Bactericidal Effect of a Novel Phage Endolysin Targeting Multi-Drug-Resistant Acinetobacter baumannii
Abstract
:1. Introduction
2. Results
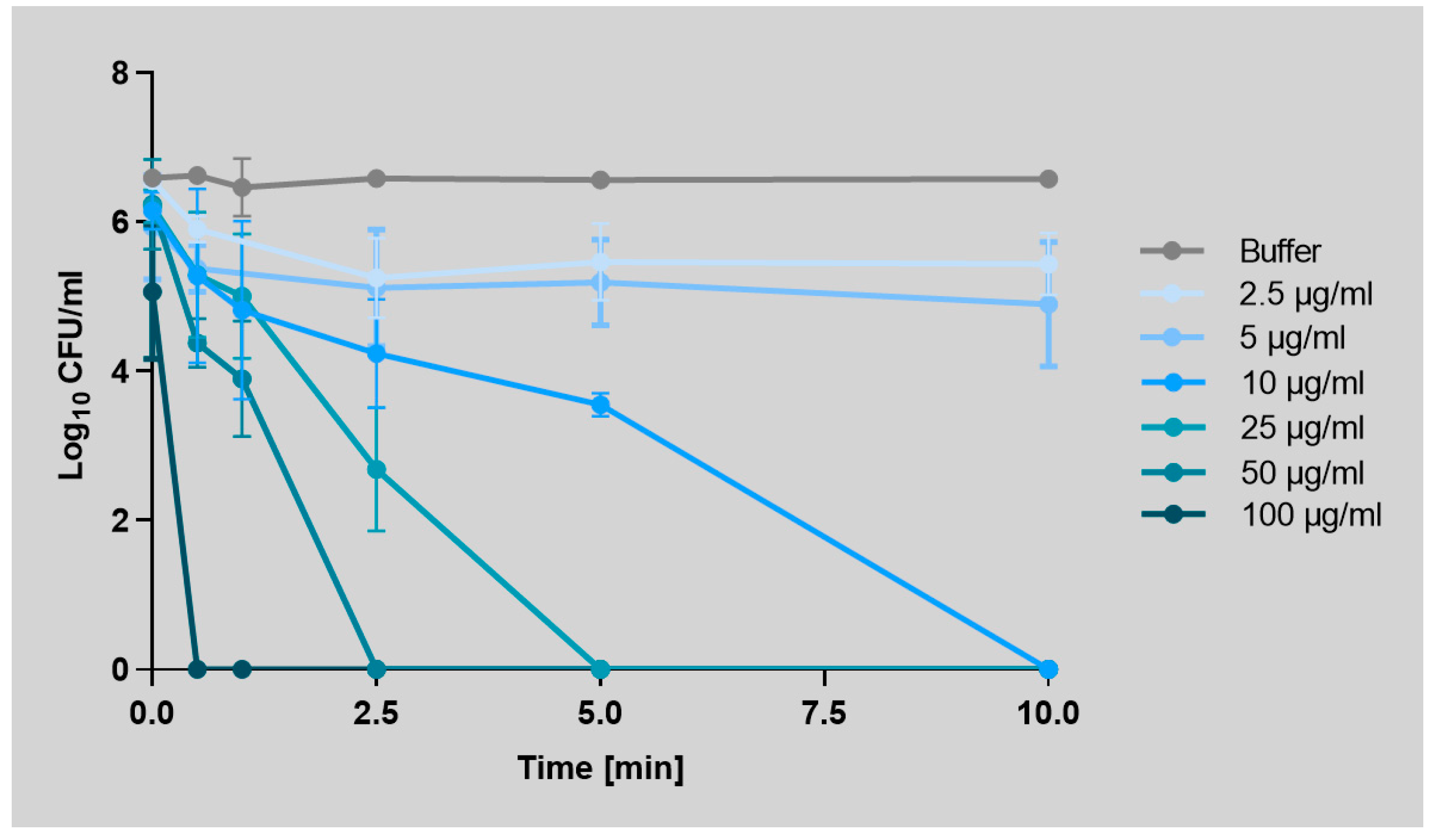
2.1. Art-Top3 Displays High Antibacterial Activity and Killing Rate Against A. baumannii
2.2. Art-Top3 Decreases Mature A. baumannii Biofilm In Vitro
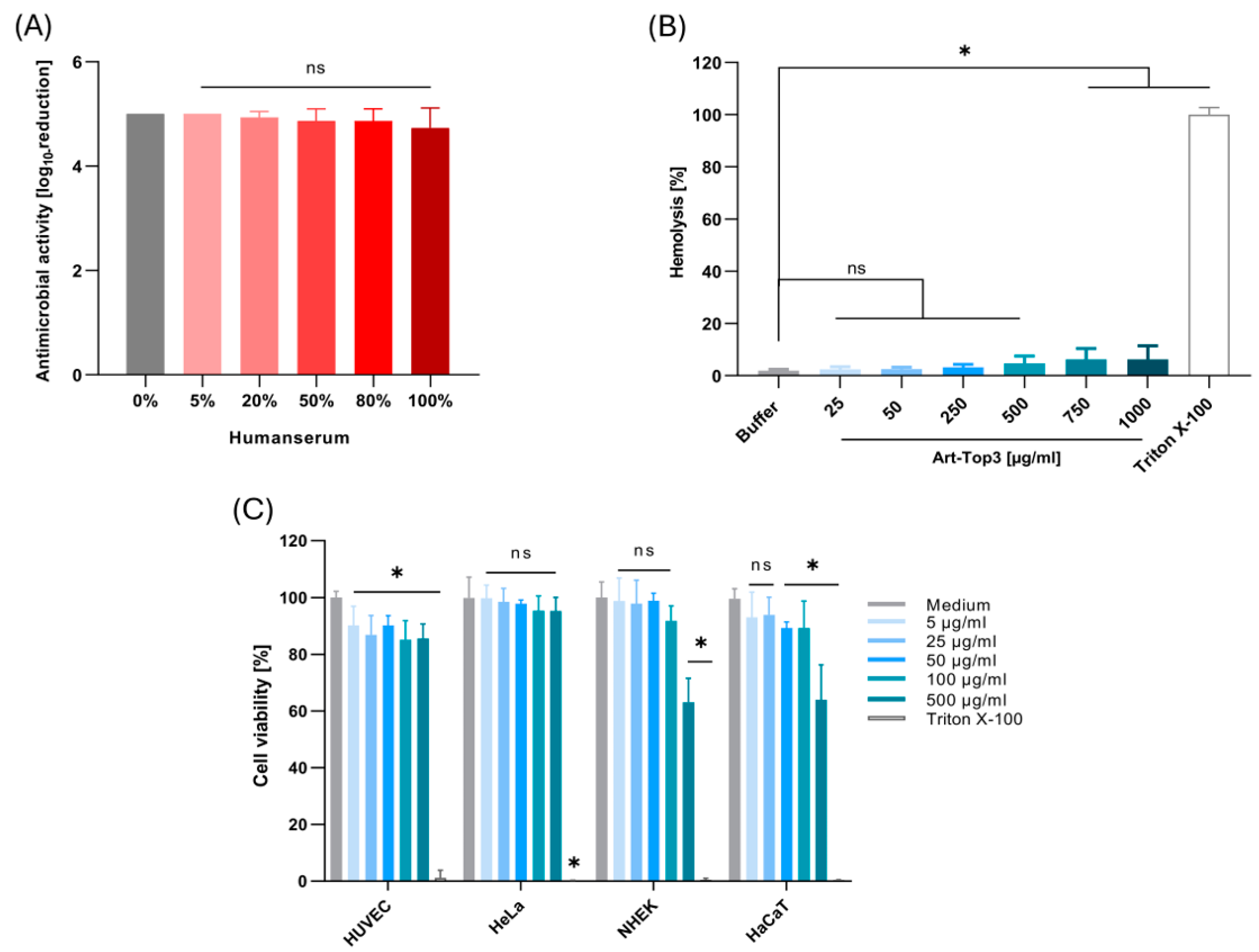
2.3. Art-Top3 Displays Low Toxicity Towards Human Cells
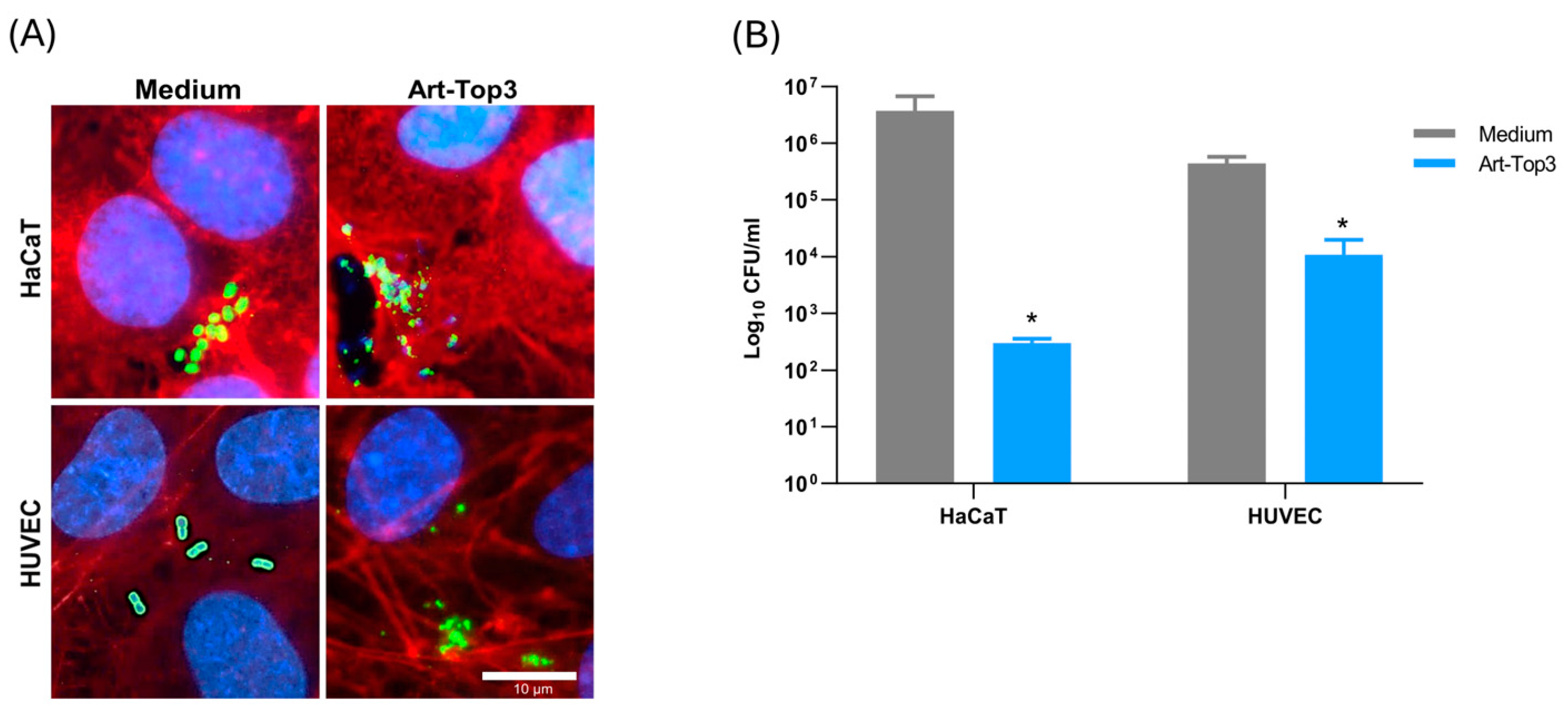
2.4. Art-Top3 Reduces Adhesion of A. baumannii to Human Cells
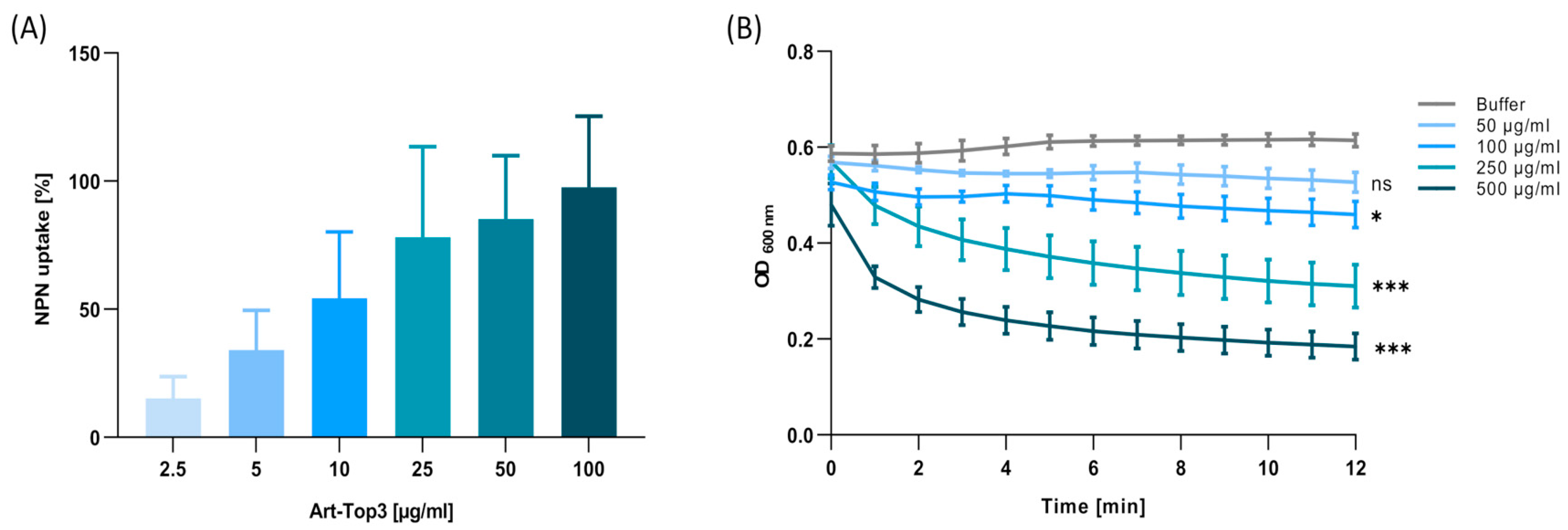
2.5. Art-Top3 Permeabilizes the Outer Membrane and Degrades Peptidoglycan
2.6. Art-Top3 Ameliorates Survival of Galleria mellonella Larvae After Infection with Multidrug-Resistant A. baumannii
3. Discussion
4. Materials and Methods
4.1. Engineering of Artilysin®
4.2. Bacterial Strains and Culture Conditions
4.3. Antimicrobial Susceptibility Testing
4.4. Antibacterial Activity Assay
4.5. Evaluation of Biofilm Formation
4.6. Biocompatibility Assay
4.7. Cell Culture and Infection
4.8. Human Serum Assay
4.9. Outer Membrane Permeability Assay
4.10. Muralytic Assay
4.11. Galleria Mellonella Infection Model
4.12. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter Baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef]
- Alsan, M.; Klompas, M. Acinetobacter baumannii: An Emerging and Important Pathogen. J. Clin. Outcomes Manag. 2010, 17, 363–369. [Google Scholar]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2016, 30, 409. [Google Scholar] [CrossRef]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef]
- Tietgen, M.; Kramer, J.S.; Brunst, S.; Djahanschiri, B.; Wohra, S.; Higgins, P.G.; Weidensdorfer, M.; Riedel-Christ, S.; Pos, K.M.; Gonzaga, A.; et al. Identification of the Novel Class D β-Lactamase OXA-679 Involved in Carbapenem Resistance in Acinetobacter calcoaceticus. J. Antimicrob. Chemother. 2019, 74, 1494–1502. [Google Scholar] [CrossRef]
