Abstract
Background/Objectives: The discovery of new molecules to which bacteria have not yet developed resistance is a significant medical priority. Synthetic flavonoids are good candidates for developing new antimicrobials. Our study investigates a series of newly synthesized tricyclic flavonoids with several different substituents on the flavonoid core. Methods: By varying the nature of the substituents on rings A and B, a structure–activity relationship study using different microbial strains has been performed. The antibacterial and antifungal properties of these compounds have been investigated against Gram-positive and Gram-negative bacteria and several Candida strains. Results: All seven tested compounds have been found to exhibit the highest antimicrobial activity against S. aureus, with an inhibition zone of up to 23 mm. The tricyclic flavonoids 5c, 5e, and 5f showed good antifungal properties against C. parapsilosis, with an inhibition zone of around 17 mm. Conclusions: All the data support the idea that flavonoids 5 are reliable candidates for developing effective antimicrobial agents.
1. Introduction
Regarded by the World Health Organization (WHO) as one of the top issues in public health worldwide, antimicrobial resistance (AMR) is estimated to have been associated with 4.95 million deaths in 2019 alone [1]. The inappropriate use of antibiotics in both medicine and agriculture represents one of the main causes of AMR. Given the current trend, if no steps are taken to address the issue, AMR is projected to lead to as many as 10 million deaths per year, globally, by 2050 [2]. Apart from its impact on human health, AMR has significant implications from a socioeconomic point of view, with an estimated loss in global gross domestic product of 3.8% by the year 2050 [3].
Microorganisms that have developed resistance to antibiotics have no problem surviving or even multiplying, despite the administration of antibiotics [4]. This leads to a loss in effectiveness when it comes to antimicrobials, which makes it increasingly difficult—and indeed, sometimes impossible—to treat certain infections, further adding pressure to already strained healthcare systems globally [5]. On top of that, the problem of AMR is further compounded by the emergence of multidrug-resistant (MDR) microorganisms, which are responsible for an increase in the incidence of nosocomial infections [6]. There are several limitations and challenges in the development of new antibiotics and antifungals as well as regulatory processes that impede advancement in this critical field. The degree of resistance expression of the bacterial strain and its capacity to survive through resistance mechanisms are two of the many elements that contribute to the expression of antibiotic resistance against drug agents [7]. Due to natural processes, first-generation antibiotics have been affected by antibiotic resistance in a variety of therapeutic applications [8].
Although there has been some progress in the fight against antimicrobial resistance, β-lactamic antibiotics and related compounds are not a viable solution against MDR strains anymore. Referring to a recent WHO report, in the last seven years, only thirteen new antibiotics have been authorized and only two (vaborbactam and lefamulin) represent new classes of antibiotics [9]. Furthermore, the newly approved drugs are not more effective and do not exhibit new mechanisms of action against the present threats.
Among the many problematic microbes, Candida pathogenic species have a significant impact on public health [10]. With a mortality rate of 40–60%, particularly in immunocompromised patients, C. albicans is the most widely encountered pathogen in the Candida genus [11]. Candida infections can be treated using antifungals such as azoles, allylamines, echinocandins and polyenes, as well as nucleoside analogs [12]. The effectiveness of antifungal drugs is, however, challenged by multidrug resistance. This is well documented for Candida species, particularly in the case of azoles [10]. It was found that one of the main causes of MDR in Candida spp. is its ability to form biofilms [13]. Within biofilms, it was found that cells can withstand antifungal concentrations up to 103 larger than planktonic cells [14]. The lack of effective treatments poses a significant challenge for modern medicine when it comes to Candida infections.
In this context, the need for innovative approaches in developing novel antimicrobials efficient in treating infectious diseases is a major goal.
From this point of view, flavonoids—a large group of naturally occurring heterocyclic organic compounds found in fruits, vegetable, tea, and wine—are potentially good candidates as antimicrobial agents. Associated with the multitude of substitution patterns on the C6-C3-C6 backbone, more than 12,000 flavonoids are known. The attention that they receive is a direct consequence of the many biological activities that this class of compounds displays. Flavonoids have a wide variety of chemical and biochemical properties that provide them antimicrobial properties [15,16,17]. These compounds are also known to be good antioxidants [18,19,20,21,22,23]. Moreover, certain studies suggest that flavonoids could offer protection against cancer, as well as cardiovascular diseases [24,25,26,27,28,29,30,31,32].
Benzopyran derivatives, like SERBAs or selective estrogen receptor β agonists, are compounds that interact with estrogen receptor subtypes α and β. The interest for this type of compound leads to derivative 1, a selective estrogen receptor β agonist (SERBA-1, Figure 1) [33]. Studies about the structure–activity relationship have been presented [34,35]. It has been shown that a cyclopenthane ring at the 3,4-carbon positions (labeled D, Figure 1) leads to a substantial rise in binding affinity for estrogen receptor β. By combining improvements in the D ring with those in the A ring, improvements in binding selectivity have been obtained (e.g., 2, Figure 1) [36].
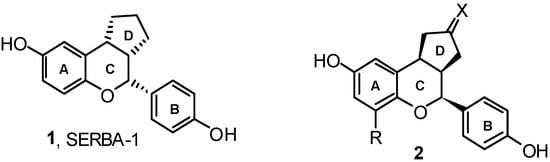
Figure 1.
Polycyclic flavonoids.
