hospital admission to MV, days | |||||
| Mean (SD) | 2.0 (2.3) | 3.0 (3.9) | 0.052 | ||
| Median [IQR] | 1 [1, 2] | 1 [1, 3] | 0.081 | ||
| MV duration, days | |||||
| Mean (SD) | 5.7 (8.9) | 6.0 (8.8) | 0.846 | ||
| Median [IQR] | 2 [1, 6] | 2 [1, 7] | 0.566 | ||
| Septic shock POA | 20 | 1.21% | 138 | 5.69% | <0.001 |
| Vasopressors | 110 | 6.65% | 184 | 7.58% | 0.261 |
| Time from hospital admission to vasopressors, days | |||||
| Mean (SD) | 2.5 (2.9) | 3.0 (4.5) | 0.338 | ||
| Median [IQR] | 1 [1, 2] | 1 [1, 3] | 0.424 | ||
| Vasopressors duration, days | |||||
| Mean (SD) | 1.6 (1.6) | 1.5 (1.2) | 0.568 | ||
| Median [IQR] | 1 [1, 1] | 1 [1, 1] | 0.831 | ||
| Dialysis | 31 | 1.88% | 107 | 4.41% | <0.001 |
| Time from hospital admission to dialysis, days | |||||
| Mean (SD) | 2.4 (3.0) | 2.0 (2.9) | 0.498 | ||
| Median [IQR] | 1 [1, 2] | 1 [1, 2] | 0.611 | ||
| Dialysis duration, days | |||||
| Mean (SD) | 1.7 (1.6) | 2.2 (3.2) | 0.406 | ||
| Median [IQR] | 1 [1, 2] | 1 [1, 2] | 0.267 | ||
| Favorable Outcomes | % | Unfavorable Outcomes | % | p-Value | |
|---|---|---|---|---|---|
| N = 1653 | N = 2427 | ||||
| Co-incident or co-prevalent COVID-19 | 169 | 10.22% | 293 | 12.07% | 0.067 |
| Organism | |||||
| MRSA | 557 | 33.70% | 1087 | 44.79% | <0.001 |
| MSSA | 1074 | 64.97% | 1325 | 54.59% | |
| Unknown | 22 | 1.33% | 15 | 0.62% | |
| Complicated BSI | 369 | 22.32% | 966 | 39.80% | <0.001 |
| Persistent bacteremia | 179 | 10.83% | 436 | 17.96% | <0.001 |
| TEE | 13 | 0.79% | 30 | 1.24% | 0.167 |
| H/o hemodialysis | 31 | 1.88% | 326 | 13.43% | <0.001 |
| Secondary BSI | 189 | 11.43% | 395 | 16.28% | <0.001 |
| SSTI | 8 | 0.48% | 14 | 0.58% | 0.691 |
| Joint | 22 | 1.33% | 49 | 2.02% | 0.099 |
| Bone | 26 | 1.57% | 56 | 2.31% | 0.101 |
| Vascular | 8 | 0.48% | 14 | 0.58% | 0.691 |
| CSF | 0 | 0.00% | 5 | 0.21% | 0.085 |
| Other CNS | 0 | 0.00% | 1 | 0.04% | 1.000 |
| Heart | 55 | 3.33% | 92 | 3.79% | 0.436 |
| Lung | 33 | 2.00% | 70 | 2.88% | 0.076 |
| Pleura | 8 | 0.48% | 14 | 0.58% | 0.691 |
| CLABSI | 40 | 2.42% | 98 | 4.04% | 0.005 |
| Primary BSI | 1464 | 88.57% | 2032 | 83.72% | <0.001 |
| Acquisition location | |||||
| Community acquired | 1416 | 85.66% | 2074 | 85.46% | 0.853 |
| Hospital acquired | 237 | 14.34% | 353 | 14.54% | |
| Treatment by day 2 of infection onset | |||||
| Drug | |||||
| Vancomycin | 1413 | 85.48% | 2152 | 88.67% | 0.003 |
| Daptomycin | 50 | 3.02% | 205 | 8.45% | <0.001 |
| Ceftaroline | 6 | 0.36% | 105 | 4.33% | <0.001 |
| Cefazolin | 577 | 34.91% | 756 | 31.15% | 0.012 |
| Oxacillin | 60 | 3.63% | 76 | 3.13% | 0.384 |
| Nafcillin | 63 | 3.81% | 121 | 4.99% | 0.076 |
| Other (none of the above) | 64 | 3.87% | 65 | 2.68% | 0.032 |
| IET | 55 | 3.33% | 48 | 1.98% | 0.007 |