- Bonnin, R.A.; Nordmann, P.; Poirel, L. Screening and Deciphering Antibiotic Resistance in Acinetobacter baumannii: A State of the Art. Expert. Rev. Anti-Infect. Ther. 2013, 11, 571–583. [Google Scholar] [CrossRef]
- Poirel, L.; Bonnin, R.A.; Nordmann, P. Genetic Basis of Antibiotic Resistance in Pathogenic Acinetobacter Species. IUBMB Life 2011, 63, 1061–1067. [Google Scholar] [CrossRef]
- Lin, M.-F.; Lan, C.-Y. Antimicrobial Resistance in Acinetobacter baumannii: From Bench to Bedside. World J. Clin. Cases 2014, 2, 787–814. [Google Scholar] [CrossRef]
- Göttig, S.; Gruber, T.M.; Higgins, P.G.; Wachsmuth, M.; Seifert, H.; Kempf, V.A.J. Detection of Pan Drug-Resistant Acinetobacter baumannii in Germany. J. Antimicrob. Chemother. 2014, 69, 2578–2579. [Google Scholar] [CrossRef]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as Emerging Alternative Therapeutic Agents to Counter Drug-Resistant Infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The Advantages and Challenges of Using Endolysins in a Clinical Setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Popova, A.V.; Zhilenkov, E.L.; Myakinina, V.P.; Krasilnikova, V.M.; Volozhantsev, N.V. Isolation and Characterization of Wide Host Range Lytic Bacteriophage AP22 Infecting Acinetobacter baumannii. FEMS Microbiol. Lett. 2012, 332, 40–46. [Google Scholar] [CrossRef]
- Yele, A.B.; Thawal, N.D.; Sahu, P.K.; Chopade, B.A. Novel Lytic Bacteriophage AB7-IBB1 of Acinetobacter baumannii: Isolation, Characterization and Its Effect on Biofilm. Arch. Virol. 2012, 157, 1441–1450. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Grymonprez, B.; Biebl, M.; Pirnay, J.