Regardless of their potential, the use of natural flavonoids in medicine is hindered by issues such as limited bioavailability, poor solubility, and stability [37]. As such, new flavonoids with potent antimicrobial properties designed to address the aforementioned issues have been synthesized. Over the past twelve years, we developed a novel type of tricyclic flavonoid containing a 1,3-dithiolium ring (similar to D in Figure 1) and featuring various substituents on the C6-C3-C6 backbone. We effectively demonstrated that these synthetic flavonoids possess strong antibacterial and antifungal abilities and examined how they function [38,39,40].
We are reporting here an extension of our studies about the development of new tricyclic flavonoids with good antimicrobial activities against several microbial strains. By varying the nature of the substituents on the rings A and B, we intended to perform a study on the structure–activity relationship using different microbial strains.
2. Results and Discussion
2.1. Tricyclic Flavonoids
The synthesis of 4-chromanone and flavanone derivatives has been previously achieved through the interaction of type 2 dithiocarbamates with aminals 3 [41]. Based on our prior research, we aimed to broaden our investigations to include other new tricyclic flavonoids similar in structure to those illustrated in Figure 1. As a result, 3-dithiocarbamic flavanones 4 have been obtained by reacting 1-(3-bromo-2-hydroxy-5-methylphenyl)-1-oxaethan-2-yl N,N-diethylaminocarbodithioate (2) with aminals 3 (Scheme 1). The preparation of the latter compounds followed established methods described in the literature [42,43]. Thus, by reacting 2-bromo-1-(3-bromo-2-hydroxy-5-methylphenyl)ethan-1-one (1) [44] with sodium N,N-diethyldithiocarbamate in acetone, phenacyl aminocarbodithioate 2 has been successfully synthesized in good yields. The reaction of 1-(3-bromo-2-hydroxy-5-methylphenyl)-1-oxaethan-2-yl N,N-diethylaminocarbodithioate (2) with aminals 3 resulted in the formation of 3-substituted dithiocarbamic flavanones 4a–g as a mixture of diastereoisomers (Scheme 1). The reaction mixture underwent reflux heating for 3.5 h, and following recrystallization from ethanol, 3-dithiocarbamic flavanones 4a–g were obtained, with yields ranging from 75% to 84%. NMR spectroscopy clearly shows the ring closure of the benzopyran core. In addition to the NMR pattern of the newly para-substituted aromatic ring B, the loss of the methylene group’s signal from dithiocarbamate 2 (4.79 ppm) and the presence of the distinctive signals of vicinal hydrogen atoms at the C-2 and C-3 positions of the benzopyran ring for both diastereoisomers are observed between 5.8 and 6.3 ppm. Mass spectra also supported the ring closure to the benzopyrane ring.
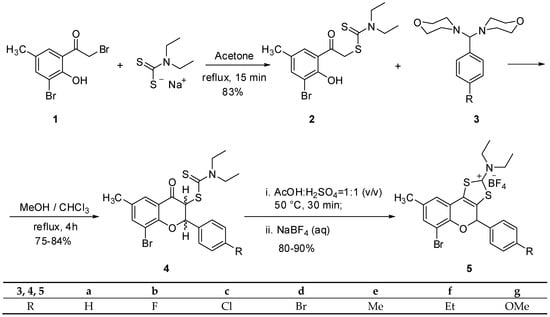
Scheme 1.
Tricyclic flavonoids 5a–g.
Based on the stereochemical principles, these flavanones should have two diastereoisomers that originate from the relative orientation of H-2 and H-3 hydrogen atoms of the flavanones 4, which can be directed either to opposite sides or to the same side of the benzopyrane core (Figure 2). The relative orientations of the two substituents induce an influence on the magnitude of their coupling constants. It is reasonable to assume that the most stable isomer should be the anti diastereomer 4′, as opposed to the syn diastereomer 4″. However, it has been found that in the case of two flavanones, 4b and 4g, the syn isomer has been the major one. The diastereoisomeric ratio and coupling constants of flavanones 4 are presented in Table 1.
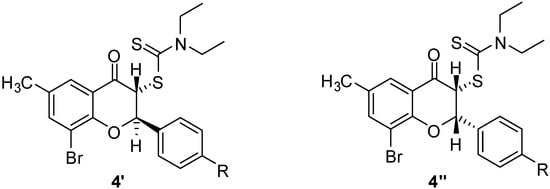
Figure 2.
Diastereoisomers of flavanones 4.

Table 1.
Coupling constants and diastereoisomers ratio of flavanones 4.
Phenacyl dithiocarbamates are valuable precursors for 2-dialkylamino-1,3-dithiolium-2-yl cations [45,46]. Cyclocondensation under acidic conditions of these substrates is the method employed for the preparation of 1,3-dithiolium cations [47]. The cyclization of 3-dithiocarbamic flavanones 4 to tricyclic flavonoids 5 has been proven by spectral data.
IR spectroscopy indicated the disappearance of the carbonyl absorption bands (1690–1705 cm−1) and the appearance of the characteristic broad absorption bands (ca. 1080–1095 cm−1) of the tetrafluoroborate anion. The 1H NMR spectra of tricyclic flavonoids 5 show the disappearance of the C-3 hydrogens from the benzopyran core. Furthermore, the signals of the C-2 hydrogens have been shifted to ca. 6.8 ppm, where they can be found as singlets. The 13C NMR spectra indicate the absence of the carbonyl and thiocarbonyl atoms and present a new signal at ca. 184 ppm, corresponding to the 1,3-dithiol-2-ylium carbon atom.
