Colistin Treatment Outcomes in Gram-Negative Bacterial Infections in the Northeast of Romania: A Decade of Change Through Pandemic Challenges
Abstract
1. Introduction
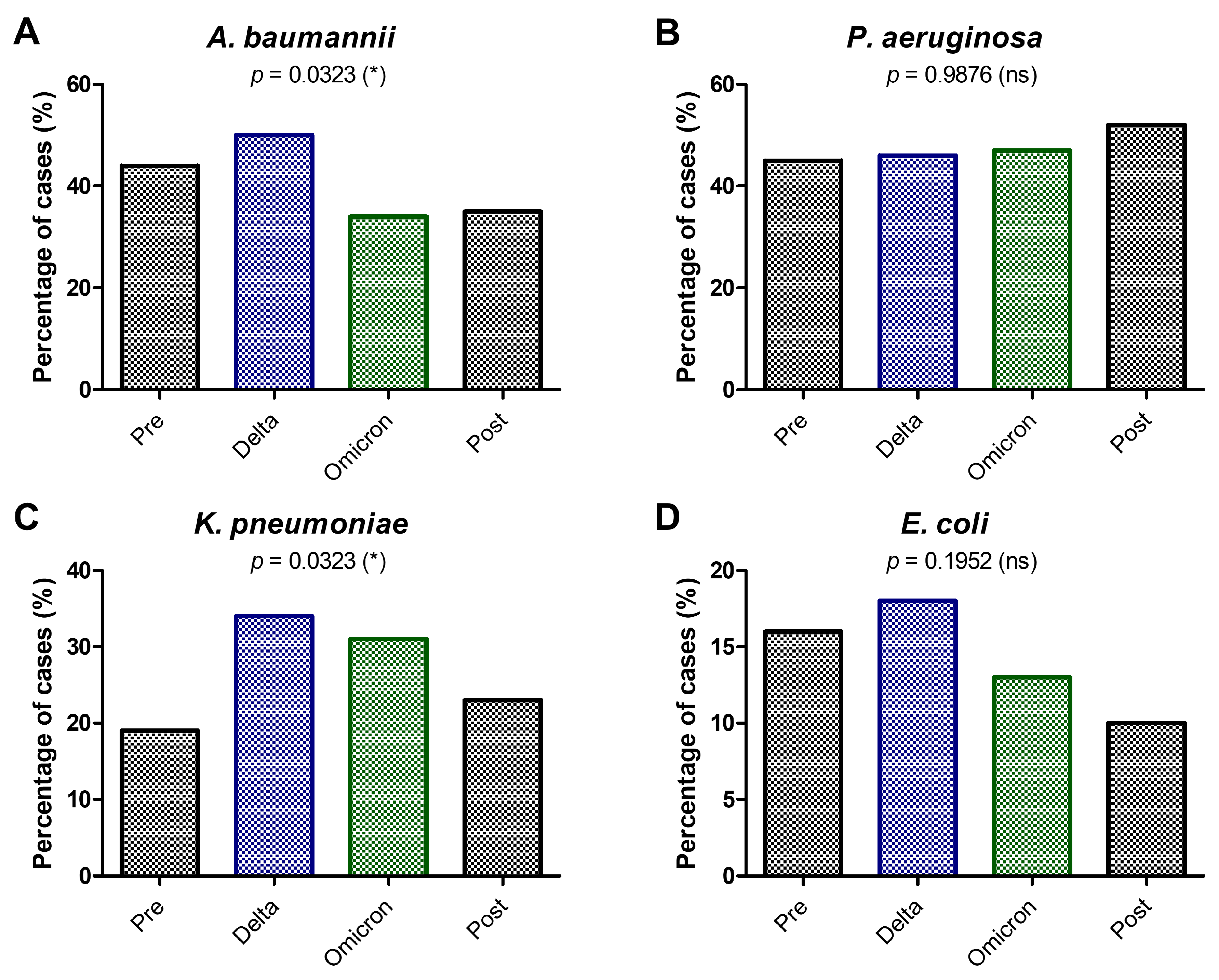
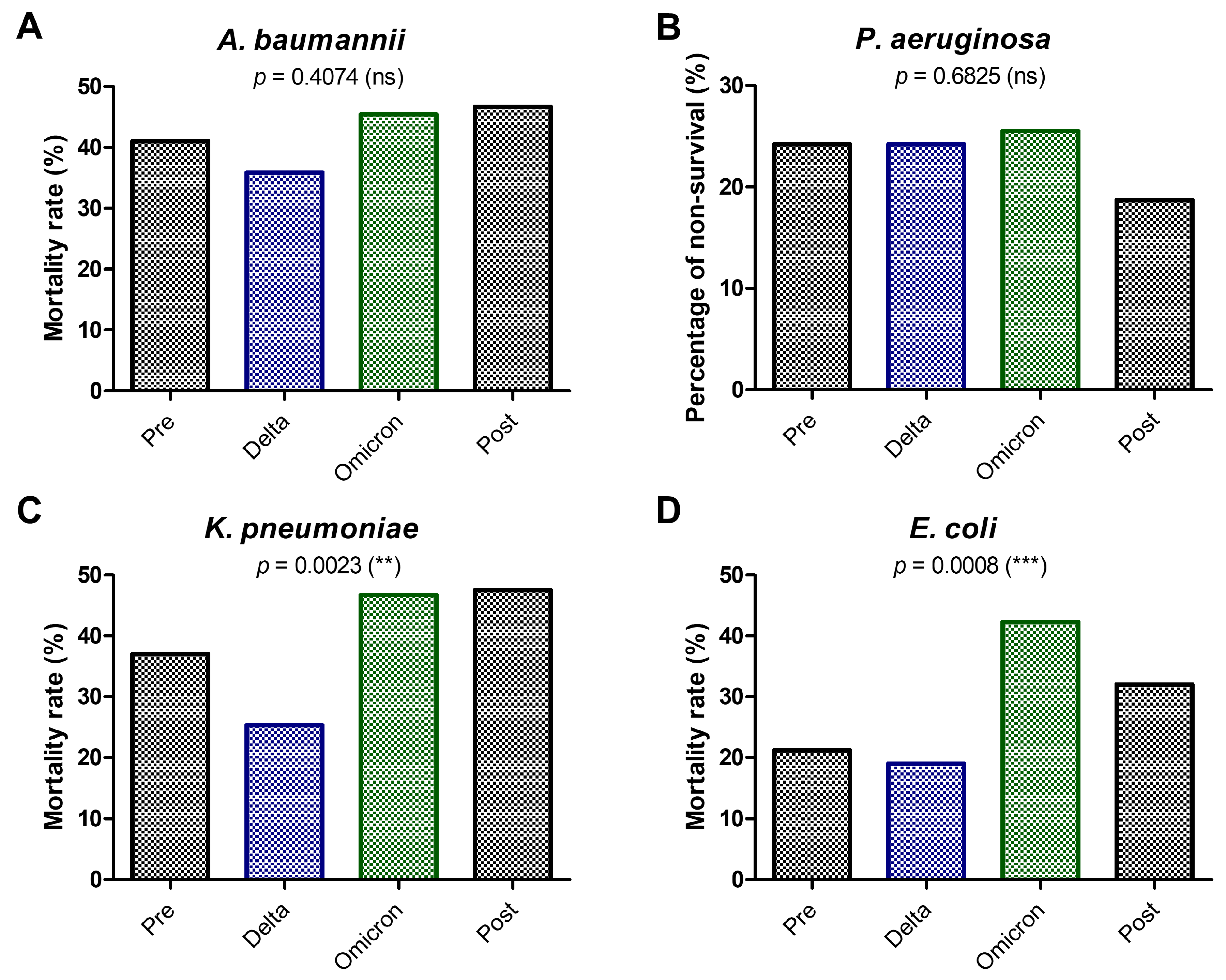
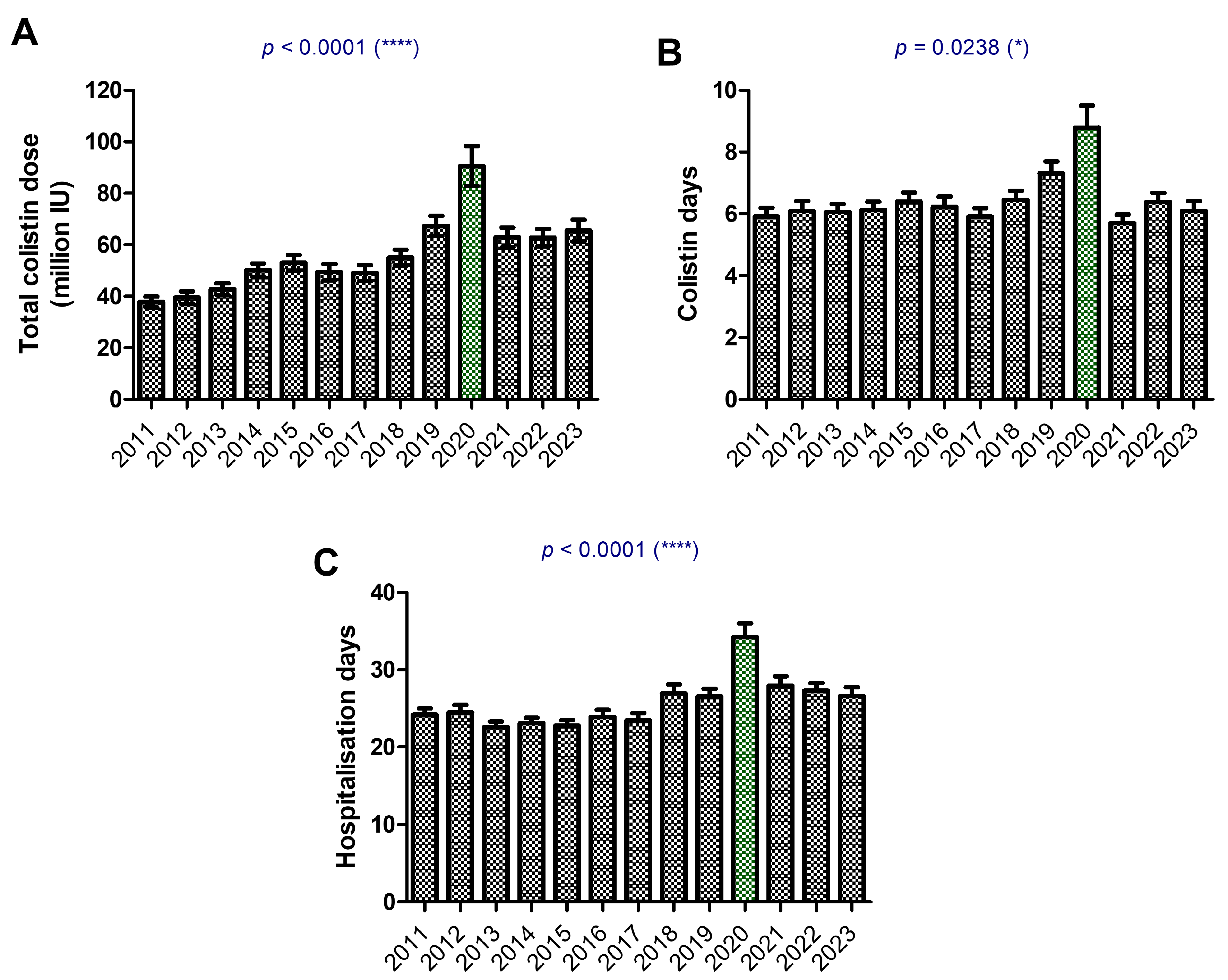
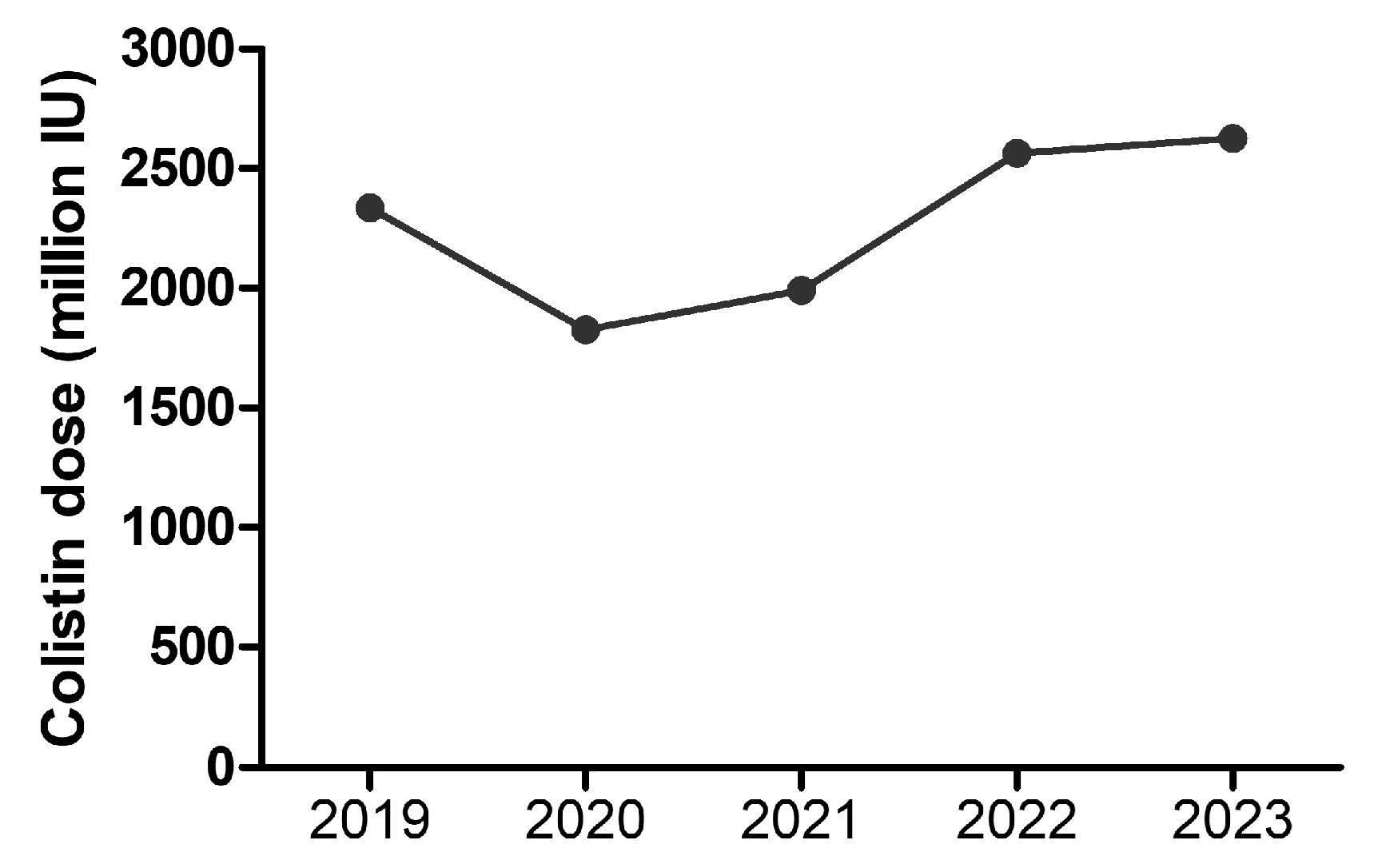
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolate Characterization
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Echegorry, M.; Marchetti, P.; Sanchez, C.; Olivieri, L.; Faccone, D.; Martino, F.; Sarkis Badola, T.; Ceriana, P.; Rapoport, M.; Lucero, C.; et al. National Multicenter Study on the Prevalence of Carbapenemase-Producing Enterobacteriaceae in the Post-COVID-19 Era in Argentina: The RECAPT-AR Study. Antibiotics 2024, 13, 1139. [Google Scholar] [CrossRef]
- CDC. COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022; U.S. Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022. [Google Scholar]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024.
