Antibiotic Usage for Treatment of Acute Upper Respiratory Tract Infections in Children in Lithuania from 2018 to 2022
Abstract
1. Introduction
2. Results
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Statistical Analysis
4.2. Ethics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grief, S.N. Upper respiratory infections. Prim. Care 2013, 40, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Institute of Hygiene, Ministry of Health of the Lithuanian Republic. Health of the Lithuanian Population and Performance of Health Care Facilities in 2022. Institute of Hygiene. Available online: https://www.hi.lt/uploads/Statistikos_leidiniai_Sveikatos_statistika/la2022.pdf (accessed on 3 December 2023).
- Long, J.C.; Williams, H.M.; Jani, S.; Arnolda, G.; Ting, H.P.; Molloy, C.J.; Hibbert, P.D.; Churruca, K.; Ellis, L.A.; Braithwaite, J. Assessing the appropriateness of the management of upper respiratory tract infection in Australian children: A population-based sample survey. BMJ Open 2019, 9, e026915. [Google Scholar] [CrossRef]
- Fahey, T.; Stocks, N.; Thomas, T. Systematic review of the treatment of upper respiratory tract infection. Arch. Dis. Child. 1998, 79, 225–230. [Google Scholar] [CrossRef]
- Kuchar, E.; Miśkiewicz, K.; Szenborn, L.; Kurpas, D. Respiratory tract infections in children in primary healthcare in Poland. Adv. Exp. Med. Biol. 2015, 835, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Youngster, I.; Avorn, J.; Belleudi, V.; Cantarutti, A.; Díez-Domingo, J.; Kirchmayer, U.; Park, B.