The Long-Term Survivorship and Cause of Failure of Metal-on-Metal Total Hip Arthroplasty
Abstract
1. Introduction
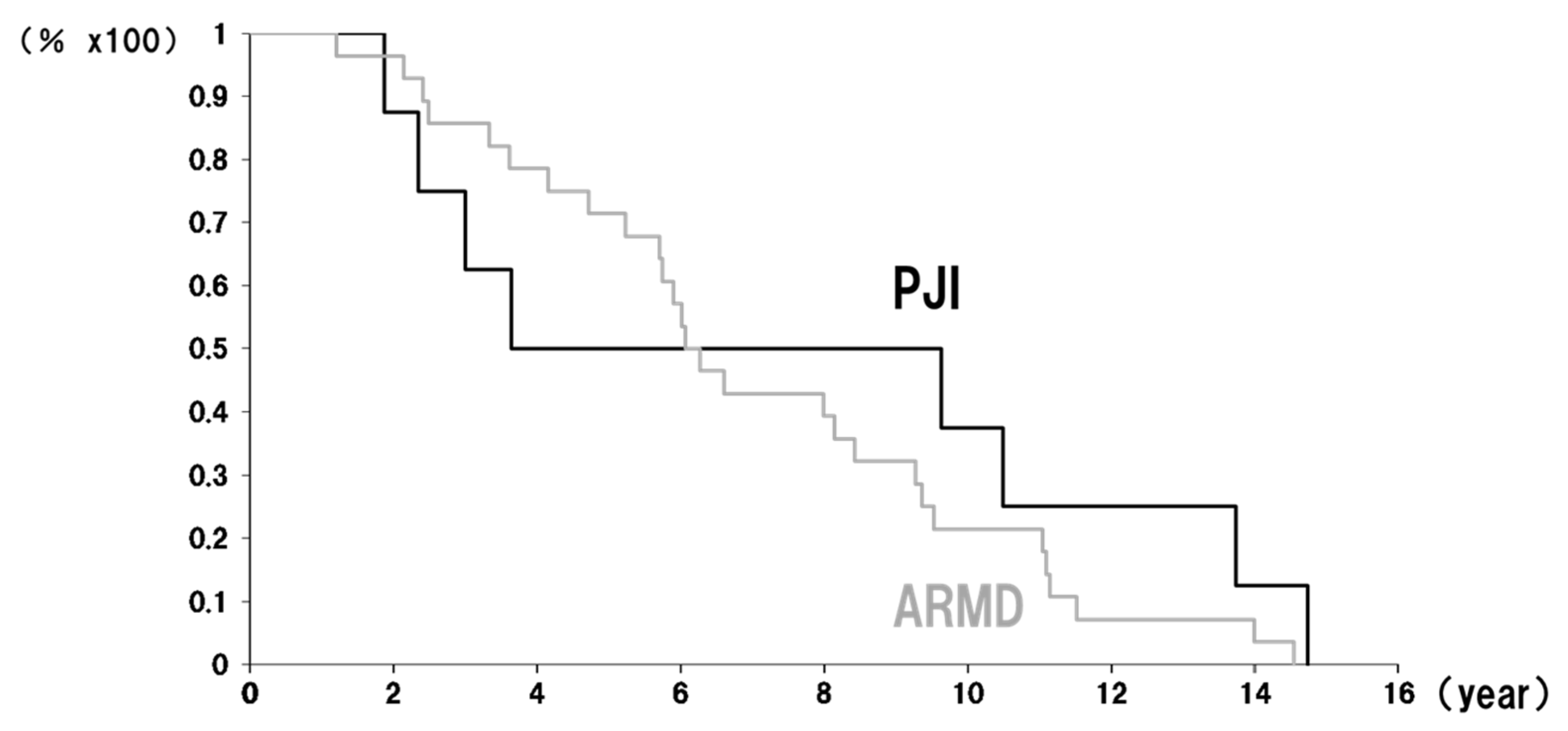
2. Results
3. Discussion
4. Materials and Methods
4.1. Clinical Evaluation
4.2. Radiological Evaluation
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cannella, A.; Greco, T.; Polichetti, C.; De Martino, I.; Mascio, A.; Maccauro, G.; Perisano, C. A Rare Case of Adverse Reaction to Metal Debris in a Ceramic-on-Ceramic Total Hip Replacement. J. Funct. Biomater. 2022, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Prieto, H.A.; Berbari, E.F.; Sierra, R.J. Acute delayed infection: Increased risk in failed metal on metal total hip arthroplasty. J. Arthroplast. 2014, 29, 1808–1812. [Google Scholar] [CrossRef] [PubMed]
- Judd, K.T.; Noiseux, N. Concomitant infection and local metal reaction in patients undergoing revision of metal on metal total hip arthroplasty. Iowa Orthop. J. 2011, 31, 59–63. [Google Scholar] [PubMed]
- Browne, J.A.; Bechtold, C.D.; Berry, D.J.; Hanssen, A.D.; Lewallen, D.G. Failed metal-on-metal hip arthroplasties: A spectrum of clinical presentations and operative findings. Clin. Orthop. Relat. Res. 2010, 468, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Grammatopoulos, G.; Adshead, S.; Tsialogiannis, E.; Tsiridis, E. Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol. Med. 2012, 18, 145–155. [Google Scholar] [CrossRef]
- Langton, D.J.; Jameson, S.S.; Joyce, T.J.; Hallab, N.J.; Natu, S.; Nargol, A.V.F. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J. Bone Jt. Surg. Ser. B 2010, 92, 38–46. [Google Scholar] [CrossRef]
- Bozza, N.; Guindani, N.; Pezzotta, G.; Alberto, F.; Castelli, C.C. 15-year follow-up of MoM 36-mm THA: Clinical, laboratory, and radiological (CT and MRI) prospective assessment. HIP Int. 2020, 30, 42–51. [Google Scholar] [CrossRef]
- Wyles, C.C.; Van Demark, R.E., 3rd; Sierra, R.J.; Trousdale, R.T. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 509–516. [Google Scholar] [CrossRef]
- de Steiger, R.N.; Hang, J.R.; Miller, L.N.; Graves, S.E.; Davidson, D.C. Five-year results of the ASR XL Acetabular System and the ASR Hip Resurfacing System: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J. Bone Jt. Surg. Am. 2011, 93, 2287–2293. [Google Scholar] [CrossRef]
- Galbraith, J.G.; Butler, J.S.; Browne, T.J.; Mulcahy, D.; Harty, J.A. Infection or metal hypersensitivity? The diagnostic challenge of failure in metal-on-metal bearings. Acta Orthop. Belg. 2011, 77, 145–151. [Google Scholar]
- Mikhael, M.M.; Hanssen, A.D.; Sierra, R.J. Failure of metal-on-metal total hip arthroplasty mimicking hip infection. A report of two cases. J. Bone Jt. Surg. Am. 2009, 91, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Wyles, C.C.; Larson, D.R.; Houdek, M.T.; Sierra, R.J.; Trousdale, R.T. Utility of synovial fluid aspirations in failed metal-on-metal total hip arthroplasty. J. Arthroplast. 