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; van Kleef, E.; et al. Antimicrobial Resistance and COVID-19: Intersections and Implications. eLife 2021, 10, E64139. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the Potential Long-Term Impact on Antimicrobial Resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-Infections in People with COVID-19: A Systematic Review and Meta-Analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Vâţă, A.; Roşu, F.M.; Dorneanu, O.S.; Lehaci, A.E.; Luca, Ş.; Loghin, I.I.; Miftode, I.D.; Luca, C.M.; Miftode, E.G. Antibiotic Usage in the COVID-19 Intensive Care Unit of an Infectious Diseases Hospital from Nord-Eastern Romania. Medicina 2023, 59, 645. [Google Scholar] [CrossRef] [PubMed]
- Musuroi, C.; Musuroi, S.-I.; Baditoiu, L.; Crainiceanu, Z.; Muntean, D.; Voinescu, A.; Izmendi, O.; Sirmon, A.; Licker, M. The Profile of Bacterial Infections in a Burn Unit during and after the COVID-19 Pandemic Period. Antibiotics 2024, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2022–2020 Data, Surveill Rep [Internet]. 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Joint-WHO-ECDC-AMR-report-2022.pdf (accessed on 20 January 2025).
- Mohd Asri, N.A.; Ahmad, S.; Mohamud, R.; Mohd Hanafi, N.; Mohd Zaidi, N.F.; Irekeola, A.A.; Shueb, R.H.; Yee, L.C.; Mohd Noor, N.; Mustafa, F.H.; et al. Global Prevalence of Nosocomial Multidrug-Resistant Klebsiella pneumoniae: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 1508. [Google Scholar] [CrossRef]
- Della Rocca, M.T.; Foglia, F.; Crudele, V.; Greco, G.; De Filippis, A.; Franci, G.; Finamore, E.; Galdiero, M. Antimicrobial Resistance Changing Trends of Klebsiella pneumoniae Isolated over the Last 5 Years. New Microbiol. 2022, 45, 338–343. [Google Scholar]
- Wang, M.G.; Earley, M.; Chen, L.; Hanson, B.M.; Yu, Y.S.; Liu, Z.Y.; Salcedo, S.; Cober, E.; Li, L.J.; Kanj, S.S.; et al. Clinical Outcomes and Bacterial Characteristics of Carbapenem-Resistant Complex among Patients from Different Global Regions (CRACKLE-2): A Prospective, Multicentre, Cohort Study. Lancet Infect. Dis. 2022, 22, 401–412. [Google Scholar] [CrossRef]
- Ahmed, M.A.G.E.S.; Zhong, L.L.; Shen, C.; Yang, Y.Q.; Doi, Y.; Tian, G.B. Colistin and Its Role in the Era of Antibiotic Resistance: An Extended Review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Ah, Y.M.; Kim, A.J.; Lee, J.Y. Colistin Resistance in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2014, 44, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.H.; Khare, K.; Saxena, P.; Debnath, P.; Mukhopadhyay, K.; Yadav, D. A Review on Colistin Resistance: An Antibiotic of Last Resort. Microorganisms 2024, 12, 772. [Google Scholar] [CrossRef]
- Uzairue, L.I.; Rabaan, A.A.; Adewumi, F.A.; Okolie, O.J.; Folorunso, J.B.; Bakhrebah, M.A.; Garout, M.; Alfouzan, W.A.; Halwani, M.A.; Alamri, A.A.; et al. Global Prevalence of Colistin Resistance in Klebsiella pneumoniae from Bloodstream Infection: A Systematic Review and Meta-Analysis. Pathogens 2022, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Narimisa, N.; Keshtkar, A.; Dadgar-Zankbar, L.; Bostanghadiri, N.; Far, Y.R.; Shahroodian, S.; Bialvaei, A.Z.; Razavi, S. Prevalence of Colistin Resistance in Clinical Isolates of Pseudomonas aeruginosa: A Systematic Review and Meta-Analysis. Front. Microbiol. 2024, 15, 1477836. [Google Scholar] [CrossRef]