2.2. Antimicrobial Activity of Tricyclic Flavonoids
Almost all the tested flavonoids exhibited both antibacterial and antifungal activity against the tested microbial strains, as presented in Table 2a. Clearly, the flavonoids were more efficient against the bacterial strains, especially against the Gram-positive bacterial strain represented by S. aureus, with an inhibition zone of up to 23 mm in the particular cases of tricyclic flavonoids 5b, 5c, 5e, and 5g (See Figure 3a). Moderate efficiency was noticed against E. faecalis (with an inhibition zone of up to 15 mm in the particular case of 5e) (as presented in Figure 3b) and E. coli (with an inhibition zone of up to 15 mm in the case of 5c). Flavonoid 5a was quite efficient against S. aureus but was not efficient against the other bacterial strains. The same flavonoid exhibited very low activity against C. parapsilosis but did not present activity against the other yeast strains. None of the flavonoids were efficient against the Gram-negative bacterial strain represented by P. aeruginosa; however, all of the tested flavonoids were quite efficient against C. parapsilosis, withan inhibition zone of up to 17 mm in the case of flavonoid 5e. The antifungal activity of 5e against the other strains was very low for the two strains of C. albicans and the two strains of C. glabrata.

Table 2.
Antimicrobial activity (mm) of the tested samples against the reference strain (a) samples and (b) controls.
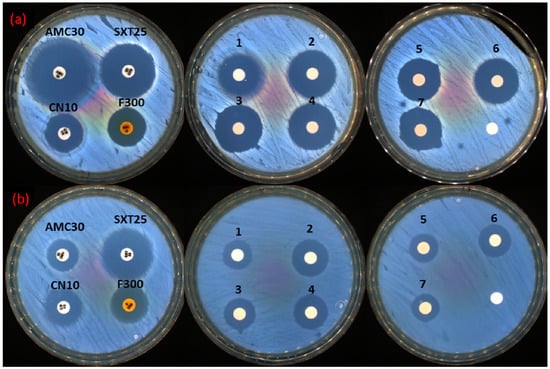
Figure 3.
Antibacterial activity of tested controls (AMC30—amoxyclav, SXT25—co-trimoxazole, CN10—gentamicin, F300—nitrofurantoin) and samples (1—5a, 2—5b, 3—5c, 4—5e, 5—5f, 6—5g, 7—5d) against (a)—S. aureus, (b)—K. pneumoniae.
When compared with the commercial controls, it was noticed that flavonoid 5a had the same efficiency as CN10 when tested against S. aureus (as presented in Table 2b, Figure 3a). Flavonoids 5b–5g had higher activity than CN10 and F300 against the same bacterial strain. In the case of E. coli, none of the flavonoids were more efficient than the controls. For E. faecalis, only flavonoid 5e had higher efficiency than CN10. Flavonoids 5f and 5g had the same activity as AMC30 against K. pneumoniae, as presented in Table 2a,b, Figure 3b.
In the case of antifungals, AP100 had the same efficiency as flavonoids 5c,d against C. albicans ATCC 90028, while 5e was better than KCA10. In addition, for this particular strain, 5f had better efficiency than all the tested controls. For the yeast strain C. albicans ATCC 10231, control AP100 had the same activity as 5d, and all the flavonoids were more efficient than the rest of the tested controls (NS100, KCA10, and FLU10). In the case of C. glabrata ATCC 15126, control sample AP100 was more efficient than all the tested flavonoids; however, flavonoids 5c–f were better than NS100, KCA10, and FLU10. A similar situation was noticed in the case of C. glabrata ATCC 20019, when comparing the flavonoids’ activity with control AP100; however, 5f was better than NS100, KCA10, and FLU10. For C. parapsilosis, flavonoids 5a and 5g had reduced efficiency when compared to AP100, while the remaining flavonoids were more efficient than the same control. Furthermore, for this yeast strain, 5d had the same activity as FLU10, while flavonoids 5c, 5e and 5f were better than this control. At the same time, all tested flavonoids exhibited better activity than NS100 and KCA10.
3. Materials and Methods
Melting points were obtained on a KSPI melting-point meter (A. KRÜSS Optronic, Hamburg, Germany) and are uncorrected. IR spectra were recorded on a Bruker Tensor 27 instrument (Bruker Optik GmbH, Ettlingen, Germany). NMR spectra were recorded on a Bruker 500 MHz spectrometer (Bruker BioSpin, Rheinstetten, Germany). Chemical shifts are reported in ppm downfield from TMS. Mass spectra were recorded on a Thermo Scientific ISQ LT instrument (Thermo Fisher Scientific Inc., Waltham, MA, USA). All reagents were commercially available and used without further purification. Elemental analysis and copies of 13C-NMR spectra are available in the Supplementary Materials.
The controls for the antimicrobial tests included the following: amoxicillin/clavulanic acid, 20/10 µg/disc (AMC30); co-trimoxazole 25 µg/disc (SXT25); gentamicin, 10 µg/disc (CN10), nitrofurantoin, 300 µg/disc (F300), from Tody Laboratories (Bucharest, Romania); amphotericin B, 100 µg/disc (AP100) from HiMedia Laboratories (Mumbai, India); nystatin, 100 µg/disc (NS100); ketoconazole, 10 µg/disc (KCA10) from Tody Laboratories (Bucharest, Romania); and fluconazole, 10 µg/disc (FLU10) from Bioanalyse Limited (Ankara, Turkey).