2013, 28, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Alijanipour, P.; Bakhshi, H.; Parvizi, J. Diagnosis of periprosthetic joint infection: The threshold for serological markers. Clin. Orthop. Relat. Res. 2013, 471, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Doorn, P.; Dorey, F.; Amstutz, H.C. Wear and morphology of ultra-high molecular weight polyethylene wear particles from total hip replacements. Proc. Inst. Mech. Eng. H 1996, 210, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Doorn, P.F.; Campbell, P.A.; Worrall, J.; Benya, P.D.; McKellop, H.A.; Amstutz, H.C. Metal wear particle characterization from metal on metal total hip replacements: Transmission electron microscopy study of periprosthetic tissues and isolated particles. J. Biomed. Mater. Res. 1998, 42, 103–111. [Google Scholar] [CrossRef]
- Aroukatos, P.; Repanti, M.; Repantis, T.; Bravou, V.; Korovessis, P. Immunologic adverse reaction associated with low-carbide metal-on-metal bearings in total hip arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 2135–2142. [Google Scholar] [CrossRef]
- Hasegawa, M.; Naito, Y.; Yamaguchi, T.; Miyazaki, S.; Wakabayashi, H.; Sudo, A. Factors associated with symptomatic pseudotumors following metal-on-metal total hip arthroplasty. BMC Musculoskelet. Disord. 2016, 17, 456. [Google Scholar] [CrossRef][Green Version]
- Hasegawa, M.; Yoshida, K.; Wakabayashi, H.; Sudo, A. Prevalence of adverse reactions to metal debris following metal-on-metal THA. Orthopedics 2013, 36, e606–e612. [Google Scholar] [CrossRef] [PubMed]
- Sutphen, S.A.; MacLaughlin, L.H.; Madsen, A.A.; Russell, J.H.; McShane, M.A. Prevalence of Pseudotumor in Patients After Metal-On-Metal Hip Arthroplasty Evaluated with Metal Ion Analysis and MARS-MRI. Radiology 2012, 265, 848–857. [Google Scholar] [CrossRef]
- Korovessis, P.; Petsinis, G.; Repanti, M.; Repantis, T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine year follow-up. J. Bone Jt. Surg. Am. 2006, 88, 1183–1191. [Google Scholar] [CrossRef]
- Furnes, O.; Paxton, E.; Cafri, G.; Graves, S.; Bordini, B.; Comfort, T.; Rivas, M.C.; Banerjee, S.; Sedrakyan, A. Distributed analysis of hip implants using six national and regional registries: Comparing metal-on-metal with metal-on-highly cross-linked polyethylene bearings in cementless total hip arthroplasty in young patients. J. Bone Jt. Surg. Am. 2014, 96 (Suppl. S1), 25–33. [Google Scholar] [CrossRef]
- Grammatopoulos, G.; Pandit, H.; Kamali, A.; Maggiani, F.; Glyn-Jones, S.; Gill, H.S.; Murray, D.W.; Athanasou, N. The correlation of wear with histological features after failed hip resurfacing arthroplasty. J. Bone Jt. Surg. Am. 2013, 95, e81. [Google Scholar] [CrossRef] [PubMed]
- Willert, H.G.; Buchhorn, G.H.; Fayyazi, A.; Flury, R.; Windler, M.; Köster, G.; Lohmann, C.H. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J. Bone Jt. Surg. Am. 2005, 87, 28–36. [Google Scholar] [CrossRef]
- Huang, P.; Lyons, M.; O’Sullivan, M. The Infection Rate of Metal-on-Metal Total Hip Replacement Is Higher When Compared to Other Bearing Surfaces as Documented by the Australian Orthopaedic Association National Joint Replacement Registry. HSS J. 2018, 14, 99–105. [Google Scholar] [CrossRef]