- Abdel-Aty, H.; El-Batal, H.; Gohar, N. Assessment of Colistin Resistance among Nosocomial Multidrug-Resistant Gram-Negative Bacilli Isolated from Different Clinical Samples. Microbes Infect. Dis. 2024, 5, 1494–1505. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Da Silva, G.J.; Domingues, S. Interplay between Colistin Resistance, Virulence and Fitness in Acinetobacter baumannii. Antibiotics 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the Last-Resort Antibiotics: Mode of Action, Resistance Emergence, and Potential Solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef]
- Rangel, K.; Chagas, T.P.G.; De-Simone, S.G. Acinetobacter baumannii Infections in Times of COVID-19 Pandemic. Pathogens 2021, 10, 1006. [Google Scholar] [CrossRef]
- Sharifipour, E.; Shams, S.; Esmkhani, M.; Khodadadi, J.; Fotouhi-Ardakani, R.; Koohpaei, A.; Doosti, Z.; EJ Golzari, S. Evaluation of Bacterial Co-Infections of the Respiratory Tract in COVID-19 Patients Admitted to ICU. BMC Infect. Dis. 2020, 20, 646. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Yang, Y.; Cai, P.; Cao, J.; Cai, X.; Zhang, Y. Etiology and Antimicrobial Resistance of Secondary Bacterial Infections in Patients Hospitalized with COVID-19 in Wuhan, China: A Retrospective Analysis. Antimicrob. Resist. Infect. Control. 2020, 9, 153. [Google Scholar] [CrossRef]
- European Centre for Disease Prevetion and Control (ECDC). Surveillance of Antimicrobial Resistance in Europe—Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) 2017; ECDC: Solna, Sweden, 2017. [Google Scholar]
- Liu, L.; Liu, B.; Li, Y.; Zhang, W. Successful Control of Resistance in Pseudomonas aeruginosa Using Antibiotic Stewardship and Infection Control Programs at a Chinese University Hospital: A 6-Year Prospective Study. Infect. Drug Resist. 2018, 11, 637–646. [Google Scholar] [CrossRef]
- Xiao, C.; Zhu, Y.; Yang, Z.; Shi, D.; Ni, Y.; Hua, L.; Li, J. Prevalence and Molecular Characteristics of Polymyxin-Resistant Pseudomonas aeruginosa in a Chinese Tertiary Teaching Hospital. Antibiotics 2022, 11, 799. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.P.; Patel, P.K.; Nori, P. Involving Antimicrobial Stewardship Programs in COVID-19 Response Efforts: All Hands on Deck. Infect. Control Hosp. Epidemiol. 2020, 41, 744–745. [Google Scholar] [CrossRef]
- Evans, T.J.; Davidson, H.C.; Low, J.M.; Basarab, M.; Arnold, A. Antibiotic Usage and Stewardship in Patients with COVID-19: Too Much Antibiotic in Uncharted Waters? J. Infect. Prev. 2021, 22, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Abdela, S.G.; Liesenborghs, L.; Tadese, F.; Abegaz, S.H.; Bayuh, F.B.; Asmamaw, E.A.; Mebrate, T.A.; Mamo, A.E.; Embiale, W.; Hunegnaw, S.; et al. Antibiotic Overuse for COVID-19: Are We Adding Insult to Injury? Am. J. Trop. Med. Hyg. 2021, 105, 1519–1520. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.C.; Huang, W.C.; Wang, H.Y.; Shiau, Y.R.; Cheng, M.F.; Lauderdale, T.L. Colistin Resistance Gene Mcr-1 in Escherichia coli Isolates from Humans and Retail Meats, Taiwan. J. Antimicrob. Chemother. 2016, 71, 2327–2329. [Google Scholar] [CrossRef]
- Chen, L.; Mathema, B.; Chavda, K.D.; DeLeo, F.R.; Bonomo, R.A.; Kreiswirth, B.N. Carbapenemase-Producing Klebsiella pneumoniae: Molecular and Genetic Decoding. Trends Microbiol. 2014, 22, 686–696. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Willems, R.J.L.; Gouliouris, T.; Ballard, S.A.; Coque, T.M.; Hammerum, A.M.; Hegstad, K.; Westh, H.T.; Howden, B.P.; Malhotra-Kumar, S.; et al. The Global Dissemination of Hospital Clones of Enterococcus faecium. Genome Med. 2021, 13, 52. [Google Scholar] [CrossRef]
- Van Der Graaf-van Bloois, L.; Duim, B.; Looft, T.; Veldman, K.T.; Zomer, A.L.; Wagenaar, J.A. Antimicrobial Resistance in Campylobacter fetus: Emergence and Genomic Evolution. Microb. Genom. 2023, 9, Mgen000934. [Google Scholar] [CrossRef]