-J.; Peiró, S.; Sanfélix-Gimeno, G.; Schröder, H.; et al. Antibiotic Use in Children—A Cross-National Analysis of 6 Countries. J. Pediatr. 2017, 182, 239–244.e1. [Google Scholar] [CrossRef]
- Collignon, P. Antibiotic resistance: Are we all doomed? Intern. Med. J. 2015, 45, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, A.; Sabbatucci, M.; Salzano, A.; Zovi, A. The importance of antibiotic treatment duration in antimicrobial resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1673–1675. [Google Scholar] [CrossRef]
- Luchen, C.C.; Chibuye, M.; Spijker, R.; Simuyandi, M.; Chisenga, C.; Bosomprah, S.; Chilengi, R.; Schultsz, C.; Mende, D.R.; Harris, V.C. Impact of antibiotics on gut microbiome composition and resistome in the first years of life in low- to middle-income countries: A systematic review. PLoS Med. 2023, 20, e1004235. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019. World Health Organization. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 3 December 2023).
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Magin, P.; Davey, A.R.; Davis, J. Evidence-based strategies for better antibiotic prescribing. Aust. J. Gen. Pract. 2022, 51, 21–24. [Google Scholar] [CrossRef] [PubMed]
- The STAR study team; Simpson, S.A.; Butler, C.C.; Hood, K.; Cohen, D.; Dunstan, F.; Evans, M.R.; Rollnick, S.; Moore, L.; Hare, M.; et al. Stemming the Tide of Antibiotic Resistance (STAR): A protocol for a trial of a complex intervention addressing the ’why’ and ’how’ of appropriate antibiotic prescribing in general practice. BMC Fam. Pract. 