The Clinical Implications of Inappropriate Therapy in Community-Onset Urinary Tract Infections and the Development of a Bayesian Hierarchical Weighted-Incidence Syndromic Combination Antibiogram
Abstract
1. Introduction
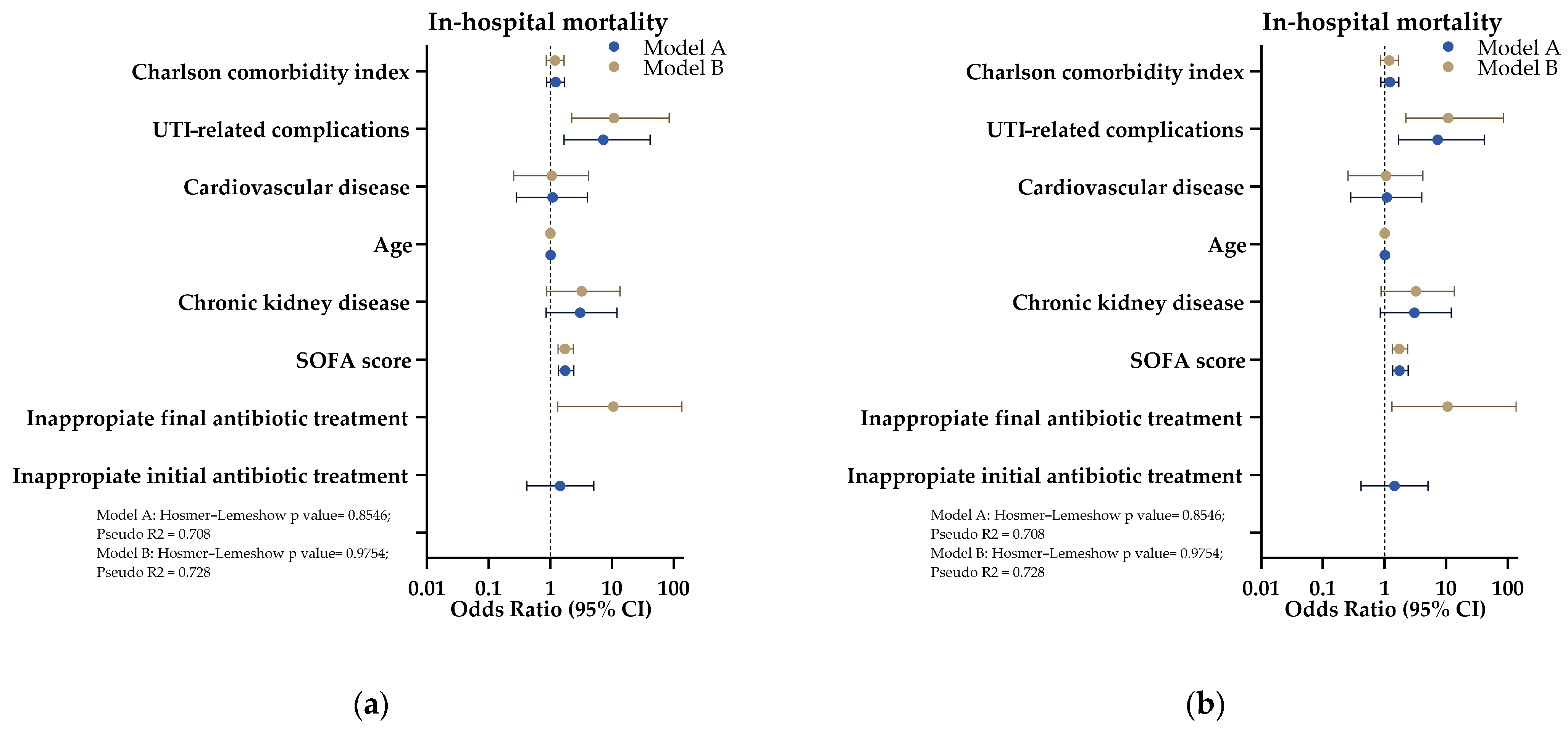
2. Results
3. Discussion
4. Materials and Methods
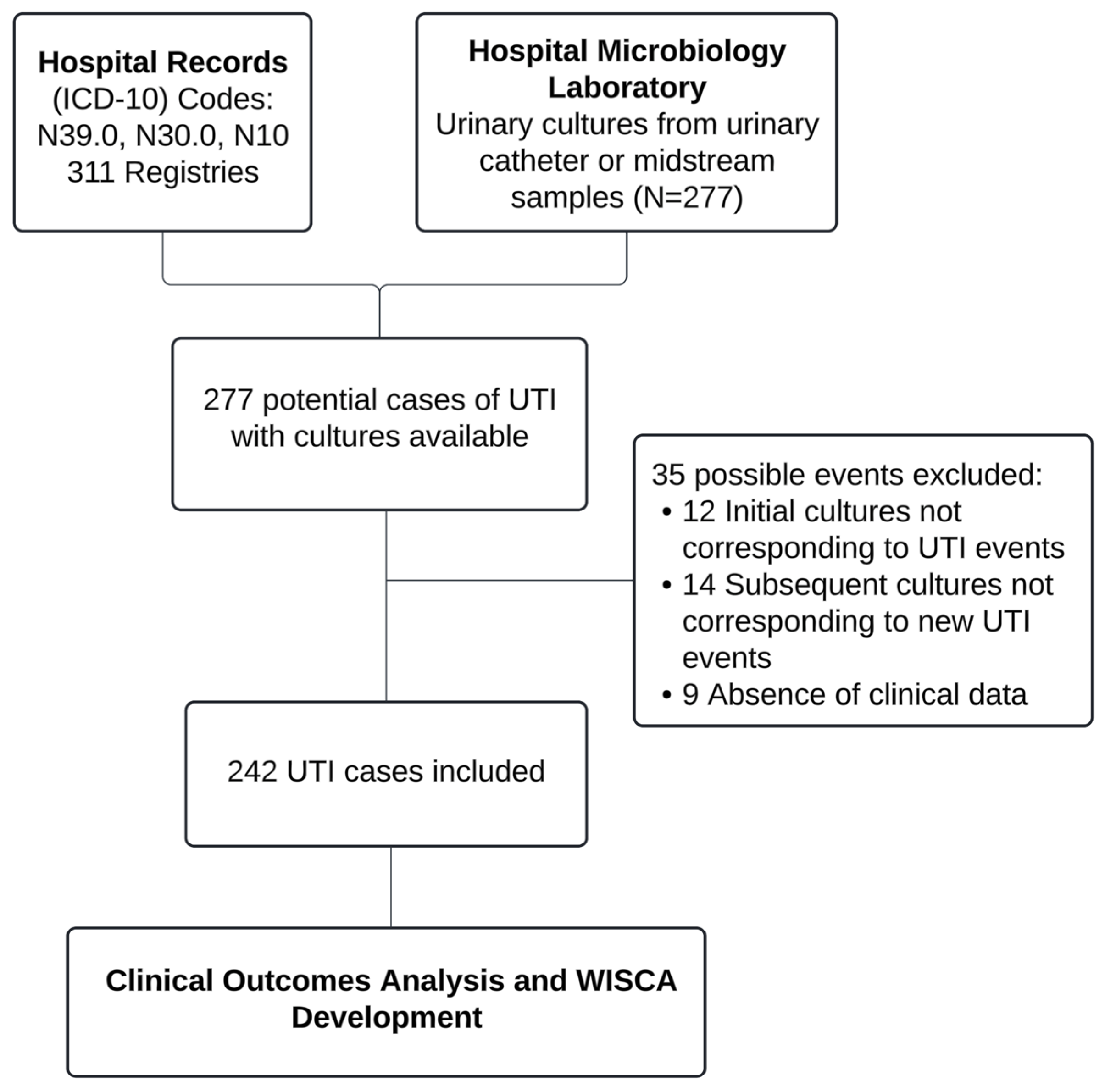
4.1. Population and Eligibility Criteria
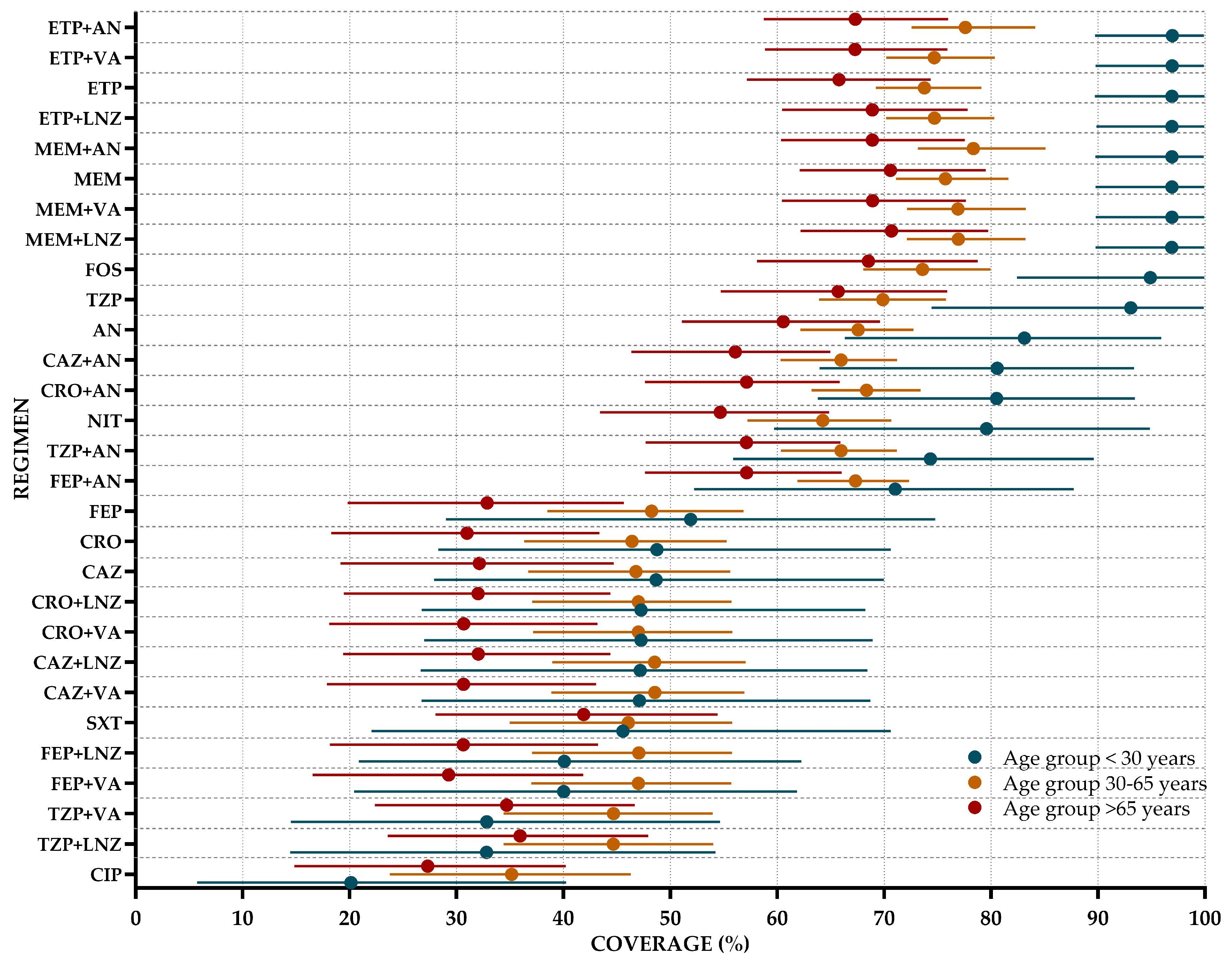
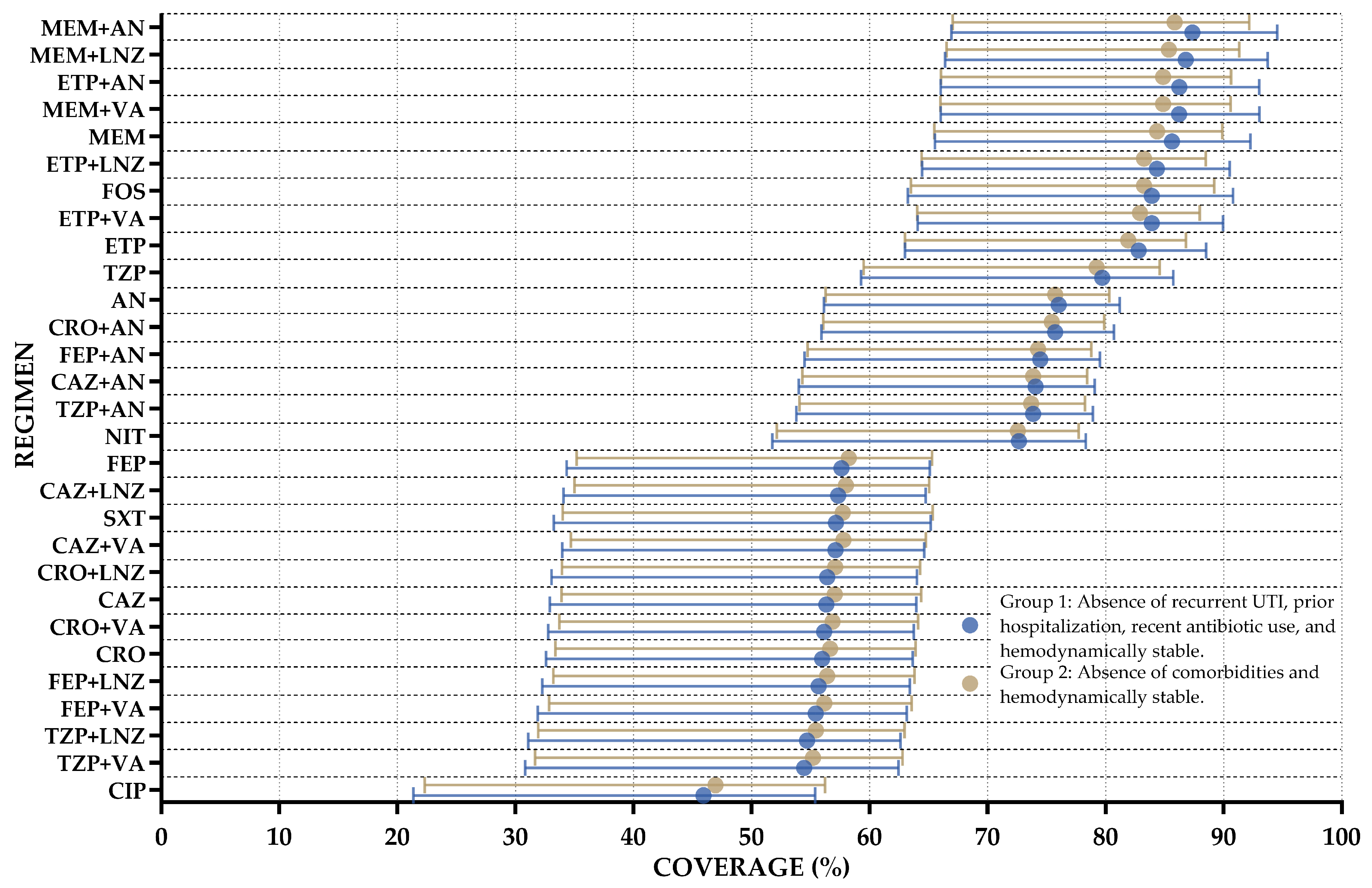
4.2. WISCA Development
4.3. Statistical Analysis
- Fixed effects for age strata (<30 years, 30–65 years, and >65 years) and sex, capturing systematic coverage differences across these patient subgroups;
- Random effects for both regimen and pathogen, allowing each regimen and pathogen to share information with one another, thereby reducing the uncertainty in regimens or pathogens with few observations.
- coveragei ∈ {0,1}: an indicator that regimen j successfully covers pathogen k in the i-th observation.
- Age and sex: patient-level covariates (age group and sex).
- α0: overall intercept.
- αpathogen[k]: random intercept for pathogen k.
- αregimen[j]: random intercept for regimen j.
- β1,β2: fixed-effect coefficients for age group and sex, respectively.
- Intercept (α0): e.g., α0 ~ Student-t(ν = 3, μ = 0, σ = 10).
- Fixed effects (β1, β2): e.g., β ~ Student-t(3,0,1) or normal(0,1).