- Cogliati Dezza, F.; Arcari, G.; Alessi, F.; Valeri, S.; Curtolo, A.; Sacco, F.; Ceccarelli, G.; Raponi, G.; Alessandri, F.; Mastroianni, C.M.; et al. Clinical Impact of COVID-19 on Multi-Drug-Resistant Gram-Negative Bacilli Bloodstream Infections in an Intensive Care Unit Setting: Two Pandemics Compared. Antibiotics 2022, 11, 926. [Google Scholar] [CrossRef]
- Wilson Dib, R.; Spallone, A.; Khawaja, F.; Feldman, A.; Cantu, S.; and Chemaly, R. The Impact of the COVID- 19 Pandemic on Hospital-Acquired Infections at a Comprehensive Cancer Center. Am. J. Infect. Control 2023, 51, 1302–1308. [Google Scholar] [CrossRef]
- O’Leary, O.; Neuhauser, M.; Srinivasan, A.; Dubendris, H.; Webb, A.; Soe, M.; Hicks, L.; Wu, H.; Kabbani, S.; and Edwards, J. Impact of the COVID-19 Pandemic on Inpatient Antibiotic Use in the United States, January 2019 Through July 2022. Clin. Infect. Dis. 2024, 78, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (U.S.). COVID-19 Reverses Progress in Fight Against Antimicrobial Resistance in U.S. 12 July 2022. [Online]. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/media/releases/2022/s0712-Antimicrobial-Resistance.html (accessed on 20 January 2025).
- Mędrzycka-Dąbrowska, W.; Lange, S.; Zorena, K.; Dąbrowski, S.; Ozga, D.; Tomaszek, L. Carbapenem-Resistant Klebsiella pneumoniae Infections in ICU COVID-19 Patients—A Scoping Review. J. Clin. Med. 2021, 10, 2067. [Google Scholar] [CrossRef]
- Balkhair, A.; Al-Muharrmi, Z.; Al’Adawi, B.; Al Busaidi, I.; Taher, H.B.; Al-Siyabi, T.; Al Amin, M.; Hassan, K.S. Prevalence and 30-Day All-Cause Mortality of Carbapenem-and Colistin-Resistant Bacteraemia Caused by Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae: Description of a Decade-Long Trend. Int. J. Infect. Dis. 2019, 85, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Gavaruzzi, F.; Ceccarelli, G.; Borrazzo, C.; Oliva, A.; Alessandri, F.; Magnanimi, E.; Pugliese, F.; Venditti, M. Multidrug-Resistant Acinetobacter baumannii Infections in COVID-19 Patients Hospitalized in Intensive Care Unit. Infection 2021, 50, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, T.A.; Shaman, M.S.B.; Suliman, D.M.; Alanazi, T.A.; Altawalbeh, S.M.; Alshareef, H.; Lahreche, D.I.; Al-Azzam, S.; Araydah, M.; Karasneh, R.; et al. The Impact of Multidrug-Resistant Acinetobacter baumannii Infection in Critically Ill Patients with or without COVID-19 Infection. Healthcare 2023, 11, 487. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, C.Y.; Hsueh, P.R. Co-Infections among Patients with COVID-19: The Need for Combination Therapy with Non-Anti-SARS-CoV-2 Agents? J. Microbiol. Immunol. Infect. 2020, 53, 505–512. [Google Scholar] [CrossRef]
- Lai, C.C.; Yu, W.L. COVID-19 Associated with Pulmonary Aspergillosis: A Literature Review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Vilbrun, S.C.; Mathurin, L.; Pape, J.W.; Fitzgerald, D.; Walsh, K.F. Case Report: Multidrug-Resistant Tuberculosis and COVID-19 Coinfection in Port-Au-Prince, Haiti. Am. J. Trop. Med. Hyg. 2020, 103, 1986–1988. [Google Scholar] [CrossRef]