2009, 10, 20. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Salman, M.; Rao, A.Z.; Asif, N.; Butt, S.A.; Shehzadi, N.; Hussain, K. Assessment of antibiotics use for children upper respiratory tract infections: A retrospective, cross-sectional study from Pakistan. Infect. Dis. 2020, 52, 473–478. [Google Scholar] [CrossRef]
- Mwanza, Z.V.; White, B.S.; Britton, P.N.; McCaskill, M.E. Timing of antibiotics in febrile children meeting sepsis criteria at a paediatric emergency department. Emerg. Med. Australas. 2023, 35, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Nocon, C.C.; Baroody, F.M. Acute rhinosinusitis in children. Curr. Allergy Asthma Rep. 2014, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, Y.; Zou, G.; Lin, M.; Zeng, J.; Deng, S.; Zachariah, R.; Walley, J.; Tucker, J.D.; Wei, X. Antibiotic prescribing for upper respiratory infections among children in rural China: A cross-sectional study of outpatient prescriptions. Glob. Health Action 2017, 10, 1287334. [Google Scholar] [CrossRef]
- Shin, S.M.; Shin, J.Y.; Kim, M.H.; Lee, S.H.; Choi, S.; Park, B.J. Prevalence of antibiotic use for pediatric acute upper respiratory tract infections in Korea. J. Korean Med. Sci. 2015, 30, 617–624. [Google Scholar] [CrossRef]