- Merritt, K.; Brown, S.A. Tissue reaction and metal sensitivity. Acta Orthop. Scand. 1980, 51, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Rushton, N.; Coakley, A.J.; Tudor, J.; Wraight, E.P. The value of technetium and gallium scanning in assessing pain after total hip replacement. J. Bone Jt. Surg. Br. 1982, 64, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Pandit, H.; Glyn-Jones, S.; McLardy-Smith, P.; Gundle, R.; Whitwell, D.; Gibbons, C.L.; Ostlere, S.; Athanasou, N.; Gill, H.S.; Murray, D.W. Pseudotumours Associated with Metal-on-Metal Hip Resurfacing. J. Bone Jt. Surg. Br. 2008, 90, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.J.; Della Valle, C.J.; Berger, R.A.; Tetreault, M.; Paprosky, W.G.; Sporer, S.M.; Jacobs, J.J. Corrosion at the head-neck taper as a cause for adverse local tissue reactions in total hip arthroplasty. J. Bone Jt. Surg. Am. 2012, 94, 1655–1661. [Google Scholar] [CrossRef]
- MacDonald, S.J.; Brodner, W.; Jacobs, J.J. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J. Arthroplast. 2004, 19 (Suppl. S3), 12–16. [Google Scholar] [CrossRef]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef]
- Nilsdotter-Augustinsson, A.; Briheim, G.; Herder, A.; Ljunghusen, O.; Wahlström, O.; Ohman, L. Inflammatory response in 85 patients with loosened hip prostheses: A prospective study comparing inflammatory markers in patients with aseptic and septic prosthetic loosening. Acta Orthop. 2007, 78, 629–639. [Google Scholar] [CrossRef]
- DeLee, J.G.; Charnley, J. Radiological Demarcation of Cemented Sockets in Total Hip Replacement. Clin. Orthop. Relat. Res. 1976, 121, 20–32. [Google Scholar] [CrossRef]
- Gruen, T.A.; McNeice, G.M.; Amstutz, H.C. “Modes of failure” of cemented stem-type femoral components: A radiographic analysis of loosening. Clin. Orthop. Relat. Res. 1979, 141, 17–27. [Google Scholar] [CrossRef]
| Case | Age, Sex, and Diagnosis | BMI (kg/m2) | Observed PT After Primary Arthroplasty | Time to Revision | Causative Bacteria | Cup Inclination | Cup Anteversion | Symptom | Cup Revised | Stem Revised |
|---|---|---|---|---|---|---|---|---|---|---|
| Case1 | 53 F RA | 16.5 | 6.5 years | 10.5 years | Listeria | 55.7 | 24.2 | Swelling | Revised (S-ROM) | |
| Case2 | 68 M RA | 27.0 | Not observed | 3.5 years | MSSA | 39.6 | 22.2 | Pain | Revised (S-ROM) | |
| Case2 | 68 M RA | 27.0 | Not observed | 3 years | MSSA | 36.7 | 14.1 | Pain | ||
| Case3 | 69 F OA | 21.8 | 5.1 years | 9.7 years | Streptococcus anginosus | 57.7 | 3.1 | Pain, Cup and stem osteolysis | 2stage | 2stage |
| Case4 | 66 F OA | 18.9 | 13.6 years | 13.7 years | MSSA | 32.7 | 2.7 | Swelling, Fistula, Cup and stem osteolysis | Revised | |
| Case5 | 60 F OA | 22.5 | 2.3 years | 2.3 years | no causative | 42.8 | 11.9 | Pain | Revised | |
| Case6 | 58 M OA | 31.7 | 1.7 years | 1.9 years | no causative | 53.2 | 6.0 | Pain | Revised | |
| Case7 | 63 F OA | 21.4 | 10.1 years | 14.7 years | MSSA | 58.8 | 27.8 | Pain, Cup osteolysis | Revised |
| Revision for PJI (3.2%) | Revision for ARMD (12.1%) | p | |