- Yousaf, Z.; Khan, A.A.; Chaudhary, H.A.; Mushtaq, K.; Parengal, J.; Aboukamar, M.; Khan, M.U.; Mohamed, M.F.H. Cavitary Pulmonary Tuberculosis with COVID-19 Coinfection. IDCases 2020, 22, E00973. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Saeed, A.; Alrashidi, A.; Alrashidi, A.; Alshaghdali, K.; Hammam, S.A.; Alreshidi, T.; Alshammary, M.; Alarfaj, A.; Thallab, R.; et al. Antimicrobial Surveillance for Bacterial Uropathogens in Ha’il, Saudi Arabia: A Five-Year Multicenter Retrospective Study. Infect. Drug Resist. 2021, 14, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Livias, K.; Pinzas-Acosta, K.; Perez-Abad, L.; Panduro-Correa, V.; Rabaan, A.A.; Pecho-Silva, S.; Dámaso-Mata, B. A Multidrug-Resistant Klebsiella pneumoniae Outbreak in a Peruvian Hospital: Another Threat from the COVID-19 Pandemic. Infect. Control Hosp. Epidemiol. 2021, 43, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Buehler, P.K.; Zinkernagel, A.S.; Hofmaenner, D.A.; Wendel Garcia, P.D.; Acevedo, C.T.; Gomez-Mejia, A.; Mairpady Shambat, S.; Andreoni, F.; Maibach, M.A.; Bartussek, J.; et al. Bacterial Pulmonary Superinfections Are Associated with Longer Duration of Ventilation in Critically Ill COVID-19 Patients. Cell Rep. Med. 2021, 2, 100229. [Google Scholar] [CrossRef]
- Vijay, S.; Bansal, N.; Rao, B.K.; Veeraraghavan, B.; Rodrigues, C.; Wattal, C.; Goyal, J.P.; Tadepalli, K.; Mathur, P.; Venkateswaran, R.; et al. Secondary Infections in Hospitalized COVID-19 Patients: Indian Experience. Infect. Drug Resist. 2021, 14, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Cai, Z.; Liu, Y.; Duan, X.; Han, S.; Liu, J.; Zhu, Y.; Jiang, Z.; Zhang, Y.; Zhuo, C.; et al. Persistent Bacterial Coinfection of a COVID-19 Patient Caused by a Genetically Adapted Pseudomonas aeruginosa Chronic Colonizer. Front. Cell. Infect. Microbiol. 2021, 11, 641920. [Google Scholar] [CrossRef]
- Bavaro, D.F.; Belati, A.; Diella, L.; Stufano, M.; Romanelli, F.; Scalone, L.; Stolfa, S.; Ronga, L.; Maurmo, L.; Dell’Aera, M.; et al. Cefiderocol-Based Combination Therapy for “Difficult-to-Treat” Gram-Negative Severe Infections: Real-Life Case Series and Future Perspectives. Antibiotics 2021, 10, 652. [Google Scholar] [CrossRef]
- Esteban Ronda, V.; Ruiz Alcaraz, S.; Ruiz Torregrosa, P.; Giménez Suau, M.; Nofuentes Pérez, E.; León Ramírez, J.M.; Andrés, M.; Moreno-Pérez, Ó.; Candela Blanes, A.; Gil Carbonell, J.; et al. Aplicación de Escalas Pronósticas de Gravedad En La Neumonía Por SARS-CoV-2. Med. Clín. 2021, 157, 99–105. [Google Scholar] [CrossRef]
- Allel, K.; Peters, A.; Conejeros, J.; Martínez, J.R.W.; Spencer-Sandino, M.; Riquelme-Neira, R.; Rivas, L.; Rojas, P.; Orellana Chea, C.; García, P.; et al. Antibiotic Consumption during the Coronavirus Disease 2019 Pandemic and Emergence of Carbapenemase-Producing Klebsiella pneumoniae Lineages among Inpatients in a Chilean Hospital: A Time-Series Study and Phylogenomic Analysis. Clin. Infect. Dis. 2023, 77, S20–S28. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters; The European Committee on Antimicrobial Susceptibility Testing: Strasbourg, France, 2024. [Google Scholar]
- CLSI. Performance Standard for Antimicrobial Susceptibility Testing, 26th ed.; CLSI: Berwyn, PA, USA, 2016. [Google Scholar]
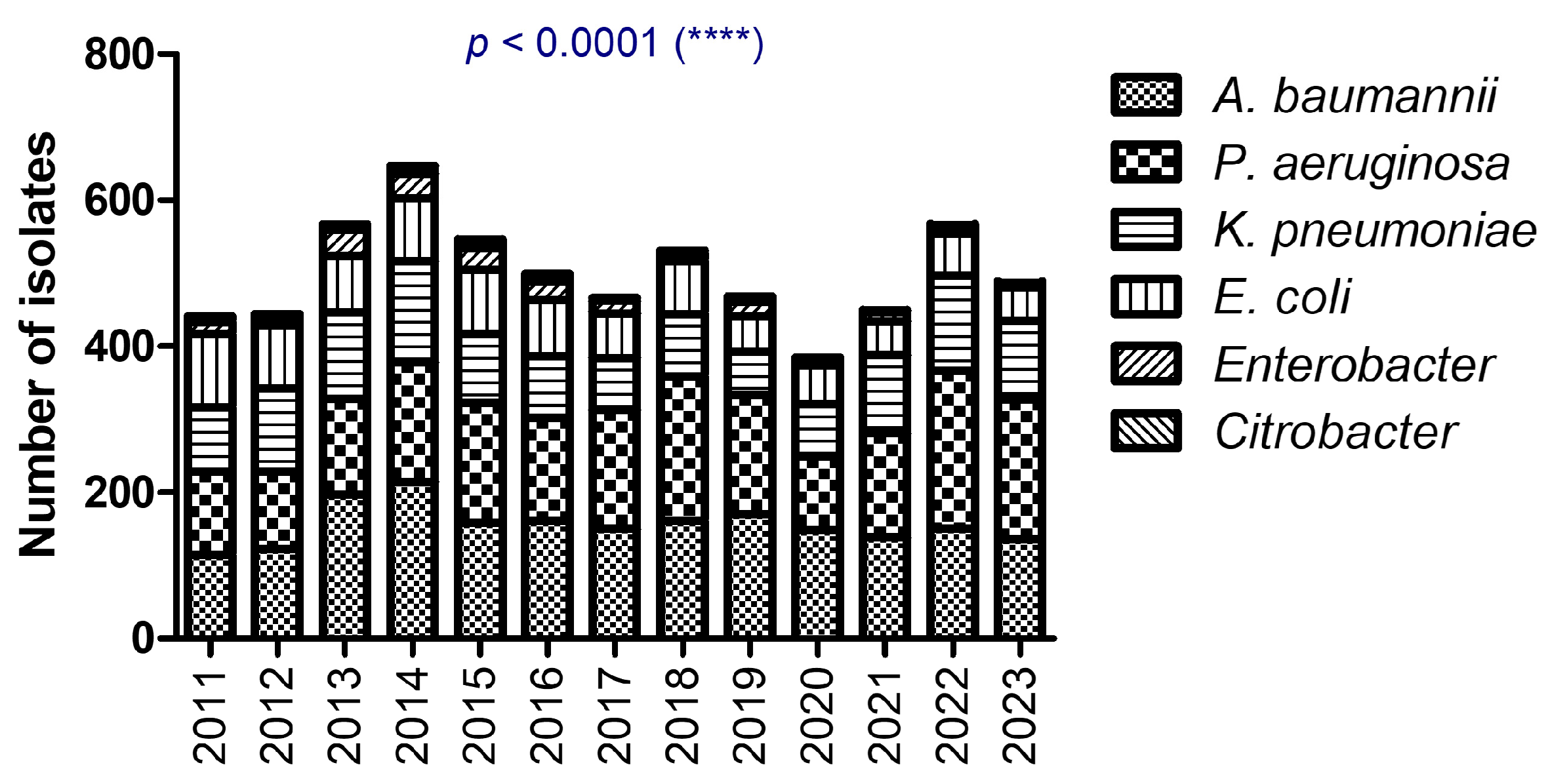
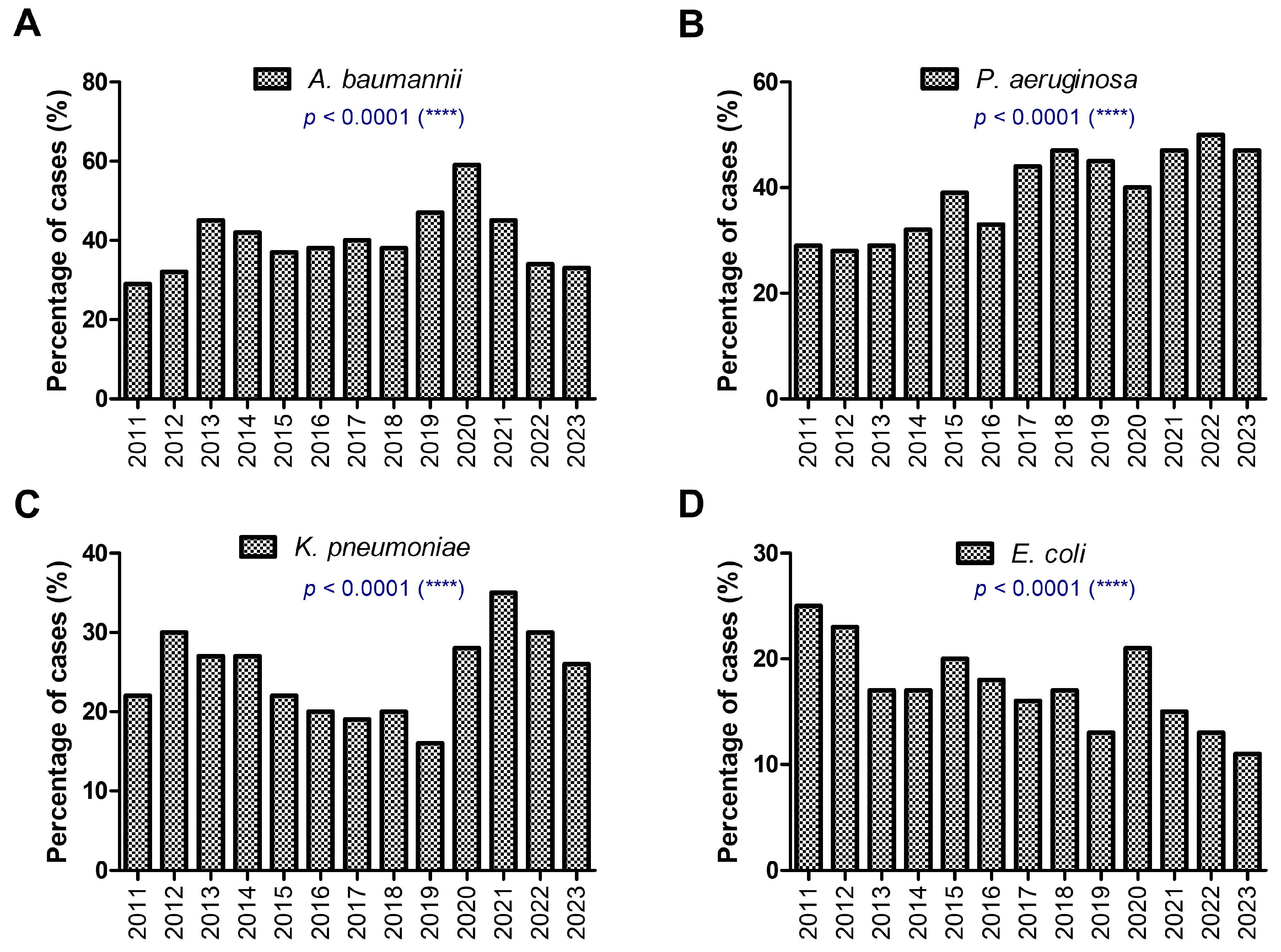
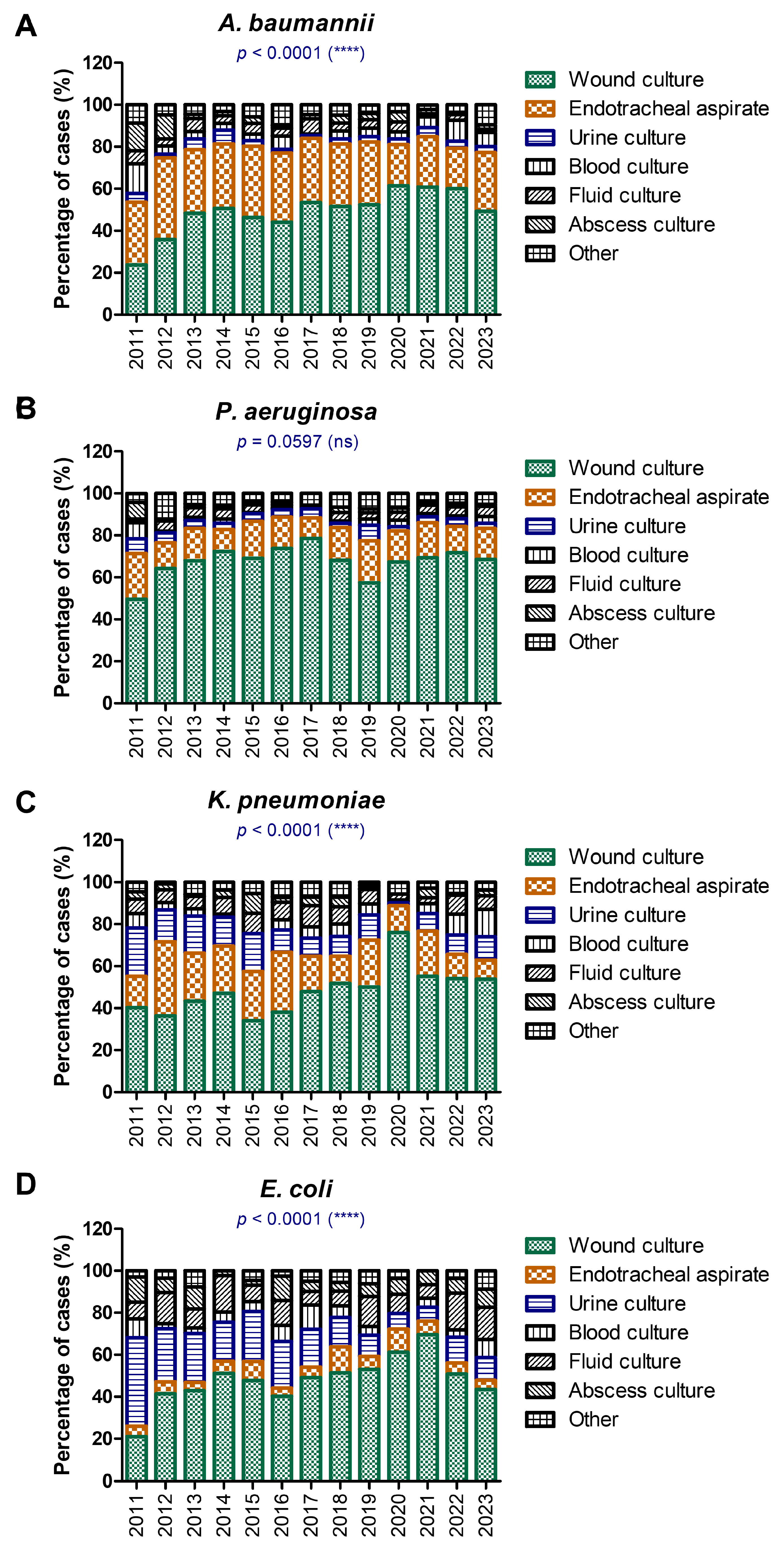

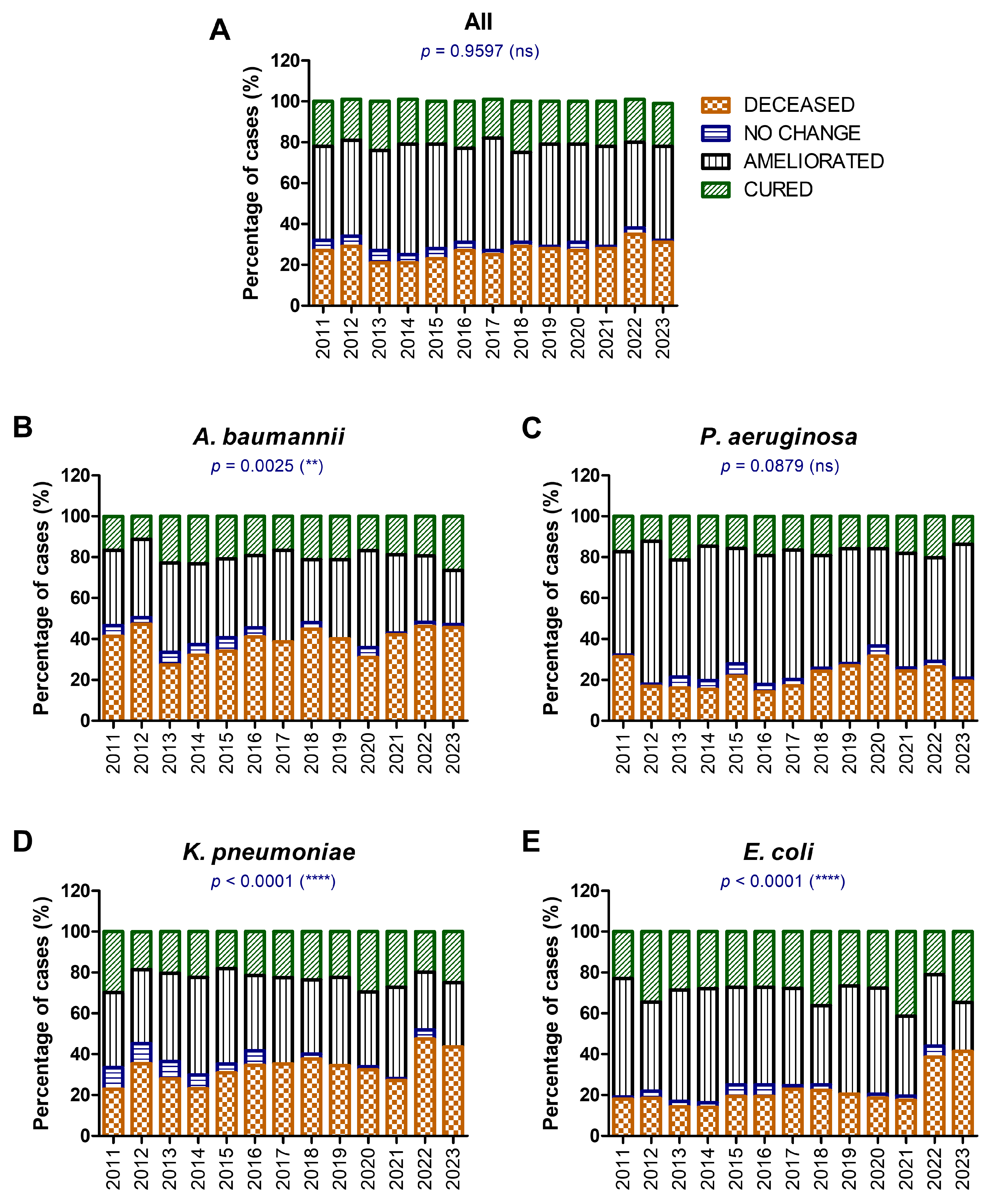


| Total, N % | F, n % M, n % | Age Median [CI] | |
|---|---|---|---|
| 2011 | 393.00 (7.73) | 177 (45.04) 216 (54.96) | 65 [55–74] |
| 2012 | 375.00 (7.38) | 148 (39.47) 227 (60.53) | 63 [54–74] |
| 2013 | 437.00 (8.60) | 151 (34.55) 286 (65.45) | 63 [52.5–75] |
| 2014 | 502.00 (9.88) | 185 (36.85) 317 (63.15) | 65 [54–75] |
| 2015 | 423.00 (8.33) | 158 (37.35) 265 (62.65) | 65 [57–74] |
| 2016 | 420.00 (8.27) | 144 (34.29) 276 (65.71) | 65 [55–74] |
| 2017 | 370.00 (7.28) | 140 (37.84) 230 (62.16) | 64 [53–75] |
| 2018 | 417.00 (8.21) | 149 (35.73) 268 (64.27) | 64 [53–73] |