- Tefera, B.B.; Getachew, M.; Kebede, B. Evaluation of drug prescription pattern using World Health Organization prescribing indicators in public health facilities found in Ethiopia: Systematic reviews and meta-analysis. J. Pharm. Policy Pract. 2021, 14, 31. [Google Scholar] [CrossRef]
- Higashi, T.; Fukuhara, S. Antibiotic prescriptions for upper respiratory tract infection in Japan. Intern. Med. 2009, 48, 1369–1375. [Google Scholar] [CrossRef]
- Sumaila, A.N.; Tabong, P.T. Rational prescribing of antibiotics in children under 5 years with upper respiratory tract infections in Kintampo Municipal Hospital in Brong Ahafo Region of Ghana. BMC Res. Notes 2018, 11, 443. [Google Scholar] [CrossRef]
- Fossum, G.H.; Lindbæk, M.; Gjelstad, S.; Dalen, I.; Kværner, K.J. Are children carrying the burden of broad-spectrum antibiotics in general practice? Prescription pattern for paediatric outpatients with respiratory tract infections in Norway. BMJ Open 2013, 3, e002285. [Google Scholar] [CrossRef] [PubMed]
- Agiro, A.; Gautam, S.; Wall, E.; Hackell, J.; Helm, M.; Barron, J.; Zaoutis, T.; Fleming-Dutra, K.E.; Hicks, L.A.; Rosenberg, A. Variation in Outpatient Antibiotic Dispensing for Respiratory Infections in Children by Clinician Specialty and Treatment Setting. Pediatr. Infect. Dis. J. 2018, 37, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Trainaviciene, V. Antibiotic Use for Upper Respiratory Tract Infections in Children. Master’s Thesis, Vilnius University, Vilnius, Lithuania, 2022. [Google Scholar]