- Random effects’ standard deviations (αpathogen, αregimen): e.g., half-Student-t or half-normal, such as
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| AMR | antimicrobial resistance |
| AN | amikacin |
| CAZ | ceftazidime |
| CCI | Charlson comorbidity index |
| CI | Confidence Interval |
| CIP | ciprofloxacin |
| CKD | chronic kidney disease |
| CLSI | Clinical and Laboratory Standards Institute |
| CoUTIs | community-onset urinary tract infections |
| CRO | ceftriaxone |
| CRBS | Catheter-Related Bloodstream Infection |
| CVD | cardiovascular disease |
| ESBL | extended-spectrum beta-lactamase |
| ESC-R EB | extended-spectrum cephalosporin-resistant Enterobacterales |
| ETP | ertapenem |
| FEP | cefepime |
| FOS | fosfomycin |
| HDI | highest density interval |
| HMC | Hamiltonian Monte Carlo |
| IIAT | inappropriate initial antibiotic treatment |
| IFAT | inappropriate final antibiotic treatment |
| ICD-10 | International Classification of Diseases 10th Edition |
| ICU | Intensive Care Unit |
| IQR | Interquartile Range |
| LNZ | linezolid |
| MCMC | Monte Carlo Markov chain |
| MDR | multidrug-resistant |
| MDROs | multidrug-resistant organisms |
| MEM | meropenem |
| MIC | minimum inhibitory concentration |
| MRSA | methicillin-resistant Staphylococcus aureus |
| NIT | nitrofurantoin |
| OR | Odds Ratio |