-P.; Defraine, V.; Michiels, J.; Cenens, W.; Aertsen, A.; Miller, S.; et al. Art-175 Is a Highly Efficient Antibacterial Against Multidrug-Resistant Strains and Persisters of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 3774–3784. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Lavigne, R. Use of Bacteriophage Endolysin EL188 and Outer Membrane Permeabilizers Against Pseudomonas aeruginosa. J. Appl. Microbiol. 2011, 110, 778–785. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Zhang, P.; Leung, S.S.Y.; Xia, J. Membrane-Permeable Antibacterial Enzyme Against Multidrug-Resistant Acinetobacter baumannii. ACS Infect. Dis. 2021, 7, 2192–2204. [Google Scholar] [CrossRef]
- Lashinsky, J.N.; Henig, O.; Pogue, J.M.; Kaye, K.S. Minocycline for the Treatment of Multidrug and Extensively Drug-Resistant A. baumannii: A Review. Infect. Dis. Ther. 2017, 6, 199–211. [Google Scholar] [CrossRef]
- Weidensdorfer, M.; Ishikawa, M.; Hori, K.; Linke, D.; Djahanschiri, B.; Iruegas, R.; Ebersberger, I.; Riedel-Christ, S.; Enders, G.; Leukert, L.; et al. The Acinetobacter Trimeric Autotransporter Adhesin Ata Controls Key Virulence Traits of Acinetobacter baumannii. Virulence 2019, 10, 68–81. [Google Scholar] [CrossRef]
- Tomaschek, F.; Higgins, P.G.; Stefanik, D.; Wisplinghoff, H.; Seifert, H. Head-to-Head Comparison of Two Multi-Locus Sequence Typing (MLST) Schemes for Characterization of Acinetobacter baumannii Outbreak and Sporadic Isolates. PLoS ONE 2016, 11, e0153014. [Google Scholar] [CrossRef]
- Blasco, L.; Ambroa, A.; Trastoy, R.; Bleriot, I.; Moscoso, M.; Fernández-Garcia, L.; Perez-Nadales, E.; Fernández-Cuenca, F.; Torre-Cisneros, J.; Oteo-Iglesias, J.; et al. In Vitro and In Bivo Efficacy of Combinations of Colistin and Different Endolysins Against Clinical Strains of Multi-Drug Resistant Pathogens. Sci. Rep. 2020, 10, 7163. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Jang, J.; Myung, H.; Song, M. Eradication of Drug-Resistant Acinetobacter baumannii by Cell-Penetrating Peptide Fused Endolysin. J. Microbiol. 2022, 60, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, Z.; Mao, C.; Guo, G.; Zeng, Z.; Fei, Y.; Wan, S.; Peng, J.; Wu, J. Antimicrobial Peptide Cec4 Eradicates the Bacteria of Clinical Carbapenem-Resistant Acinetobacter baumannii Biofilm. Front. Microbiol. 2020, 11, 1532. [Google Scholar] [CrossRef] [PubMed]
- Roca, I.; Espinal, P.; Vila-Farrés, X.; Vila, J. The Acinetobacter baumannii Oxymoron: Commensal Hospital Dweller Turned Pan-Drug-Resistant Menace. Front. Microbiol. 2012, 3, 148. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Kim, S.; Bae, S.; Kim, Y.; Chang, H.-H.; Kim, J. Antibacterial Effects of Recombinant Endolysins in Disinfecting Medical Equipment: A Pilot Study. Front. Microbiol. 2022, 12, 773640. [Google Scholar] [CrossRef]
- Gerstmans, H.; Grimon, D.; Gutiérrez, D.; Lood, C.; Rodríguez, A.; van Noort, V.; Lammertyn, J.; Lavigne, R.; Briers, Y. A VersaTile-Driven Platform for Rapid Hit-to-Lead Development of Engineered Lysins. Sci. Adv. 2020, 6, eaaz1136. [Google Scholar] [CrossRef]
- Chu, J.J.K.; Poh, W.H.; Hasnuddin, N.T.B.; Hew, E.Y.; Dam, L.C.; Sahili, A.E.; Rice, S.A.; Goh, B.C. Novel Phage Lysin Abp013 Against Acinetobacter baumannii. Antibiotics 2022, 11, 169. [Google Scholar] [CrossRef]
- Khan, F.M.; Rasheed, F.; Yang, Y.; Liu, B.; Zhang, R. Endolysins: A New Antimicrobial Agent Against Antimicrobial Resistance. Strategies and Opportunities in Overcoming the Challenges of Endolysins Against Gram-Negative Bacteria. Front. Pharmacol. 2024, 15, 1385261. [Google Scholar] [CrossRef]
- Rahman, M.u.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a Promising Solution Against Antimicrobial Resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef]