- Gerber, J.S.; Ross, R.K.; Bryan, M.; Localio, A.R.; Szymczak, J.E.; Wasserman, R.; Barkman, D.; Odeniyi, F.; Conaboy, K.; Bell, L.; et al. Association of Broad- vs. Narrow-Spectrum Antibiotics with Treatment Failure, Adverse Events, and Quality of Life in Children with Acute Respiratory Tract Infections. JAMA 2017, 318, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Hall, M.; Shah, S.S.; Parikh, K.; Tyler, A.; Neuman, M.I.; Hersh, A.L.; Brogan, T.V.; Blaschke, A.J.; Grijalva, C.G. Narrow vs broad-spectrum antimicrobial therapy for children hospitalized with pneumonia. Pediatrics 2013, 132, e1141–e1148. [Google Scholar] [CrossRef]
- Coxeter, P.D.; Mar, C.D.; Hoffmann, T.C. Parents’ Expectations and Experiences of Antibiotics for Acute Respiratory Infections in Primary Care. Ann. Fam. Med. 2017, 15, 149–154. [Google Scholar] [CrossRef]
- Adlhoch, C.; Mook, P.; Lamb, F.; Ferland, L.; Melidou, A.; Amato-Gauci, A.J.; Pebody, R. Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021. Eurosurveillance 2021, 26, 2100221. [Google Scholar] [CrossRef]
- Shmueli, M.; Lendner, I.; Ben-Shimol, S. Effect of the COVID-19 pandemic on the pediatric infectious disease landscape. Eur. J. Pediatr. 2023, 183, 1001–1009. [Google Scholar] [CrossRef]




| Year | 2018 | 2019 | 2020 | 2021 | 2022 | Total | |
|---|---|---|---|---|---|---|---|
| Age Group | |||||||
| 0–5 | 65,287 (45.68) | 62,740 (53.26) | 26,601 (51.80) | 27,362 (63.80) | 48,227 (53.37) | 230,217 (51.70) | |
| 6–10 | 39,420 (27.58) | 30,127 (25.58) | 12,933 (25.18) | 8481 (19.77) | 24,086 (26.66) | 115,047 (25.83) | |