| SD | standard deviation |
| SOFA | Sequential Organ Failure Assessment |
| SXT | trimethoprim/sulfamethoxazole |
| TZP | piperacillin/tazobactam |
| UTI | urinary tract infection |
| VA | vancomycin |
| WISCA | weighted-incidence syndromic combination antibiogram |
References
- Zeng, Z.; Zhan, J.; Zhang, K.; Chen, H.; Cheng, S. Global, regional, and national burden of urinary tract infections from 1990 to 2019: An analysis of the global burden of disease study 2019. World J. Urol. 2022, 40, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.W.; Puttagunta, S.; Aronin, S.I.; Brossette, S.; Murray, J.; Gupta, V. Impact of Empirical Antibiotic Therapy on Outcomes of Outpatient Urinary Tract Infection Due to Nonsusceptible Enterobacterales. Microbiol. Spectr. 2022, 10, e0235921. [Google Scholar] [CrossRef] [PubMed]
- Poor Clinical Outcomes Associated with Community-Onset Urinary Tract Infections Due to Extended-Spectrum Cephalosporin-Resistant Enterobacteriaceae|Infection Control & Hospital Epidemiology|Cambridge Core. Available online: https://www.cambridge.org/core/journals/infection-control-and-hospital-epidemiology/article/abs/poor-clinical-outcomes-associated-with-communityonset-urinary-tract-infections-due-to-extendedspectrum-cephalosporinresistant-enterobacteriaceae/677DF43C020E9A13BE5DA7A929D53DAC (accessed on 11 January 2025).