- Antonova, N.P.; Vasina, D.V.; Rubalsky, E.O.; Fursov, M.V.; Savinova, A.S.; Grigoriev, I.V.; Usachev, E.V.; Shevlyagina, N.V.; Zhukhovitsky, V.G.; Balabanyan, V.U.; et al. Modulation of Endolysin LysECD7 Bactericidal Activity by Different Peptide Tag Fusion. Biomolecules 2020, 10, 440. [Google Scholar] [CrossRef]
- Peng, S.-Y.; You, R.-I.; Lai, M.-J.; Lin, N.-T.; Chen, L.-K.; Chang, K.-C. Highly Potent Antimicrobial Modified Peptides Derived from the Acinetobacter baumannii Phage Endolysin LysAB2. Sci. Rep. 2017, 7, 11477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Liang, S.; Wang, J.; Zhu, Y.; Zhang, W.; Liu, S.; Schwarz, S.; Xie, F. Bactericidal Synergism between Phage Endolysin Ply2660 and Cathelicidin LL-37 Against Vancomycin-Resistant Enterococcus faecalis Biofilms. NPJ Biofilms Microbiomes 2023, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Son, S.M.; Kim, J.; Ryu, S. Development of Sensitizer Peptide-Fused Endolysin Lys1S-L9P Acting Against Multidrug-Resistant Gram-Negative Bacteria. Front. Microbiol. 2023, 14, 1296796. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Kim, D.; Kim, K.; Park, S.-J.; Akter, S.; Kim, J.; Bang, S.; Kim, S.; Kim, J.; Lee, J.C.; et al. Engineering of Lysin by Fusion of Antimicrobial Peptide (Cecropin A) Enhances Its Antibacterial Properties Against Multidrug-Resistant Acinetobacter baumannii. Front. Microbiol. 2022, 13, 988522. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Jara, S.; Monga, D.; Eliopoulos, G.M.; Moellering, R.C.; Mylonakis, E. Galleria Mellonella as a Model System to Study Acinetobacter baumannii Pathogenesis and Therapeutics. Antimicrob. Agents Chemother. 2009, 53, 2605–2609. [Google Scholar] [CrossRef]