| 11–15 | 25,979 (18.18) | 17,621 (14.96) | 8243 (16.05) | 5137 (11.98) | 14,026 (15.52) | 71,006 (15.94) | |
| 16–18 | 12,243 (08.57) | 7302 (06.20) | 3585 (06.98) | 1911 (04.46) | 4017 (04.45) | 29,058 (06.53) | |
| Total | 142,929 (100) | 117,790 (100) | 51,362 (100) | 42,891 (100) | 90,356 (100) | 445,328 (100) | |
| Year | 2018 | 2019 | 2020 | 2021 | 2022 | Total | |
|---|---|---|---|---|---|---|---|
| Age Group | |||||||
| 0–5 | 0.38 | 0.37 | 0.16 | 0.17 | 0.31 | 0.28 | |
| 6–10 | 0.28 | 0.21 | 0.09 | 0.06 | 0.17 | 0.16 | |
| 11–15 | 0.20 | 0.14 | 0.06 | 0.04 | 0.10 | 0.11 | |
| 16–18 | 0.14 | 0.09 | 0.05 | 0.02 | 0.05 | 0.07 | |
| Total | 0.27 | 0.22 | 0.10 | 0.08 | 0.17 | 0.17 | |
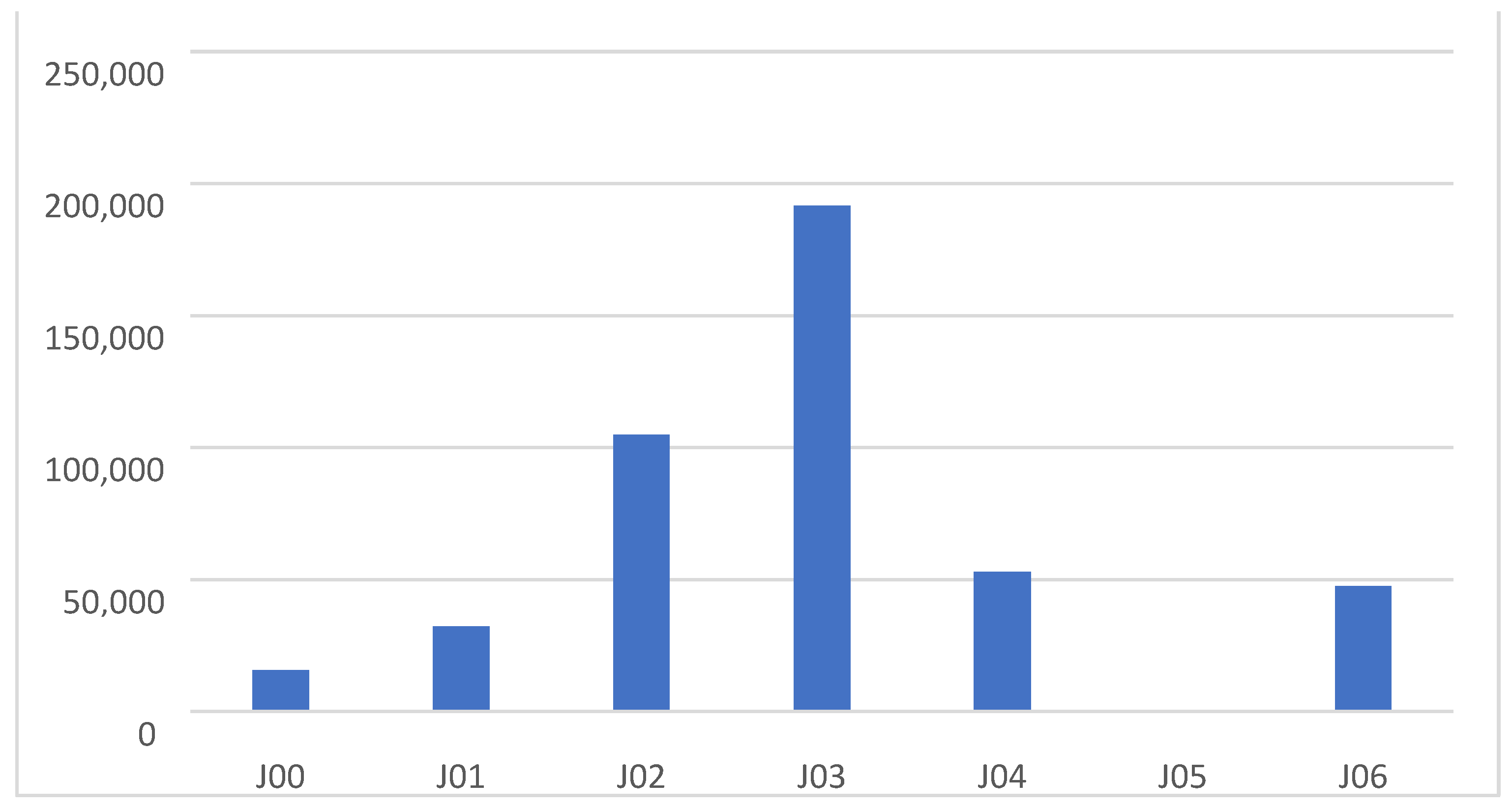
| Diagnoses | J00 | J01 | J02 | J03 | J04 | J05 | J06 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | |||||||||
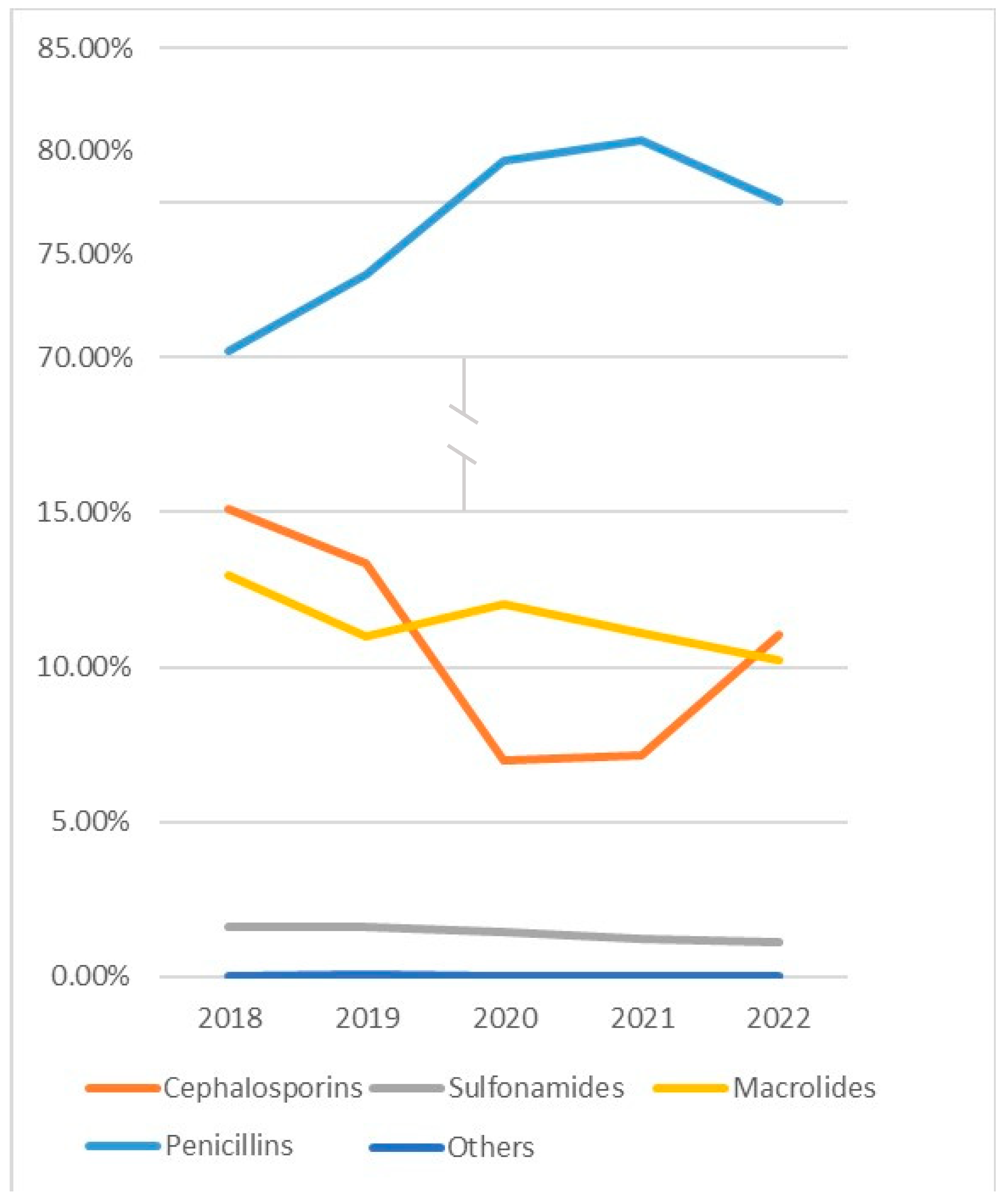
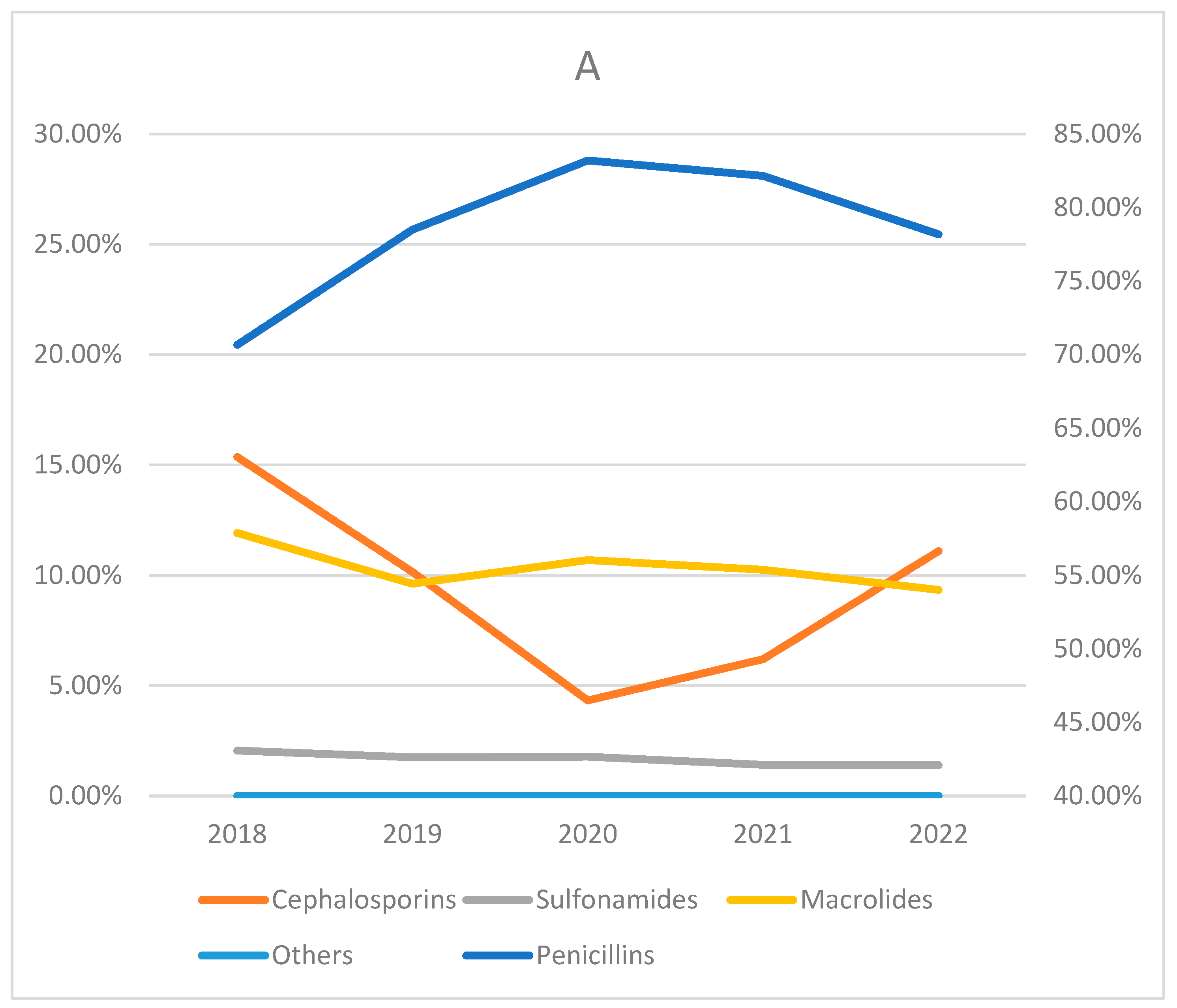
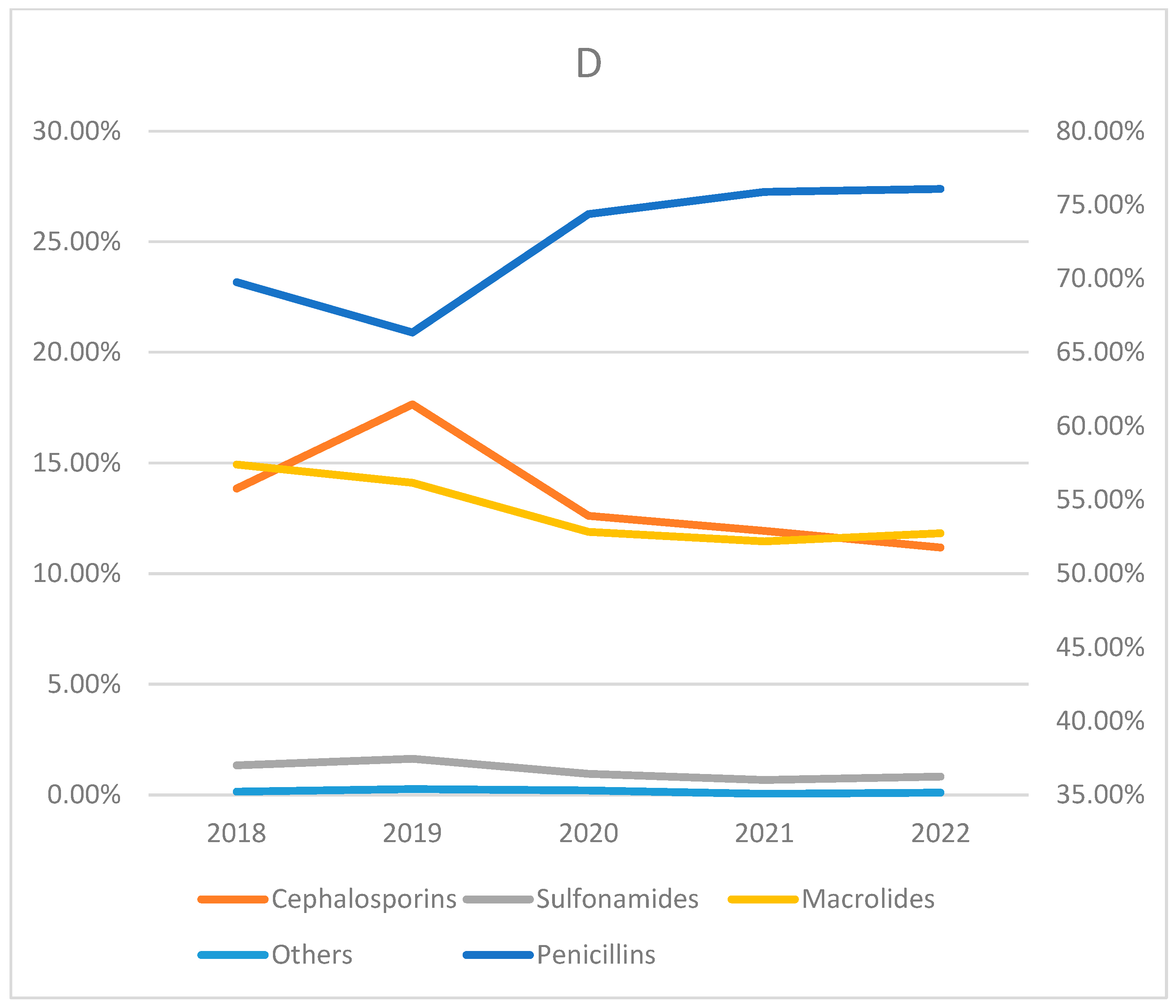
| Penicillins | 12,389 (79.31) | 25,489 (78.93) | 85,313 (81.29) | 139,911 (73.03) | 32,142 (60.72) | 368 (70.10) | 37,536 (79.15) | 333,148 (74.80) | |
| Cephalosporins | 501 (3.21) | 830 (2.57) | 10,295 (9.81) | 39,121 (20.42) | 1110 (2.10) | 18 (3.43) | 2075 (4.38) | 53,950 (12.11) | |
| Sulfonamides | 605 (3.87) | 323 (1.00) | 1803 (1.72) | 508 (0.27) | 1617 (3.05) | 4 (0.76) | 1543 (3.25) | 6403 (0.01) | |
| Macrolides | 2122 (13.58) | 5620 (17.40) | 7521 (7.17) | 12,010 (6.27) | 18,039 (34.08) | 135 (25.71) | 6254 (13.19) | 51,701 (11.60) | |