- Chiu, C.-C.; Lin, T.-C.; Wu, R.-X.; Yang, Y.-S.; Hsiao, P.-J.; Lee, Y.; Lin, J.-C.; Chang, F.-Y. Etiologies of Community-Onset Urinary Tract Infections Requiring Hospitalization and Antimicrobial Susceptibilities of Causative Microorganisms. J. Microbiol. Immunol. Infect. 2017, 50, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.; Kassis, E.; Reinhertz, G.; Gorenstein, S.; Herman, P. Community-Acquired Urinary Tract Infection in Adults: A Hospital Viewpoint. J. Hosp. Infect. 1998, 38, 193–202. [Google Scholar] [CrossRef]
- Lu, P.-L.; Liu, Y.-C.; Toh, H.-S.; Lee, Y.-L.; Liu, Y.-M.; Ho, C.-M.; Huang, C.-C.; Liu, C.-E.; Ko, W.-C.; Wang, J.-H.; et al. Epidemiology and Antimicrobial Susceptibility Profiles of Gram-Negative Bacteria Causing Urinary Tract Infections in the Asia-Pacific Region: 2009–2010 Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 2012, 40, S37–S43. [Google Scholar] [CrossRef] [PubMed]
- Fasugba, O.; Mitchell, B.G.; Mnatzaganian, G.; Das, A.; Collignon, P.; Gardner, A. Five-Year Antimicrobial Resistance Patterns of Urinary Escherichia coli at an Australian Tertiary Hospital: Time Series Analyses of Prevalence Data. PLoS ONE 2016, 11, e0164306. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Nathanson, B.H.; Sulham, K.; Shorr, A.F. Antimicrobial Susceptibility and Cross-Resistance Patterns among Common Complicated Urinary Tract Infections in U.S. Hospitals, 2013 to 2018. Antimicrob. Agents Chemother. 2020, 64, e00346-20. [Google Scholar] [CrossRef]
- Von Vietinghoff, S.; Shevchuk, O.; Dobrindt, U.; Engel, D.R.; Jorch, S.K.; Kurts, C.; Miethke, T.; Wagenlehner, F. The Global Burden of Antimicrobial Resistance—Urinary Tract Infections. Nephrol. Dial. Transplant. 2024, 39, 581–588. [Google Scholar] [CrossRef]
- Stapleton, A.E.; Wagenlehner, F.M.E.; Mulgirigama, A.; Twynholm, M. Escherichia coli Resistance to Fluoroquinolones in Community-Acquired Uncomplicated Urinary Tract Infection in Women: A Systematic Review. Antimicrob. Agents Chemother. 2020, 64, e00862-20. [Google Scholar] [CrossRef]
- Madrazo, M.; Esparcia, A.; López-Cruz, I.; Alberola, J.; Piles, L.; Viana, A.; Eiros, J.M.; Artero, A. Clinical Impact of Multidrug-Resistant Bacteria in Older Hospitalized Patients with Community-Acquired Urinary Tract Infection. BMC Infect. Dis. 2021, 21, 1232. [Google Scholar] [CrossRef]
- Silago, V.; Moremi, N.; Mtebe, M.; Komba, E.; Masoud, S.; Mgaya, F.X.; Mirambo, M.M.; Nyawale, H.A.; Mshana, S.E.; Matee, M.I. Multidrug-Resistant Uropathogens Causing Community Acquired Urinary Tract Infections among Patients Attending Health Facilities in Mwanza and Dar Es Salaam, Tanzania. Antibiotics 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Costa, E.; Freitas, A.; Almeida, A. Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics 2022, 11, 768. [Google Scholar] [CrossRef]
- Shields, R.K.; Zhou, Y.; Kanakamedala, H.; Cai, B. Burden of Illness in US Hospitals Due to Carbapenem-Resistant Gram-Negative Urinary Tract Infections in Patients with or without Bacteraemia. BMC Infect. Dis. 2021, 21, 572. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Sader, H.S.; Jones, R.N.; SENTRY Participants Group (Latin America). Urinary Tract Infection Trends in Latin American Hospitals: Report from the SENTRY Antimicrobial Surveillance Program (1997–2000). Diagn. Microbiol. Infect. Dis. 2002, 44, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Garza-González, E.; Bocanegra-Ibarias, P.; Bobadilla-del-Valle, M.; Ponce-de-León-Garduño, L.A.; Esteban-Kenel, V.; Silva-Sánchez, J.; Garza-Ramos, U.; Barrios-Camacho, H.; López-Jácome, L.E.; Colin-Castro, C.A.; et al. Drug Resistance Phenotypes and Genotypes in Mexico in Representative Gram-Negative Species: Results from the Infivar Network. PLoS ONE 2021, 16, e0248614. [Google Scholar] [CrossRef]
- Hebert, C.; Ridgway, J.; Vekhter, B.; Brown, E.C.; Weber, S.G.; Robicsek, A. Demonstration of the Weighted-Incidence Syndromic Combination Antibiogram: An Empiric Prescribing Decision Aid. Infect. Control Hosp. Epidemiol. 2012, 33, 381–388. [Google Scholar] [CrossRef]
- Randhawa, V.; Sarwar, S.; Walker, S.; Elligsen, M.; Palmay, L.; Daneman, N. Weighted-Incidence Syndromic Combination Antibiograms to Guide Empiric Treatment of Critical Care Infections: A Retrospective Cohort Study. Crit. Care 2014, 18, R112. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Bottigliengo, D.; Tellini, M.; Minotti, C.; Marchiori, M.; Cavicchioli, P.; Gregori, D.; Giaquinto, C.; Da Dalt, L.; Donà, D. Development of a Weighted-Incidence Syndromic Combination Antibiogram (WISCA) to Guide the Choice of the Empiric Antibiotic Treatment for Urinary Tract Infection in Paediatric Patients: A Bayesian Approach. Antimicrob. Resist. Infect. Control 2021, 10, 74. [Google Scholar] [CrossRef]