3.1. Chemistry
3.1.1. General Procedure for 8-Bromo-6-methyl-2-phenyl-4-oxochroman-3-yl N,N-Diethyldithiocarbamate (4a)
To a solution of 1-(5-methyl-2-hydroxyphenyl)-1-oxoethan-2-yl N,N-diethyldithiocarbamate (2) (0.376 g, 1 mmol) in a mixture of CHCl3/CH3OH (15 mL, 1:1 v/v), aminal 3a (0.262 g, 1 mmol) was added and the reaction mixture was heated under reflux for 3.5 h. After cooling, the solid material was filtered off and was recrystallized from ethanol to give 4a (0.39 g, 81%) as colorless crystals. IR (ATR, cm−1) 2981, 1693, 1492, 1465, 1202, 976, 766, 694, 562. 1H NMR (CDCl3, selected data for the major anti isomer) δ 7.63 (m, 2H), 7.52 (m, 2H), 7.35 (m, 3H), 6.00 (d, J = 6.9 Hz, 1H), 5.82 (d, J = 6.9 Hz, 1H), 3.94 (m, 2H), 3.67 (m, 2H), 2.30 (s, 3H), 1.24 (m, 6H). 13C NMR (CDCl3, selected data for the major anti isomer) δ 191.5, 187.4, 154.8, 140.5, 136.3, 132.3, 128.6, 128.5, 127.2, 126.7, 122.0, 111.5, 82.8, 58.5, 50.5, 47.2, 20.2, 12.6, 11.4. MS (EI) m/z: 463.1 (M+, 24%) for C21H2279BrNO2S2.
3.1.2. 8-Bromo-6-methyl-2-(4-fluorophenyl)-4-oxochroman-3-yl N,N-Diethyldithiocarbamate (4b)
White solid, 0.35 g, 78%. IR (ATR, cm−1) 2989, 1694, 1493, 1469, 1269, 1198, 808, 555. 1H NMR (CDCl3, selected data for the major syn isomer) δ 7.72 (d, J = 2.0 Hz, 1H), 7.61 (d, J = 2.0 Hz, 1H), 7.51 (m, 2H), 7.05 (m, 2H), 6.21 (d, J = 3.8 Hz, 1H), 5.98 (d, J = 3.8 Hz, 1H), 3.98 (m, 2H), 3.67 (m, 2H), 2.33 (s, 3H), 1.21 (m, 6H). 13C NMR (CDCl3, selected data for the major syn isomer) δ 191.0, 188.0, 163.7, 154.5, 140.4, 132.3, 131.2, 129.3, 128.8, 126.8, 121.2, 115.3, 111.6, 80.6, 57.9, 51.0, 47.0, 20.2, 12.6, 11.3. MS (EI) m/z: 481.1 (M+, 19%) for C21H2179BrFNO2S2.
3.1.3. 8-Bromo-6-methyl-2-(4-chlorophenyl)-4-oxochroman-3-yl N,N-Diethyldithiocarbamate (4c)
Pale yellow solid, 0.34 g, 79%. IR (ATR, cm−1) 2972, 1692, 1492, 1470, 1271, 1200, 803, 549. 1H NMR (CDCl3, selected data for the major anti isomer) δ 7.66 (m, 1H), 7.64 (m, 1H), 7.48 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.5 Hz, 2H), 5.93 (d, J = 7.1 Hz, 1H), 5.80 (d, J = 7.1 Hz, 1H), 3.93 (m, 2H), 3.70 (m, 2H), 2.32 (s, 3H), 1.25 (m, 6H). 13C NMR (CDCl3, selected data for the major anti isomer) δ 191.3, 187.1, 154.7, 140.6, 134.6, 133.9, 132.6, 128.8, 128.6, 126.8, 121.9, 111.4, 82.3, 58.7, 50.6, 47.2, 20.2, 12.6, 11.3. MS (EI) m/z: 497.0 (M+, 14%) for C21H2179BrClNO2S2.
3.1.4. 8-Bromo-6-methyl-2-(4-bromophenyl)-4-oxochroman-3-yl N,N-Diethyldithiocarbamate (4d)
White solid, 0.37 g, 84%. IR (ATR, cm−1) 2977, 1691, 1489, 1469, 1271, 1200, 801, 549. 1H NMR (CDCl3, selected data for the major anti isomer) δ 7.65 (d, J = 1.8 Hz, 1H), 7.63 (d, J = 1.8 Hz, 1H), 7.48 (d, J = 8.5 Hz, 2H), 7.42 (d, J = 8.5 Hz, 1H), 5.91 (d, J = 7.2 Hz, 1H), 5.79 (d, J = 7.2 Hz, 1H), 3.93 (m, 2H), 3.70 (m, 2H), 2.32 (s, 3H), 1.24 (m, 6H). 13C NMR (CDCl3, selected data for the major anti isomer) δ 191.3, 187.1, 154.7, 140.6, 135.3, 132.6, 131.6, 129.1, 126.8, 122.9, 121.9, 111.4, 82.3, 58.7, 50.6, 47.3, 20.2, 12.6, 11.4. MS (EI) m/z: 540.9 (M+, 14%) for C21H2179Br2NO2S2.
3.1.5. 8-Bromo-6-methyl-2-(4-methylphenyl)-4-oxochroman-3-yl N,N-Diethyldithiocarbamate (4e)
Pale yellow solid, 0.31 g, 77%. IR (ATR, cm−1) 2981, 1695, 1494, 1468, 1269, 1202, 798, 547. 1H NMR (CDCl3, selected data for the major anti isomer) δ 7.63 (m, 1H), 7.62 (m, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 8.0 Hz, 2H), 5.96 (d, J = 7.6 Hz, 1H), 5.79 (d, J = 7.6 Hz, 1H), 3.94 (m, 2H), 3.70 (m, 2H), 2.33 (s, 3H), 2.30 (s, 3H), 1.24 (m, 6H). 13C NMR (CDCl3, selected data for the major anti isomer) δ 191.6, 187.5, 154.8, 140.5, 138.5, 133.3, 132.2, 129.2, 127.1, 126.7, 122.0, 111.5, 82.7, 58.5, 50.4, 47.2, 21.2, 20.2, 12.6, 11.4. MS (EI) m/z: 477.1 (M+, 21%) for C22H2479BrNO2S2.