| Others | 4 (0.03) | 30 (0.09) | 21 (0.02) | 30 (0.02) | 24 (0.05) | 0 (0) | 17 (0.04) | 126 (0.0) | |
| Total | 15,621 (100) | 32,292 (100) | 104,953 (100) | 191,580 (100) | 52,932 (100) | 525 (100) | 47,425 (100) | 445,328 | |
| Diagnoses | J00 | J01 | J02 | J03 | J04 | J05 | J06 | Total | |
|---|---|---|---|---|---|---|---|---|---|
| Antibiotics | |||||||||
| Amx | 7391 (47.31) | 10,870 (33.66) | 58,232 (55.48) | 58,238 (30.40) | 21,932 (41.43) | 213 (40.57) | 27,184 (57.32) | 184,060 (41.33) | |
| Pcv | 1318 (8.44) | 517 (1.60) | 14,598 (13.91) | 53,804 (28.08) | 1519 (2.87) | 59 (11.24) | 3319 (7.00) | 75,134 (16.87) | |
| Amc | 3213 (20.57) | 13,189 (40.84) | 10,829 (10.32) | 24,216 (12.64) | 7851 (14.83) | 86 (16.38) | 6444 (13.59) | 65,828 (14.78) | |
| Stm | 467 (2.99) | 913 (2.83) | 1654 (1.58) | 3653 (1.91) | 840 (1.59) | 10 (1.90) | 589 (1.24) | 8126 (0.02) | |
| Cfr | 492 (3.15) | 747 (2.31) | 10,195 (9.71) | 38,483 (20.09) | 1078 (2.04) | 18 (3.43) | 2002 (4.22) | 53,015 (11.90) | |
| Cxm | 8 (0.05) | 78 (0.24) | 100 (0.10) | 633 (0.33) | 32 (0.06) | 0 (0) | 73 (0.15) | 924 (0.00) | |
| Sxt | 605 (3.87) | 323 (1.00) | 1803 (1.72) | 508 (0.27) | 1617 (3.05) | 4 (0.76) | 1543 (3.25) | 6403 (0.01) | |
| Ery | 4 (0.03) | 6 (0.02) | 79 (0.08) | 98 (0.05) | 104 (0.20) | 0 (0) | 14 (0.03) | 305 (0.0) | |
| Clr | 1808 (11.57) | 4269 (13.22) | 6066 (5.78) | 10,307 (5.38) | 14,493 (27.38) | 85 (16.19) | 5345 (11.27) | 42,373 (0.10) | |