- Tandogdu, Z.; Kakariadis, E.T.A.; Naber, K.; Wagenlehner, F.; Bjerklund Johansen, T.E. Appropriate Empiric Antibiotic Choices in Health Care Associated Urinary Tract Infections in Urology Departments in Europe from 2006 to 2015: A Bayesian Analytical Approach Applied in a Surveillance Study. PLoS ONE 2019, 14, e0214710. [Google Scholar] [CrossRef]
- Ridgway, J.P.; Robicsek, A.; Shah, N.; Smith, B.A.; Singh, K.; Semel, J.; Acree, M.E.; Grant, J.; Ravichandran, U.; Peterson, L.R. A Randomized Controlled Trial of an Electronic Clinical Decision Support Tool for Inpatient Antimicrobial Stewardship. Clin. Infect. Dis. 2021, 72, e265–e271. [Google Scholar] [CrossRef] [PubMed]
- Bielicki, J.A.; Sharland, M.; Heath, P.T.; Walker, A.S.; Agarwal, R.; Turner, P.; Cromwell, D.A. Evaluation of the Coverage of 3 Antibiotic Regimens for Neonatal Sepsis in the Hospital Setting Across Asian Countries. JAMA Netw. Open 2020, 3, e1921124. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.C.M.; Jones, M.; Snelling, T.L.; Duguid, R.; Moore, N.; Dickson, B.; Wu, Y.; Saunders, J.; Wijeratne, P.; Douangnouvong, A.; et al. Coverage Gaps in Empiric Antibiotic Regimens Used to Treat Serious Bacterial Infections in Neonates and Children in Southeast Asia and the Pacific. Lancet Reg. Health Southeast Asia 2024, 22, 100291. [Google Scholar] [CrossRef]
- Liberati, C.; Donà, D.; Maestri, L.; Petris, M.G.; Barbieri, E.; Gallo, E.; Gallocchio, J.; Pierobon, M.; Calore, E.; Zin, A.; et al. Application of the Weighted-Incidence Syndromic Combination Antibiogram (WISCA) to Guide the Empiric Antibiotic Treatment of Febrile Neutropenia in Oncological Paediatric Patients: Experience from Two Paediatric Hospitals in Northern Italy. Ann. Clin. Microbiol. Antimicrob. 2024, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Cook, A.; Sharland, M.; Yau, Y.; Group, P.; Bielicki, J.; PediBSI Group. Improving Empiric Antibiotic Prescribing in Pediatric Bloodstream Infections: A Potential Application of Weighted-Incidence Syndromic Combination Antibiograms (WISCA). Expert Rev. Anti Infect. Ther. 2022, 20, 445–456. [Google Scholar] [CrossRef]
- Bielicki, J.A.; Sharland, M.; Johnson, A.P.; Henderson, K.L.; Cromwell, D.A. Antibiotic Resistance and Prescribing in European Children project Selecting Appropriate Empirical Antibiotic Regimens for Paediatric Bloodstream Infections: Application of a Bayesian Decision Model to Local and Pooled Antimicrobial Resistance Surveillance Data. J. Antimicrob. Chemother. 2016, 71, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Salm, J.; Ikker, F.; Noory, E.; Beschorner, U.; Kramer, T.S.; Westermann, D.; Zeller, T. Weighted-Incidence Syndromic Combination Antibiogram (WISCA) to Support Empirical Antibiotic Therapy Decisions in Infected Ischemic Leg Ulcers—A Feasibility Study. J. Clin. Med. 2024, 13, 6219. [Google Scholar] [CrossRef]
- Eliakim-Raz, N.; Babitch, T.; Shaw, E.; Addy, I.; Wiegand, I.; Vank, C.; Torre-Vallejo, L.; Joan-Miquel, V.; Steve, M.; Grier, S.; et al. Risk Factors for Treatment Failure and Mortality Among Hospitalized Patients with Complicated Urinary Tract Infection: A Multicenter Retrospective Cohort Study (RESCUING Study Group). Clin. Infect. Dis. 2019, 68, 29–36. [Google Scholar] [CrossRef]
- Reuken, P.A.; Stallmach, A.; Bruns, T. Mortality after Urinary Tract Infections in Patients with Advanced Cirrhosis—Relevance of Acute Kidney Injury and Comorbidities. Liver Int. 2013, 33, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, V.; Malini, A. Antimicrobial Resistance Pattern in Escherichia coli Causing Urinary Tract Infection among Inpatients. Indian J. Med. Res. 2014, 139, 945–948. [Google Scholar] [PubMed]
- Seifert, H.; von Linstow, M.-L.; Janssen, H.; Dowzicky, M. Antimicrobial Susceptibility among Gram-Negative Isolates in Pediatric Patients in Europe from 2013–2018 Compared to 2004–2012: Results from the ATLAS Surveillance Study. Int. J. Antimicrob. Agents 2021, 58, 106441. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Zhao, C.; Tiseo, K.; Pires, J.; Van Boeckel, T.P. Predictive Mapping of Antimicrobial Resistance for Escherichia Coli, Salmonella, and Campylobacter in Food-Producing Animals, Europe, 2000–2021. Emerg. Infect. Dis. 2024, 30, 96–104. [Google Scholar] [CrossRef]