- Leukert, L.; Tietgen, M.; Krause, F.F.; Schultze, T.G.; Fuhrmann, D.C.; Debruyne, C.; Salcedo, S.P.; Visekruna, A.; Wittig, L.; Göttig, S. Infection of Endothelial Cells with Acinetobacter baumannii Reveals Remodelling of Mitochondrial Protein Complexes. Microbiol. Spectr. 2023, 11, e0517422. [Google Scholar] [CrossRef]
- Jeong, T.-H.; Hong, H.-W.; Kim, M.S.; Song, M.; Myung, H. Characterization of Three Different Endolysins Effective Against Gram-Negative Bacteria. Viruses 2023, 15, 679. [Google Scholar] [CrossRef]
- Sykilinda, N.N.; Nikolaeva, A.Y.; Shneider, M.M.; Mishkin, D.V.; Patutin, A.A.; Popov, V.O.; Boyko, K.M.; Klyachko, N.L.; Miroshnikov, K.A. Structure of an Acinetobacter Broad-Range Prophage Endolysin Reveals a C-Terminal α-Helix with the Proposed Role in Activity Against Live Bacterial Cells. Viruses 2018, 10, 309. [Google Scholar] [CrossRef]
- Hugh, R.; Reese, R. A Comparison of 120 Strains of Bacterium Anitratum Schaub and Hauber with the Type Strain of This Species. Int. J. Bacteriol. 1968, 18, 207–229. [Google Scholar] [CrossRef]
- Vaca, D.J.; Frenzel, F.; Ballhorn, W.; Torres, S.G.; Leisegang, M.S.; Günther, S.; Bender, D.; Kraiczy, P.; Göttig, S.; Kempf, V.A.J. Adhesion of Human Pathogenic Bacteria to Endothelial Cells Is Facilitated by Fibronectin Interaction. Microbes Infect. 2023, 25, 105172. [Google Scholar] [CrossRef]
- Hong, M.J.; Kim, M.K.; Park, Y. Comparative Antimicrobial Activity of Hp404 Peptide and Its Analogs Against Acinetobacter baumannii. Int. J. Mol. Sci. 2021, 22, 5540. [Google Scholar] [CrossRef] [PubMed]


| MIC [µg/mL] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strain | Resistance | Art-Top3 | Piperacillin-Tazobactam | Ceftazidime | Imipenem | Ciprofloxacin | Amikacin | Minocycline | Colistin |
| 1355 | MDR | 2.5 | ≥32 | ≥32 | ≥32 | 4 | ≥32 | 0.125 | 0.25 |
| 1594 | MDR0 | 2.5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 0.125 | 1 |
| ATCC 19606T | WT | 5 | 24 | 12 | 1 | 0.25 | 16 | 0.125 | 0.5 |