| Antimicrobial course completed | 1332 | 80.58% | 1877 | 77.34% | 0.013 |
| Evidence of source control procedure | 16 | 0.97% | 40 | 1.65% | 0.067 |
| Post-infection onset outcomes | |||||
| ICU LOS, days | |||||
| Mean (SD) | 5.1 (6.2) | ||||
| Median [IQR] | 3 [2, 6] | ||||
| Hospital LOS, days | |||||
| Mean (SD) | 9.2 (3.7) | 15.5 (14.2) | <0.001 | ||
| Median [IQR] | 8 [7, 11] | 12 [8, 18] | <0.001 | ||
| Hospital costs, $ | |||||
| Mean (SD) | 18,371 (11,249) | 37,800 (56,910) | <0.001 | ||
| Median [IQR] | 15,682 [11,125, 22,182] | 26,546 [16,698, 43,124] | <0.001 | ||
| Covariate | Odds Ratio | 95% CI | p > |z| |
|---|---|---|---|
| Demographics | |||
| Age ≥ 80 | 0.687 | (0.564 to 0.836) | <0.001 |
| Comorbidities | |||
| Charlson Index (per unit) | 1.056 | (1.026 to 1.087) | <0.001 |
| Weight loss | 1.658 | (1.349 to 2.037) | <0.001 |
| Deficiency anemias | 1.499 | (1.306 to 1.722) | <0.001 |
| Valvular disease | 1.322 | (1.122 to 1.559) | 0.001 |
| Infection Characteristics | |||
| Septic shock present on admission | 3.498 | (2.145 to 5.704) | <0.001 |
| Complicated BSI | 2.476 | (2.047 to 2.994) | <0.001 |
| MRSA (vs. MSSA or unknown) | 1.473 | (1.281 to 1.694) | <0.001 |
| Secondary BSI | 0.704 | (0.549 to 0.904) | 0.006 |
| Treatment by day 2 of infection onset | |||
| Daptomycin | 2.725 | (1.943 to 3.821) | <0.001 |
| Vancomycin | 1.451 | (1.179 to 1.786) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zilberberg, M.D.; Nathanson, B.H.; Wagenaar, R.; Posthumus, J.; Shorr, A.F. Unfavorable Outcomes and Their Risk Factors in Hospitalized Patients with Staphylococcus aureus Bacteremia in the US: A Multicenter Retrospective Cohort Study, 2020–2022. Antibiotics 2025, 14, 326. https://doi.org/10.3390/antibiotics14030326
Zilberberg MD, Nathanson BH, Wagenaar R, Posthumus J, Shorr AF. Unfavorable Outcomes and Their Risk Factors in Hospitalized Patients with Staphylococcus aureus Bacteremia in the US: A Multicenter Retrospective Cohort Study, 2020–2022. Antibiotics. 2025; 14(3):326. https://doi.org/10.3390/antibiotics14030326
Chicago/Turabian StyleZilberberg, Marya D., Brian H. Nathanson, Rolf Wagenaar, Jan Posthumus, and Andrew F. Shorr. 2025. "Unfavorable Outcomes and Their Risk Factors in Hospitalized Patients with Staphylococcus aureus Bacteremia in the US: A Multicenter Retrospective Cohort Study, 2020–2022" Antibiotics 14, no. 3: 326. https://doi.org/10.3390/antibiotics14030326
APA StyleZilberberg, M. D., Nathanson, B. H., Wagenaar, R., Posthumus, J., & Shorr, A. F. (2025). Unfavorable Outcomes and Their Risk Factors in Hospitalized Patients with Staphylococcus aureus Bacteremia in the US: A Multicenter Retrospective Cohort Study, 2020–2022. Antibiotics, 14(3), 326. https://doi.org/10.3390/antibiotics14030326