- Álvarez-Marín, R.; López-Cerero, L.; Guerrero-Sánchez, F.; Palop-Borras, B.; Rojo-Martín, M.D.; Ruiz-Sancho, A.; Herrero-Rodríguez, C.; García, M.V.; Lazo-Torres, A.M.; López, I.; et al. Do Specific Antimicrobial Stewardship Interventions Have an Impact on Carbapenem Resistance in Gram-Negative Bacilli? A Multicentre Quasi-Experimental Ecological Study: Time-Trend Analysis and Characterization of Carbapenemases. J. Antimicrob. Chemother. 2021, 76, 1928–1936. [Google Scholar] [CrossRef]
- Rice, L.B. Antimicrobial Stewardship and Antimicrobial Resistance. Med. Clin. N. Am. 2018, 102, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Esparcia, A.; Artero, A.; Eiros, J.M.; Balaguer, M.; Madrazo, M.; Alberola, J.; Nogueira, J.M. Influence of Inadequate Antimicrobial Therapy on Prognosis in Elderly Patients with Severe Urinary Tract Infections. Eur. J. Intern. Med. 2014, 25, 523–527. [Google Scholar] [CrossRef]
- Álvarez-Artero, E.; Campo-Nuñez, A.; García-García, I.; García-Bravo, M.; Cores-Calvo, O.; Galindo-Pérez, I.; Pendones-Ulerio, J.; López-Bernus, A.; Belhassen-García, M.; Pardo-Lledías, J. Urinary Tract Infection Caused by Enterococcus spp.: Risk Factors and Mortality. An Observational Study. Rev. Clin. Esp. 2021, 221, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Lamas Ferreiro, J.L.; Álvarez Otero, J.; González González, L.; Novoa Lamazares, L.; Arca Blanco, A.; Bermúdez Sanjurjo, J.R.; Rodríguez Conde, I.; Fernández Soneira, M.; de la Fuente Aguado, J. Pseudomonas Aeruginosa Urinary Tract Infections in Hospitalized Patients: Mortality and Prognostic Factors. PLoS ONE 2017, 12, e0178178. [Google Scholar] [CrossRef]
- Ronco, C.; Bellomo, R.; Kellum, J.A. Acute Kidney Injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-L.; Hsiao, C.-Y.; Wu, C.-Y.; Yen, C.-L.; Tsai, C.-Y.; Jenq, C.-C.; Lin, H.-L. Delayed Fever and Acute Kidney Injury in Patients with Urinary Tract Infection. J. Clin. Med. 2020, 9, 3486. [Google Scholar] [CrossRef]
- Rouphael, N.; Winokur, P.; Keefer, M.C.; Traenkner, J.; Drobeniuc, A.; Doi, Y.; Munsiff, S.; Fowler, V.G.; Evans, S.; Oler, R.E.; et al. Daily Fosfomycin versus Levofloxacin for Complicated Urinary Tract Infections. mBio 2023, 14, e0167723. [Google Scholar] [CrossRef]
- Hatlen, T.J.; Flor, R.; Nguyen, M.H.; Lee, G.H.; Miller, L.G. Oral Fosfomycin Use for Pyelonephritis and Complicated Urinary Tract Infections: A 1 Year Review of Outcomes and Prescribing Habits in a Large Municipal Healthcare System. J. Antimicrob. Chemother. 2020, 75, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Pogue, J.M.; Mohr, J.F. Intravenous Fosfomycin for the Treatment of Hospitalized Patients with Serious Infections. Expert. Rev. Anti Infect. Ther. 2017, 15, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Burgos, R.M.; Rodvold, K.A. ZTI-01 (Fosfomycin for Injection) in the Treatment of Hospitalized Patients with Complicated Urinary Tract Infections. Future Microbiol. 2019, 14, 461–475. [Google Scholar] [CrossRef] [PubMed]
- ten Doesschate, T.; van Werkhoven, H.; Meijvis, S.; Stalenhoef, J.; van Zuilen, A.; de Vries, A.; Bonten, M. Fosfomycin-Trometamol for Urinary Tract Infections in Kidney Transplant Recipients. Transplantation 2019, 103, 1272. [Google Scholar] [CrossRef] [PubMed]
- Zykov, I.N.; Samuelsen, Ø.; Jakobsen, L.; Småbrekke, L.; Andersson, D.I.; Sundsfjord, A.; Frimodt-Møller, N. Pharmacokinetics and Pharmacodynamics of Fosfomycin and Its Activity against Extended-Spectrum-β-Lactamase-, Plasmid-Mediated AmpC-, and Carbapenemase-Producing Escherichia coli in a Murine Urinary Tract Infection Model. Antimicrob. Agents Chemother. 2018, 62, e02560-17. [Google Scholar] [CrossRef]
- Bouiller, K.; Zayet, S.; Lalloz, P.-E.; Potron, A.; Gendrin, V.; Chirouze, C.; Klopfenstein, T. Efficacy and Safety of Oral Fosfomycin-Trometamol in Male Urinary Tract Infections with Multidrug-Resistant Enterobacterales. Antibiotics 2022, 11, 198. [Google Scholar] [CrossRef]
- Babiker, A.; Clarke, L.; Doi, Y.; Shields, R.K. Fosfomycin for Treatment of Multidrug-Resistant Pathogens Causing Urinary Tract Infection: A Real-World Perspective and Review of the Literature. Diagn. Microbiol. Infect. Dis. 2019, 95, 114856. [Google Scholar] [CrossRef] [PubMed]