| 698 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 0.064 | 1 |
| 893 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 0.5 | 1 |
| 981 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 0.5 | 0.25 |
| 1284 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 1 | 1 |
| 1372 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 0.125 | 0.5 |
| 2778 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 16 | 2 |
| 3378 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 16 | 0.125 |
| 6863 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 0.25 | 0.5 |
| 6904 | MDR | 5 | ≥32 | ≥32 | ≥32 | ≥32 | ≥32 | 0.75 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia Torres, S.; Henrich, D.; Verboket, R.D.; Marzi, I.; Hahne, G.; Kempf, V.A.J.; Göttig, S. Bactericidal Effect of a Novel Phage Endolysin Targeting Multi-Drug-Resistant Acinetobacter baumannii. Antibiotics 2025, 14, 162. https://doi.org/10.3390/antibiotics14020162
Garcia Torres S, Henrich D, Verboket RD, Marzi I, Hahne G, Kempf VAJ, Göttig S. Bactericidal Effect of a Novel Phage Endolysin Targeting Multi-Drug-Resistant Acinetobacter baumannii. Antibiotics. 2025; 14(2):162. https://doi.org/10.3390/antibiotics14020162
Chicago/Turabian StyleGarcia Torres, Sara, Dirk Henrich, Rene D. Verboket, Ingo Marzi, Gernot Hahne, Volkhard A. J. Kempf, and Stephan Göttig. 2025. "Bactericidal Effect of a Novel Phage Endolysin Targeting Multi-Drug-Resistant Acinetobacter baumannii" Antibiotics 14, no. 2: 162. https://doi.org/10.3390/antibiotics14020162
APA StyleGarcia Torres, S., Henrich, D., Verboket, R. D., Marzi, I., Hahne, G., Kempf, V. A. J., & Göttig, S. (2025). Bactericidal Effect of a Novel Phage Endolysin Targeting Multi-Drug-Resistant Acinetobacter baumannii. Antibiotics, 14(2), 162. https://doi.org/10.3390/antibiotics14020162