3.1.6. 8-Bromo-6-methyl-2-(4-ethylphenyl)-4-oxochroman-3-yl N,N-Diethyldithiocarbamate (4f)
White solid, 0.3 g, 75%. IR (ATR, cm−1) 2978, 1691, 1491, 1416, 1261, 1236, 796, 553. 1H NMR (CDCl3, selected data for the major anti isomer) δ 7.64 (m, 1H), 7.62 (m, 1H), 7.43 (d, J = 8.1 Hz, 2H), 7.18 (d, J = 8.1 Hz, 2H), 5.98 (m, 1H), 5.79 (m, 1H), 3.95 (m, 2H), 3.70 (m, 2H), 2.63 (q, J = 7.6 Hz, 2H), 2.31 (s, 3H), 1.24 (m, 6H), 1.22 (t, J = 7.6 Hz, 3H). 13C NMR (CDCl3, selected data for the major anti isomer) δ 191.6, 187.5, 154.9, 144.7, 140.5, 133.5, 132.2, 128.0, 127.2, 126.7, 122.0, 111.5, 82.7, 58.4, 50.4, 47.2, 28.5, 20.2, 15.3, 12.6, 11.4. MS (EI) m/z: 491.1 (M+, 16%) for C23H2679BrNO2S2.
3.1.7. 8-Bromo-6-methyl-2-(4-methoxyphenyl)-4-oxochroman-3-yl N,N-Diethyldithiocarbamate (4g)
White solid, 0.29 g, 79%. IR (ATR, cm−1) 2967, 1692, 1493, 1416, 1265, 1240, 1034, 817, 546. 1H NMR (CDCl3, selected data for the major syn isomer) δ 7.71 (m, 1H), 7.58 (m, 1H), 7.41 (d, J = 8.7 Hz, 2H), 6.88 (d, J = 8.7 Hz, 2H), 6.27 (d, J = 4.2 Hz, 1H), 5.97 (d, J = 4.2 Hz, 1H), 3.95 (m, 2H), 3.78 (s, 3H), 3.72 (m, 2H), 2.31 (s, 3H), 1.23 (m, 6H). 13C NMR (CDCl3, selected data for the major syn isomer) δ 191.5, 188.1, 159.7, 154.5, 140.3, 132.3, 128.5, 127.5, 126.7, 121.4, 113.7, 111.6, 81.1, 58.4, 55.2, 50.8, 47.1, 20.2, 12.6, 11.4. MS (EI) m/z: 493.0 (M+, 21%) for C22H2479BrNO3S2.
3.1.8. General Procedure for 2-N,N-Diethylamino-8-bromo-6-methyl-4-phenyl-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5a)
To a mixture of sulfuric acid (1 mL) and acetic acid (1 mL), flavanone 4a (0.232 g, 0.5 mmol) was added and the resulting solution was heated to 50 °C for 30 min. The reaction mixture was then left to cool to room temperature and a solution of sodium tetrafluoroborate (0.3 g) in water (10 mL) was added dropwise, under vigorous stirring. The resulting precipitate was then filtered, washed thoroughly with water, and recrystallized from ethanol, yielding the desired tetrafluoroborate 5a in the form of colorless crystals (0.22 g, 88%). m.p. 249–250 °C. IR (ATR, cm−1) 1540, 1444, 1041, 710, 421. 1H NMR (DMSO-d6) δ 7.47 (m, 5H), 7.27 (s, 1H), 7.18 (d, J = 8.2 Hz, 1H), 6.95 (d, J = 8.2 Hz, 1H), 6.71 (s, 1H), 3.93 (m, 2H), 3.84 (m, 2H), 2.28 (s, 3H), 1.39 (t, J = 7.1 Hz, 3H), 1.31 (t, J = 7.1 Hz, 3H). 13C NMR (DMSO-d6) δ 184.9, 149.3, 137.4, 132.9, 132.7, 130.3, 129.5, 128.5, 127.9, 127.6, 125.4, 117.5, 116.6, 75.5, 54.7, 54.5, 20.4, 10.8, 10.6. MS (EI) m/z: 446.0 (M+-BF4, 5%) for C21H2179BrNOS2]+.
3.1.9. 2-N,N-Diethylamino-8-bromo-6-methyl-4-(4-fluorophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5b)
White solid, m.p. 201–202 °C (0.21 g, 82%). IR (ATR, cm−1) 1559, 1464, 1224, 1051, 763, 561, 518. 1H NMR (DMSO-d6) δ 7.55 (m, 2H), 7.49 (m, 1H), 7.31 (m, 2H), 7.28 (m, 1H), 6.91 (s, 1H), 3.94 (m, 2H), 3.88 (m, 2H), 2.27 (s, 3H), 1.41 (t, J = 7.0 Hz, 3H), 1.34 (t, J = 7.0 Hz, 3H). 13C NMR (DMSO-d6) δ 185.0, 164.2, 145.7, 135.6, 134.5, 133.2, 130.3, 128.3, 128.2, 125.0, 117.9, 116.6, 110.8, 75.2, 54.7, 54.5, 20.1, 10.8, 10.6. MS (EI) m/z: 463.9 (M+-BF4, 7%) for C21H2079BrFNOS2]+.