- McCowan, C.; Bakhshi, A.; McConnachie, A.; Malcolm, W.; Barry, S.J.; Santiago, V.H.; Leanord, A.E. Coli Bacteraemia and Antimicrobial Resistance Following Antimicrobial Prescribing for Urinary Tract Infection in the Community. BMC Infect. Dis. 2022, 22, 805. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Hsiao, C.-Y.; Hung, M.-C.; Hung, S.-C.; Wang, H.-P.; Huang, Y.-J.; Wang, J.-T. Bacteremic Urinary Tract Infection Caused by Multidrug-Resistant Enterobacteriaceae Are Associated with Severe Sepsis at Admission: Implication for Empirical Therapy. Medicine 2016, 95, e3694. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.Q.; Nguyen, N.T.Q.; Hughes, C.M.; O’Neill, C. Trends and Impact of Antimicrobial Resistance on Older Inpatients with Urinary Tract Infections (UTIs): A National Retrospective Observational Study. PLoS ONE 2019, 14, e0223409. [Google Scholar] [CrossRef]
- Smithson, A.; Ramos, J.; Niño, E.; Culla, A.; Pertierra, U.; Friscia, M.; Bastida, M.T. Characteristics of Febrile Urinary Tract Infections in Older Male Adults. BMC Geriatr. 2019, 19, 334. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef] [PubMed]
- Kranz, J.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; Wagenlehner, F.M.E.; et al. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur. Urol. 2024, 86, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, L.E.; AMMI Canada Guidelines Committee. Complicated Urinary Tract Infection in Adults. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; Lewis, J.S., II, Weinstein, M.P., Bobenchik, A.M., Campeau, S., Cullen, S.K., Dingle, T., Galas, M.F., Humphries, R.M., Kirn, T.J., Jr., Limbago, B., et al., Eds.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024; Volume M100, pp. 59–67. [Google Scholar]
- Frimodt-Møller, N.; Bjerrum, L. Treating Urinary Tract Infections in the Era of Antibiotic Resistance. Expert Rev. Anti Infect. Ther. 2023, 21, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines on Urological Infections—Uroweb. Available online: https://uroweb.org/guidelines/urological-infections/chapter/the-guideline (accessed on 11 January 2025).
- Salles, M.J.C.; Zurita, J.; Mejía, C.; Villegas, M.V. Latin America Working Group on Bacterial Resistance Resistant Gram-Negative Infections in the Outpatient Setting in Latin America. Epidemiol. Infect. 2013, 141, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Monrreal, M.G.; Mendez-Pfeiffer, P.; Barrios-Villa, E.; Arenas-Hernández, M.M.P.; Enciso-Martínez, Y.; Sepúlveda-Moreno, C.O.; Bolado-Martínez, E.; Valencia, D. Uropathogenic Escherichia coli in Mexico, an Overview of Virulence and Resistance Determinants: Systematic Review and Meta-Analysis. Arch. Med. Res. 2023, 54, 247–260. [Google Scholar] [CrossRef]


| Variable | Obs. (n = 242) | Total (n = 242) | Women (n = 165) | Men (n = 77) | Significance * |
|---|---|---|---|---|---|
| Sociodemographic and clinical characteristics | |||||
| Age—mean (IQR) | 165/77 | 53 (40.0–63.0) | 51 (38.0–63.0) | 55.0 (47.0–67.0) | * |
| Complicated UTI—n (%) | 165/77 | 240 (99.17) | 163 (98.78) | 77 (100.00) | NS |
| Permanent urinary catheter—n (%) | 165/77 | 28 (11.57) | 11 (6.66) | 17 (22.08) | NS |
| Recurrent UTI—n (%) | 165/77 | 93 (38.43) | 58 (35.2) | 35 (45.45) | NS |
| Pyelonephritis—n (%) | 165/77 | 235 (97.11) | 158 (95.8) | 77 (100.00) | NS |
| Initial inappropriate antibiotic treatment—n (%) | 164/75 | 72 (29.75) | 44 (26.67) | 28 (36.36) | NS |
| Days to correction of empirical treatment—median (IQR) | 44/28 | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.00 (2.75–4.0) | NS |
| Inappropriate final antibiotic treatment in UTI event—n (%) | 164/77 | 20 (8.26) | 11 (6.67) | 9 (11.69) | NS |
| Previous antibiotic treatment within 90 days—n (%) | 165/77 | 88 (36.36) | 50 (30.30) | 38 (49.35) | NS |
| Previous hospitalization within 90 days—n (%) | 165/77 | 108 (44.63) | 69 (41.82) | 39 (50.65) | NS |
| Severity of illness | |||||
| Hypotension—n (%) | 165/77 | 56 (23.14) | 37 (22.42) | 19 (24.68) | NS |
| Need for vasopressors—n (%) | 165/77 | 45 (18.59) | 31 (18.79) | 14 (18.18) | NS |
| SOFA score—median (IQR) | 165/77 | 2 (0.0–6.0) | 2 (0.0–6.0) | 3.0 (0.0–7.0) | NS |
| Comorbidities | 165/77 | 190 (78.51) | 129 (78.18) | 61 (79.22) | NS |
| Charlson comorbidity index—median (IQR) | 165/77 | 3 (1.0–5.0) | 3 (1.0–5.0) | 3 (2.0–5.0) | NS |
| Diabetes mellitus—n (%) | 165/77 | 150 (61.98) | 105 (63.64) | 45 (58.44) | NS |
| Hypertension—n (%) | 165/77 | 82 (33.88) | 62 (37.58) | 20 (25.97) | NS |
| Cardiovascular disease—n (%) | 165/77 | 48 (19.83) | 35 (21.21) | 13 (16.88) | NS |
| Acute kidney injury—n (%) | 165/77 | 105 (43.39) | 65 (39.39) | 40 (51.95) | NS |