| 2019 | 358.00 (7.05) | 144 (40.22) 214 (59.78) | 64 [52–72] |
| 2020 | 247.00 (4.86) | 98 (39.68) 149 (60.32) | 63 [52–73] |
| 2021 | 304.00 (5.98) | 110 (36.18) 194 (63.82) | 63 [53–74] |
| 2022 | 431.00 (8.48) | 148 (34.34) 283 (65.66) | 65 [54–73] |
| 2023 | 404.00 (7.95) | 157 (38.86) 247 (61.14) | 66 [54–73] |
| Model | AUC | S.E. | p-Value | 95% CI |
|---|---|---|---|---|
| Pre-pandemic | ||||
| Model_1 | 0.811 | 0.014 | <0.0001 | 0.785–0.838 |
| Model_2 | 0.756 | 0.015 | <0.0001 | 0.726–0.786 |
| Model_3 | 0.793 | 0.014 | <0.0001 | 0.765–0.821 |
| Delta | ||||
| Model_1 | 0.817 | 0.023 | <0.0001 | 0.773–0.862 |
| Model_2 | 0.785 | 0.024 | <0.0001 | 0.738–0.832 |
| Model_3 | 0.800 | 0.025 | <0.0001 | 0.751–0.849 |
| Omicron | ||||
| Model_1 | 0.796 | 0.019 | <0.001 | 0.758–0.833 |
| Model_2 | 0.772 | 0.020 | <0.001 | 0.732–0.811 |
| Model_3 | 0.772 | 0.020 | <0.001 | 0.733–0.811 |
| Post-pandemic | ||||
| Model_1 | 0.866 | 0.024 | <0.0001 | 0.819–0.912 |
| Model_2 | 0.836 | 0.026 | <0.0001 | 0.785–0.887 |
| Model_3 | 0.847 | 0.024 | <0.0001 | 0.800–0.895 |
| Variable (Pre) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender (M) | 0.899 | 0.655–1.232 | 0.509 | |||
| Age | 1.036 | 1.024–1.047 | <0.0001 | 1.037 | 1.025–1.048 | <0.0001 |
| Hospitalization days | 0.960 | 0.948–0.972 | <0.0001 | 0.960 | 0.947–0.972 | <0.0001 |
| Colistin dose | 0.996 | 0.989–1.002 | 0.185 | |||
| Colistin days | 1.117 | 1.045–1.193 | 0.001 | 1.077 | 1.044–1.110 | <0.0001 |
| A. baumannii | 7.083 | 4.637–10.81 | <0.0001 | 6.786 | 4.495–10.245 | <0.0001 |
| P. aeruginosa | 2.367 | 1.585–3.534 | <0.0001 | 2.240 | 1.516–3.310 | <0.0001 |
| K. pneumoniae | 3.045 | 2.046–4.530 | <0.0001 | 3.023 | 2.037–4.483 | <0.0001 |
| E. coli | 1.890 | 1.166–3.060 | 0.010 | 1.889 | 1.171–3.045 | 0.009 |
| Co-infection with colistin-resistant GNB | 1.690 | 1.050–2.719 | 0.031 | 1.626 | 1.016–2.600 | 0.043 |
| Wound culture | 0.439 | 0.228–0.842 | 0.013 | 0.315 | 0.214–0.463 | <0.0001 |
| Endotracheal aspirate | 4.552 | 2.290–9.047 | <0.0001 | 3.152 | 2.077–4.782 | <0.0001 |
| Urine culture | 0.861 | 0.352–2.105 | 0.743 | |||
| Blood culture | 2.418 | 0.957–6.108 | 0.062 | |||
| Fluid culture | 2.300 | 0.983–5.382 | 0.055 | |||
| Abscess culture | 1.195 | 0.430–3.312 | 0.732 | |||
| Variable (Delta) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender (M) | 0.938 | 0.555–1.584 | 0.811 | |||
| Age | 1.037 | 1.018–1.056 | <0.0001 | 1.027 | 1.010–1.043 | 0.001 |
| Hospitalization days | 0.953 | 0.933–0.971 | <0.0001 | 0.975 | 0.961–0.988 | <0.0001 |
| Colistin dose | 1.001 | 0.992–1.009 | 0.811 | |||
| Colistin days | 1.091 | 0.989–1.203 | 0.081 | |||
| A. baumannii | 6.620 | 3.344–13.102 | <0.0001 | 5.548 | 3.070–10.024 | <0.0001 |
| P. aeruginosa | 2.046 | 1.086–3.850 | 0.027 | 1.709 | 0.997–2.926 | 0.051 |
| K. pneumoniae | 1.444 | 0.807–2.579 | 0.215 | |||
| E. coli | 1.357 | 0.610–3.017 | 0.454 | |||
| Co-infection with colistin-resistant GNB | 1.477 | 0.656–3.324 | 0.346 | |||
| Wound culture | 0.534 | 0.129–2.206 | 0.386 | |||
| Endotracheal aspirate | 4.369 | 1.029–18.533 | 0.046 | 6.159 | 3.499–10.838 | <0.0001 |
| Urine culture | 0.677 | 0.112–4.087 | 0.671 | |||
| Blood culture | 3.683 | 0.689–19.662 | 0.127 | |||
| Fluid culture | 3.823 | 0.692–21.109 | 0.124 | |||
| Abscess culture | 0.198 | 0.016–2.378 | 0.202 | |||
| SARS-CoV-2 infection confirmed | 2.011 | 1.046–3.864 | 0.036 | 1.827 | 1.002–3.346 | 0.049 |
| Variable (Omicron) | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender (M) | 0.854 | 0.537–1.356 | 0.504 | |||
| Age | 1.044 | 1.027–1.060 | <0.0001 | 1.031 | 1.014–1.048 | <0.0001 |