3.1.10. 2-N,N-Diethylamino-8-bromo-6-methyl-4-(4-chlorophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5c)
White solid, m.p. 221–222 °C (0.26 g, 86%). IR (ATR, cm−1) 1549, 1455, 1273, 1044, 783, 519. 1H NMR (DMSO-d6) δ 7.52 (m, 4H), 7.49 (m, 1H), 7.31 (m, 1H), 6.93 (s, 1H), 3.94 (m, 2H), 3.89 (m, 2H), 2.27 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (DMSO-d6) δ 185.1, 145.7, 135.9, 135.6, 135.1, 134.6, 129.7, 129.6, 128.3, 128.0, 125.1, 117.9, 110.8, 75.1, 54.7, 54.6, 20.1, 10.8, 10.6. MS (EI) m/z: 480.0 (M+-BF4, 8%) for C21H2079BrClNOS2]+.
3.1.11. 2-N,N-Diethylamino-8-bromo-6-methyl-4-(4-bromophenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5d)
White solid, m.p. 235–237 °C (0.23 g, 90%). IR (ATR, cm−1) 1548, 1477, 1282, 1029, 781, 519. 1H NMR (DMSO-d6) δ 7.66 (d, J = 8.4 Hz, 2H), 7.49 (m, 1H), 7.45 (d, J = 8.4 Hz, 2H), 7.31 (m, 1H), 6.91 (s, 1H), 3.94 (m, 2H), 3.89 (m, 2H), 2.27 (s, 3H), 1.41 (t, J = 7.1 Hz, 3H), 1.34 (t, J = 7.1 Hz, 3H). 13C NMR (DMSO-d6) δ 185.1, 145.7, 136.3, 135.6, 134.6, 132.5, 129.9, 128.3, 127.9, 125.1, 123.8, 117.9, 110.8, 75.2, 54.7, 54.5, 20.1, 10.8, 10.6. MS (EI) m/z: 523.8 (M+-BF4, 4%) for C21H2079Br2NOS2]+.
3.1.12. 2-N,N-Diethylamino-8-bromo-6-methyl-4-(4-methylphenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5e)
White solid, m.p. 245–246 °C (0.19 g, 82%). IR (ATR, cm−1) 1548, 1449, 1283, 1045, 788, 560. 1H NMR (DMSO-d6) δ 7.48 (m, 1H), 7.38 (d, J = 7.8 Hz, 2H), 7.30 (m, 1H), 7.26 (d, J = 7.8 Hz, 2H), 6.84 (s, 1H), 3.93 (m, 2H), 3.87 (m, 2H), 2.31 (s, 3H), 2.27 (s, 3H), 1.40 (t, J = 7.1 Hz, 3H), 1.33 (t, J = 7.1 Hz, 3H). 13C NMR (DMSO-d6) δ 185.1, 146.1, 140.1, 135.5, 134.3, 134.0, 130.0, 128.8, 127.9, 127.8, 125.0, 118.0, 110.8, 75.9, 54.7, 54.5, 21.2, 20.1, 10.8, 10.6. MS (EI) m/z: 460.0 (M+-BF4, 9%) for C22H2379BrNOS2]+.
3.1.13. 2-N,N-Diethylamino-8-bromo-6-methyl-4-(4-ethylphenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5f)
White solid, m.p. 205–207 °C (0.18 g, 83%). IR (ATR, cm−1) 1548, 1449, 1283, 1044, 785, 518. 1H NMR (DMSO-d6) δ 7.49 (m, 1H), 7.40 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.28 (m, 1H), 6.86 (s, 1H), 3.93 (m, 2H), 3.87 (m, 2H), 2.61 (q, J = 7.5 Hz, 2H), 2.27 (s, 3H), 1.40 (t, J = 7.1 Hz, 3H), 1.33 (t, J = 7.1 Hz, 3H), 1.17 (t, J = 7.6 Hz, 3H). 13C NMR (DMSO-d6) δ 185.0, 146.2, 146.0, 135.5, 134.4, 134.3, 128.9, 128.7, 128.0, 127.9, 125.0, 118.0, 110.8, 75.9, 54.7, 54.5, 28.3, 20.1,15.8, 10.8, 10.6. MS (EI) m/z: 474.1 (M+-BF4, 4%) for C23H2579BrNOS2]+.
3.1.14. 2-N,N-Diethylamino-8-bromo-6-methyl-4-(4-methoxyphenyl)-4H-1,3-dithiol[4,5-c]chromen-2-ylium Tetrafluoroborate (5g)
White solid, m.p. 219–220 °C (0.14 g, 83%). IR (ATR, cm−1) 1542, 1456, 1254, 1179, 1051, 792, 519. 1H NMR (DMSO-d6) δ 7.48 (m, 1H), 7.42 (d, J = 8.6 Hz, 2H), 7.30 (m, 1H), 7.00 (d, J = 8.6 Hz, 2H), 6.82 (s, 1H), 3.93 (m, 2H), 3.87 (m, 2H), 3.77 (s, 3H), 2.27 (s, 3H), 1.40 (t, J = 7.1 Hz, 3H), 1.32 (t, J = 7.1 Hz, 3H). 13C NMR (DMSO-d6) δ 184.9, 160.8, 146.1, 135.5, 134.2, 129.6, 129.1, 128.8, 127.9, 125.0, 118.0, 114.8, 110.8, 75.8, 55.7, 54.7, 54.5, 20.1, 10.8, 10.6. MS (EI) m/z: 476.0 (M+-BF4, 6%) for C22H2379BrNO2S2]+.