|---|---|---|---|
| Male/Female | 2 patients (3 hips)/5 patients | 4 patients/25 patients (26 hips) | ns |
| Age at revision | Mean 62.4 (58–69) | Mean 64.5 (52–69) | ns |
| Preoperative diagnosis | OA 5 patients RA 2 patients (3 hips) | OA 26 patients (27 hips) RA 2 patients Fx 1 patient | ns |
| CRP (≦0.14 mg/dL) | CRP-positive 7 patients CRP-negative 1 patient | CRP-positive 13 patients (14 hips) CRP-negative 16 patients | ns |
| Revision for | PJI (8 Hips) | ARMDs (30 Hips) | p |
|---|---|---|---|
| Frequency (%) | 3.2% | 12.1% | ns |
| PT-positive (%) | 75% | 70% | ns |
| Revision for | PJI (7 Patients) | ARMDs (29 Patients) | p |
| CRP-positive (%) | 85.7% | 44.8% | ns |
| CRP level (mean ± SD) | 3.89 ± 3.06 | 0.41 ± 0.67 | <0.005 |
| WBC counts (mean ± SD) | 8060 ± 3181 | 5651 ± 1357 | <0.05 |
| Neutrophil counts (mean ± SD) | 5927 ± 3181 | 3664 ± 1280 | <0.05 |
| Lymphocyte counts (mean ± SD) | 1390 ± 319 | 1410 ± 484 | ns |
| Neutrophil-to-WBC ratios (mean ± SD) | 71.0 ± 8.5 | 64.2 ± 9.4 | ns |
| Lymphocyte-to-WBC ratios (mean ± SD) | 19.2 ± 6.9 | 25.5 ± 8.0 | ns |
| Male/Female | 39 Patients/191 Patients | ||
|---|---|---|---|
| Age (years) | Mean 64.1 (34–85) | ||
| Preoperative diagnosis | Osteoarthritis (OA) | 223 hips | |
| Rheumatoid arthritis (RA) | 15 hips | ||
| Osteonecrosis (ON) | 7 hips | ||
| Secondary OA in post hip fracture | 2 hips | ||
| Implant | Pinnacle | 82 patients | 89 hips |
| Cormet | 98 patients | 108 hips | |
| Conserve plus | 50 patients | 50 hips | |
| Follow-up periods | Mean 10.5 years | ||
| Metal on Polyethylene | Ceramic on Polyethylene | |
|---|---|---|
| 68 patients/69 hips | 78 patients/78 hips | |
| Age (years) | Mean 60.8 | Mean 64.9 |
| Preoperative diagnosis | ||
| Osteoarthritis (OA) | 57 hips | 72 hips |
| Rheumatoid arthritis (RA) | 5 hips | 2 hips |
| Osteonecrosis (ON) | 3 hips (2 patients) | |
| Rapidly destructive coxarthrosis | 2 hips | 3 hips |
| Secondary OA in infected hip | 2 hips | |
| Pigmented villonodular synovitis | 1 hip | |
| Follow-up periods | Mean 11.7 years | Mean 10.7 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakabayashi, H.; Hasegawa, M.; Naito, Y.; Tone, S.; Sudo, A. The Long-Term Survivorship and Cause of Failure of Metal-on-Metal Total Hip Arthroplasty. Antibiotics 2025, 14, 161. https://doi.org/10.3390/antibiotics14020161
Wakabayashi H, Hasegawa M, Naito Y, Tone S, Sudo A. The Long-Term Survivorship and Cause of Failure of Metal-on-Metal Total Hip Arthroplasty. Antibiotics. 2025; 14(2):161. https://doi.org/10.3390/antibiotics14020161
Chicago/Turabian StyleWakabayashi, Hiroki, Masahiro Hasegawa, Yohei Naito, Shine Tone, and Akihiro Sudo. 2025. "The Long-Term Survivorship and Cause of Failure of Metal-on-Metal Total Hip Arthroplasty" Antibiotics 14, no. 2: 161. https://doi.org/10.3390/antibiotics14020161
APA StyleWakabayashi, H., Hasegawa, M., Naito, Y., Tone, S., & Sudo, A. (2025). The Long-Term Survivorship and Cause of Failure of Metal-on-Metal Total Hip Arthroplasty. Antibiotics, 14(2), 161. https://doi.org/10.3390/antibiotics14020161