| Hospitalization days | 0.952 | 0.935–0.968 | <0.0001 | 0.954 | 0.935–0.972 | <0.0001 |
| Colistin dose | 1.011 | 1.004–1.018 | 0.002 | 1.008 | 1.004–1.012 | <0.0001 |
| Colistin days | 1.032 | 0.955–1.113 | 0.424 | |||
| A. baumannii | 3.365 | 1.812–6.250 | <0.0001 | 3.809 | 2.214–6.551 | <0.0001 |
| P. aeruginosa | 1.242 | 0.692–2.227 | 0.468 | |||
| K. pneumoniae | 4.731 | 2.672–8.374 | <0.0001 | 1.091 | 0.649–1.833 | 0.742 |
| E. coli | 4.091 | 2.029–8.245 | <0.0001 | 1.000 | 0.496–2.010 | 0.999 |
| Co-infection with colistin-resistant GNB | 1.867 | 0.814–4.282 | 0.140 | |||
| Wound culture | 0.602 | 0.208–1.738 | 0.348 | |||
| Endotracheal aspirate | 12.399 | 3.858–39.842 | <0.0001 | 5.949 | 3.358–10.535 | <0.0001 |
| Urine culture | 0.776 | 0.218–2.752 | 0.695 | |||
| Blood culture | 2.043 | 0.613–6.805 | 0.245 | |||
| Fluid culture | 1.232 | 0.327–4.628 | 0.758 | |||
| Abscess culture | 2.066 | 0.422–10.093 | 0.370 | |||
| SARS-CoV-2 infection confirmed | 1.125 | 0.471–2.683 | 0.790 | |||
| Variable (Post). | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Gender (M) | 1.064 | 0.469–2.410 | 0.882 | |||
| Age | 1.072 | 1.038–1.107 | <0.0001 | 1.069 | 1.037–1.101 | <0.0001 |
| Hospitalization days | 0.943 | 0.910–0.975 | 0.001 | 0.943 | 0.913–0.973 | <0.0001 |
| Colistin dose | 1.016 | 1.003–1.027 | 0.012 | 1.008 | 1.002–1.014 | 0.007 |
| Colistin days | 0.911 | 0.795–1.043 | 0.177 | |||
| A. baumannii | 11.610 | 3.255- | <0.0001 | 7.998 | 3.368–18.987 | <0.0001 |
| P. aeruginosa | 1.479 | 0.452–4.835 | 0.517 | |||
| K. pneumoniae | 6.368 | 2.104–19.263 | 0.001 | 4.644 | 1.967–10.959 | <0.0001 |
| E. coli | 1.800 | 0.439–7.372 | 0.414 | |||
| Co-infection with colistin-resistant GNB | 7.860 | 1.774–34.816 | 0.007 | 10.087 | 2.654–38.329 | 0.001 |
| Wound culture | 0.099 | 0.024–0.394 | 0.001 | 0.105 | 0.046–0.233 | <0.0001 |
| Endotracheal aspirate | 0.887 | 0.229–3.427 | 0.862 | |||
| Urine culture | 0.505 | 0.081–3.127 | 0.463 | |||
| Blood culture | 2.361 | 0.432–12.890 | 0.321 | |||
| Fluid culture | 1.437 | 0.189–10.880 | 0.726 | |||
| Abscess culture | 2.392 | 0.135–42.221 | 0.552 | |||
| SARS-CoV-2 infection confirmed | 6.623 | 0.834–52.545 | 0.074 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlad, M.A.; Iancu, L.S.; Dorneanu, O.S.; Duhaniuc, A.; Pavel-Tanasa, M.; Tuchilus, C.G. Colistin Treatment Outcomes in Gram-Negative Bacterial Infections in the Northeast of Romania: A Decade of Change Through Pandemic Challenges. Antibiotics 2025, 14, 275. https://doi.org/10.3390/antibiotics14030275
Vlad MA, Iancu LS, Dorneanu OS, Duhaniuc A, Pavel-Tanasa M, Tuchilus CG. Colistin Treatment Outcomes in Gram-Negative Bacterial Infections in the Northeast of Romania: A Decade of Change Through Pandemic Challenges. Antibiotics. 2025; 14(3):275. https://doi.org/10.3390/antibiotics14030275
Chicago/Turabian StyleVlad, Madalina Alexandra, Luminita Smaranda Iancu, Olivia Simona Dorneanu, Alexandru Duhaniuc, Mariana Pavel-Tanasa, and Cristina Gabriela Tuchilus. 2025. "Colistin Treatment Outcomes in Gram-Negative Bacterial Infections in the Northeast of Romania: A Decade of Change Through Pandemic Challenges" Antibiotics 14, no. 3: 275. https://doi.org/10.3390/antibiotics14030275
APA StyleVlad, M. A., Iancu, L. S., Dorneanu, O. S., Duhaniuc, A., Pavel-Tanasa, M., & Tuchilus, C. G. (2025). Colistin Treatment Outcomes in Gram-Negative Bacterial Infections in the Northeast of Romania: A Decade of Change Through Pandemic Challenges. Antibiotics, 14(3), 275. https://doi.org/10.3390/antibiotics14030275