3.2. Antimicrobial Activity
The antimicrobial activity of the samples was determined by the disk diffusion assay method [48,49] against 10 microbial reference Gram-positive bacterial strains represented by Staphylococcus aureus ATCC 25923 and Enterococcus faecalis ATCC 29212. The Gram-negative bacterial strains were represented by Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 10031, and Salmonella typhimurium ATCC 14028, and the yeast strains were represented by Candida albicans ATCC 90028, Candida albicans ATCC 10231, Candida glabrata ATCC 20019, Candida glabrata ATCC 15126, and Candida parapsilosis ATCC 22019. All microorganisms were stored at −80 °C in 20% glycerol. The bacterial strains were refreshed on nutrient agar (NA) at 37 °C, and the yeast strains were refreshed on Sabouraud dextrose agar (SDA). Microbial suspensions were prepared with these cultures in a sterile solution to obtain turbidity optically comparable to that of 0.5 McFarland standards. Volumes of 0.1 mL from each inoculum were spread onto NA/SDA plates, and 15 µL of the samples (concentration 3 mg/mL) were added. To evaluate the antimicrobial properties, the growth inhibition was measured under standard conditions after 24 h of incubation at 37 °C. All tests were carried out in triplicate to verify the results. Standard controls were tested in the same conditions. After incubation, the samples were analyzed with SCAN1200®, version 8.6.10.0 (Interscience, Saint Nom la Bretêche, France) and were expressed as the mean ± standard deviation (SD) performed with GraphPad Prism software version 7.00 for Windows (GraphPad Software, La Jolla, CA, USA, www.graphpad.com).
4. Conclusions
Our study focused its investigation on the synthesis and antimicrobial properties of a series of novel tricyclic flavonoids. By changing the nature of the substituents on the flavonoid rings A and B, a structure–activity relationship study using different microbial strains was performed. The antibacterial and antifungal properties of these compounds have been investigated against Gram-positive and Gram-negative bacteria and several Candida strains. All seven tested compounds were found to exhibit the highest antimicrobial activity against S. aureus, with an inhibition zone of around 20 mm for flavonoids 5a, 5d, 5f and around 23 mm for 5b, 5c, 5e, and 5g. The tricyclic flavonoids 5c, 5e, and 5f showed good antifungal properties against C. parapsilosis, with an inhibition zone of around 17 mm. Among these compounds, the tricyclic flavonoid 5e with an ethyl substituent at the ring B exhibited the highest activity against both S. aureus and C. parapsilosis strains, with an inhibition zone of 23.03 and 17.43 mm, respectively. These results support the idea that the tricyclic flavonoids 5 are reliable candidates for developing effective antimicrobial agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14030307/s1. Elemental analysis and copies of 13C-NMR spectra.
Author Contributions
Conceptualization, L.G.S. and M.L.B.; methodology, I.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was supported by grants from the Ministry of Research, Innovation and Digitization, CNCS/CCCDI—UEFISCDI, project number PN-IV-P8-8.1-PRE-HE-ORG-2023-0048 and project number PN-IV-P7-7.1-PED-2024-1393, within PNCDI IV. The authors thank the CERNESIM Center, within the Interdisciplinary Research Institute at the “Alexandru Ioan Cuza” University of Iasi, for recording the NMR experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- Bank, T.W. Drug-Resistant Infections: A Threat to Our Economic Future; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Zhu, Y.; Huang, W.E.; Yang, Q. Clinical perspective of antimicrobial resistance in bacteria. Infect. Drug. Resist. 2022, 15, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Chinemerem, N.D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic resistance: The challenges and some emerging strategies for tackling a global menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Hwang, I.Y.; Tan, M.H.; Koh, E.; Ho, C.L.; Poh, C.L.; Chang, M.W. Reprogramming Microbes to Be Pathogen-Seeking Killers. ACS Synth. Biol. 2014, 3, 228–237. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Gilbert, D.N.; Spellberg, B. Seven Ways to Preserve the Miracle of Antibiotics. Clin. Infect. Dis. 2013, 56, 1445–1450. [Google Scholar] [CrossRef]
- World Health Organization. 2023 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; WHO: Geneva, Switzerland, 2024. [Google Scholar]
- de Oliveira Santos, G.C.; Vasconcelos, C.C.; Lopes, A.J.O.; de Sousa Cartágenes, M.d.S.; Filho, A.K.D.B.; do Nascimento, F.R.F.; Ramos, R.M.; Pires, E.R.R.B.; de Andrade, M.S.; Rocha, F.M.G.; et al. Candida Infections and therapeutic strategies: Mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Tobudic, S.; Kratzer, C.; Lassnigg, A.; Presterl, E. Antifungal susceptibility of Candida albicans in biofilms. Mycoses 2012, 55, 199–204. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2015, 62, e1–e50. [Google Scholar] [CrossRef]
- Douglas, L.J. Candida biofilms and their role in infection. Trends. Microbiol. 2003, 11, 30–36. [Google Scholar] [CrossRef]