| Chronic kidney disease—n (%) | 165/77 | 68 (28.10) | 49 (29.70) | 19 (24.68) | NS |
| Chronic liver disease—n (%) | 165/77 | 4 (1.65) | 3 (1.82) | 1 (1.30) | NS |
| Pregnancy—n (%) | 165/77 | 9 (3.72) | 9 (5.45) | 0.0 (0.0) | NS |
| Immunosuppression—n (%) | 165/77 | 14 (5.79) | 10 (6.06) | 4 (5.95) | NS |
| Cancer—n (%) | 165/77 | 15 (6.20) | 11 (6.67) | 4 (5.95) | NS |
| Central nervous system neurological disease—n (%) | 165/77 | 28 (11.57) | 11 (6.67) | 17 (22.08) | NS |
| Previous diagnosis of peripheral neuropathy—n (%) | 165/77 | 26 (10.74) | 23 (13.94) | 3 (3.90) | * |
| Outcome | |||||
| Urinary tract infection-related complications | 165/77 | 68 (28.10) | 47 (28.48) | 21 (27.27) | NS |
| Local complication of UTI—n (%) | 165/77 | 29 (11.98) | 20 (12.12) | 9 (11.69) | NS |
| System complication of UTI—n (%) | 165/77 | 48 (19.83) | 34 (20.60) | 14 (18.18) | NS |
| Hospital stay—median (IQR) | 165/77 | 6.5 (4.0–10.0) | 7.0 (4.0–10.0) | 6.0 (3.0–12.0) | NS |
| Hospital stay > 15 days—n (%) | 165/77 | 29 (11.98) | 17 (10.30) | 12 (15.56) | NS |
| In-hospital mortality—n (%) | 165/77 | 34 (14.05) | 24 (14.55) | 10 (12.99) | NS |
| Variable | Obs. (n = 242) | Total (n = 242) | Women (n = 165) | Men (n = 77) | Significance * |
|---|---|---|---|---|---|
| Microorganism—n (%) | |||||
| Escherichia coli | 165/77 | 172 (71.07) | 129 (78.18) | 43 (55.84) | ** |
| Klebsiella pneumoniae | 165/77 | 24 (9.91) | 14 (8.48) | 10 (12.98) | NS |
| Pseudomonas aeruginosa | 165/77 | 11 (4.54) | 3 (1.81) | 8 (10.38) | ** |
| Proteus mirabilis | 165/77 | 7 (2.89) | 6 (3.63) | 1 (1.29) | NS |
| Candida glabrata | 165/77 | 6 (2.47) | 5 (3.03) | 1 (1.29) | NS |
| Enterococcus faecalis | 165/77 | 5 (2.06) | 1 (0.60) | 4 (5.19) | * |
| Citrobacter freundii | 165/77 | 4 (1.65) | 0 (0.00) | 4 (5.19) | * |
| Staphylococcus aureus | 165/77 | 3 (1.23) | 2 (1.21) | 1 (1.29) | NS |
| Candida tropicalis | 165/77 | 3 (1.23) | 1 (0.60) | 2 (2.59) | NS |
| Morganella morganii | 165/77 | 2 (0.82) | 0 (0.00) | 2 (2.59) | NS |
| Enterobacter cloacae | 165/77 | 1 (0.41) | 1 (0.60) | 0 (0.00) | NS |
| Staphylococcus saprophyticus | 165/77 | 1 (0.41) | 1 (0.60) | 0 (0.00) | NS |
| Acinetobacter baumannii complex | 165/77 | 1 (0.41) | 1 (0.60) | 0 (0.00) | NS |
| Candida parapsilosis | 165/77 | 1 (0.41) | 0 (0.00) | 1 (1.29) | NS |
| Enterococcus faecium | 165/77 | 1 (0.41) | 1 (0.60) | 0 (0.00) | NS |
| Resistance Profile—n (%) ** | |||||
| MDR Enterobacterales | 151/60 | 98 (46.44%) | 70 (46.35) | 28 (46.66) | NS |
| 3rd Gen Cephalosporin-Resistant Enterobacterales | 151/60 | 130 (61.61) | 91 (60.26) | 39 (65.0) | NS |
| MDR Pseudomonas | 8/3 | 3 (27.27) | 0 (0.00) | 3 (100) | NS |
| MDR Enterococcus | 2/4 | 1 (16.66) | 1 (50.0) | 0 (0.00) | NS |
| MRSA | 2/1 | 2 (66.66) | 2 (100) | 0 (0.00) | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Quiroz, A.; Avila-Cardenas, B.B.; De Arcos-Jiménez, J.C.; Perales-Guerrero, L.; Martínez-Ayala, P.; Briseno-Ramirez, J. The Clinical Implications of Inappropriate Therapy in Community-Onset Urinary Tract Infections and the Development of a Bayesian Hierarchical Weighted-Incidence Syndromic Combination Antibiogram. Antibiotics 2025, 14, 187. https://doi.org/10.3390/antibiotics14020187
Gómez-Quiroz A, Avila-Cardenas BB, De Arcos-Jiménez JC, Perales-Guerrero L, Martínez-Ayala P, Briseno-Ramirez J. The Clinical Implications of Inappropriate Therapy in Community-Onset Urinary Tract Infections and the Development of a Bayesian Hierarchical Weighted-Incidence Syndromic Combination Antibiogram. Antibiotics. 2025; 14(2):187. https://doi.org/10.3390/antibiotics14020187
Chicago/Turabian StyleGómez-Quiroz, Adolfo, Brenda Berenice Avila-Cardenas, Judith Carolina De Arcos-Jiménez, Leonardo Perales-Guerrero, Pedro Martínez-Ayala, and Jaime Briseno-Ramirez. 2025. "The Clinical Implications of Inappropriate Therapy in Community-Onset Urinary Tract Infections and the Development of a Bayesian Hierarchical Weighted-Incidence Syndromic Combination Antibiogram" Antibiotics 14, no. 2: 187. https://doi.org/10.3390/antibiotics14020187
APA StyleGómez-Quiroz, A., Avila-Cardenas, B. B., De Arcos-Jiménez, J. C., Perales-Guerrero, L., Martínez-Ayala, P., & Briseno-Ramirez, J. (2025). The Clinical Implications of Inappropriate Therapy in Community-Onset Urinary Tract Infections and the Development of a Bayesian Hierarchical Weighted-Incidence Syndromic Combination Antibiogram. Antibiotics, 14(2), 187. https://doi.org/10.3390/antibiotics14020187