| Azm | 310 (1.98) | 1345 (4.17) | 1375 (1.31) | 1605 (0.84) | 3442 (6.50) | 50 (9.52) | 895 (1.89) | 9022 (0.02) | |
| Dox | 3 (0.02) | 17 (0.05) | 18 (0.02) | 17 (0.01) | 18 (0.03) | 0 (0) | 11 (0.02) | 84 (0.0) | |
| Others | 2 (0.01) | 18 (0.06) | 4 (0) | 18 (0.01) | 6 (0.01) | 0 (0) | 6 (0.01) | 54 (0.00) | |
| Total | 15,621 (100) | 32,292 (100) | 104,953 (100) | 191,580 (100) | 52,932 (100) | 525 (100) | 47,425 (100) | 445,328 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alčauskas, T.; Garuolienė, K.; Burokienė, S. Antibiotic Usage for Treatment of Acute Upper Respiratory Tract Infections in Children in Lithuania from 2018 to 2022. Antibiotics 2025, 14, 310. https://doi.org/10.3390/antibiotics14030310
Alčauskas T, Garuolienė K, Burokienė S. Antibiotic Usage for Treatment of Acute Upper Respiratory Tract Infections in Children in Lithuania from 2018 to 2022. Antibiotics. 2025; 14(3):310. https://doi.org/10.3390/antibiotics14030310
Chicago/Turabian StyleAlčauskas, Tadas, Kristina Garuolienė, and Sigita Burokienė. 2025. "Antibiotic Usage for Treatment of Acute Upper Respiratory Tract Infections in Children in Lithuania from 2018 to 2022" Antibiotics 14, no. 3: 310. https://doi.org/10.3390/antibiotics14030310
APA StyleAlčauskas, T., Garuolienė, K., & Burokienė, S. (2025). Antibiotic Usage for Treatment of Acute Upper Respiratory Tract Infections in Children in Lithuania from 2018 to 2022. Antibiotics, 14(3), 310. https://doi.org/10.3390/antibiotics14030310