- Ngo-Mback, M.N.L.; Babii, C.; Jazet Dongmo, P.M.; Kouipou Toghueo, M.R.; Stefan, M.; Fekam Boyom, F. Anticandidal and synergistic effect of essential oil fractions from three aromatic plants used in Cameroon. J. Mycol. Med. 2020, 30, 100940. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Fini, A.; Gori, A.; Guidi, L.; Landi, M.; Sebastiani, F.; Tattini, M. Are Flavonoids Effective Antioxidants in Plants? Twenty Years of Our Investigation. Antioxidants 2020, 9, 1098. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Friends in Arms: Flavonoids and the Auxin/Cytokinin Balance in Terrestrialization. Plants 2023, 12, 517. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Narbona, E.; del Valle, J.C.; Arista, M.; Buide, M.L.; Ortiz, P.L. Major Flower Pigments Originate Different Colour Signals to Pollinators. Front. Ecol. Evol. 2021, 9, 743850. [Google Scholar] [CrossRef]
- Ramaroson, M.L.; Koutouan, C.; Helesbeux, J.J.; Le Clerc, V.; Hamama, L.; Geoffriau, E.; Briard, M. Role of Phenylpropanoids and Flavonoids in Plant Resistance to Pests and Diseases. Molecules 2022, 27, 8371. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Croft, K.D.; Ward, N.; Considine, M.J.; Hodgson, J.M. Dietary flavonoids and nitrate: Effects on nitric oxide and vascular function. Nutr. Rev. 2015, 73, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Jagtap, S.; Meganathan, K.; Wagh, V.; Winkler, J.; Hescheler, J.; Sachinidis, A. Chemoprotective Mechanism of the Natural Compounds, Epigallocatechin- 3-O-Gallate, Quercetin and Curcumin Against Cancer and Cardiovascular Diseases. Curr. Med. Chem. 2009, 16, 1451–1462. [Google Scholar] [CrossRef]
- Norman, B.H.; Dodge, J.A.; Richardson, T.I.; Borromeo, P.S.; Lugar, C.W.; Jones, S.A.; Chen, K.; Wang, Y.; Durst, G.L.; Barr, R.J.; et al. Benzopyrans Are Selective Estrogen Receptor β Agonists with Novel Activity in Models of Benign Prostatic Hyperplasia. J. Med. Chem. 2006, 49, 6155–6157. [Google Scholar] [CrossRef]
- Richardson, T.I.; Norman, B.H.; Lugar, C.W.; Jones, S.A.; Wang, Y.; Durbin, J.D.; Krishnan, V.; Dodge, J.A. Benzopyrans as selective estrogen receptor beta agonists (SERBAs). Part 2: Structure-activity relationship studies on the benzopyran scaffold. Bioorg. Med. Chem. Lett. 2007, 17, 3570–3574. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.I.; Dodge, J.A.; Durst, G.L.; Pfeifer, L.A.; Shah, J.; Wang, Y.; Durbin, J.D.N.; Krishnan, V.; Norman, B.H. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Bioorg. Med. Chem. Lett. 2007, 17, 4824–4828. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.I.; Dodge, J.A.; Wang, Y.; Durbin, J.D.; Krishnan, V.; Norman, B.H. Estrogen receptor beta ligand-binding domain complexed to a benzopyran ligand. Bioorg. Med. Chem. Lett. 2007, 17, 5563–5566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, J.; Xie, Y. Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: An overview. Int. J. Pharm. 2019, 570, 118642. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Hopf, H.; Jones, P.G.; Sarbu, L.G.; Babii, C.; Mihai, A.C.; Stefan, M.; Birsa, L.M. Antibacterial structure-activity relationship studies of several tricyclic sulfur-containing flavonoids. Beilstein J. Org. Chem. 2016, 12, 1065–1071. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Sarbu, L.G.; Jones, P.G.; Birsa, L.M.; Hopf, H. [2.2]Paracyclophane-Bis(triazole) Systems: Synthesis and Photochemical Behavior. Chem. Eur. J. 2017, 23, 12338–12345. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Apostu, M.O.; Birsa, L.M.; Stefan, M. The antibacterial properties of sulfur containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. [Google Scholar] [CrossRef]
- Birsa, M.L. Synthesis of some new substituted flavanones and related 4-chromanones by a novel synthetic method. Synth. Commun. 2002, 32, 115–118. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Asaftei, I.V.; Sandu, I.; Sarbu, L.G. Synthesis of (4-Methylpiperazin-1-yl)carbodithioates and of their 1,3-Dithiolium Derivatives. Rev. Chim. 2014, 65, 1046–1048. [Google Scholar]
- Sarbu, L.G.; Hopf, H.; Gruenenberg, J.; Birsa, M.L. Reduction of pseudo-geminal bis(ethynyl) substituted [2.2]paracyclophanes. Synlett 2015, 26, 87–90. Available online: http://www.thieme-connect.de/products/ejournals/abstract/10.1055/s-0034-1378935 (accessed on 20 December 2024).
- Toader, E.; Bahrin, L.G.; Jones, P.G.; Hopf, H.; Sarbu, L.G.; Stoleriu, G. Synthesis of New Morpholine Containing Flavonoids with Potential Biological Applications. Rev. Chim. 2016, 67, 1520–1522. [Google Scholar]
- Seliger, H.; Happ, E.; Cascaval, A.; Birsa, M.L.; Nicolaescu, T.; Poinescu, I.; Cojocariu, C. Synthesis and characterization of new photostabilizers from 2,4-dihydroxybenzophenone. Eur. Polym. J. 1999, 35, 827–833. [Google Scholar] [CrossRef]
- Sarbu, L.G.; Birsa, A.; Hopf, H.; Birsa, M.L. New bridges in [2.2]paracyclophanes: The interaction of chalcogenide halides with pseudo-geminal triple bonds. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 1246–1250. [Google Scholar] [CrossRef]
- Birsa, M.L. A new approach to preparation of 1,3-dithiolium salts. Synth. Commun. 2001, 31, 1271–1275. [Google Scholar] [CrossRef]
- Bauer, A.W.; Perry, D.M.; Kirby, W.M.M. Single disc antibiotic sensitivity testing of Staphylococci. A.M.A. Arch. Intern. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Berwyn, PA, USA, 2022; ISSN 978-1-68440-134-5/978-1-68440-135-2. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).