Synergistic Antibiofilm Effects of Chestnut and Linden Honey with Lavender Essential Oil Against Multidrug-Resistant Otitis Media Pathogens
Abstract
1. Introduction
2. Results
2.1. Pollen Profile and Physicochemical Parameters of Honey Samples
2.2. Antibiotic Sensitivity of Test Bacteria
2.3. Minimum Inhibitory Concentrations (MICs)
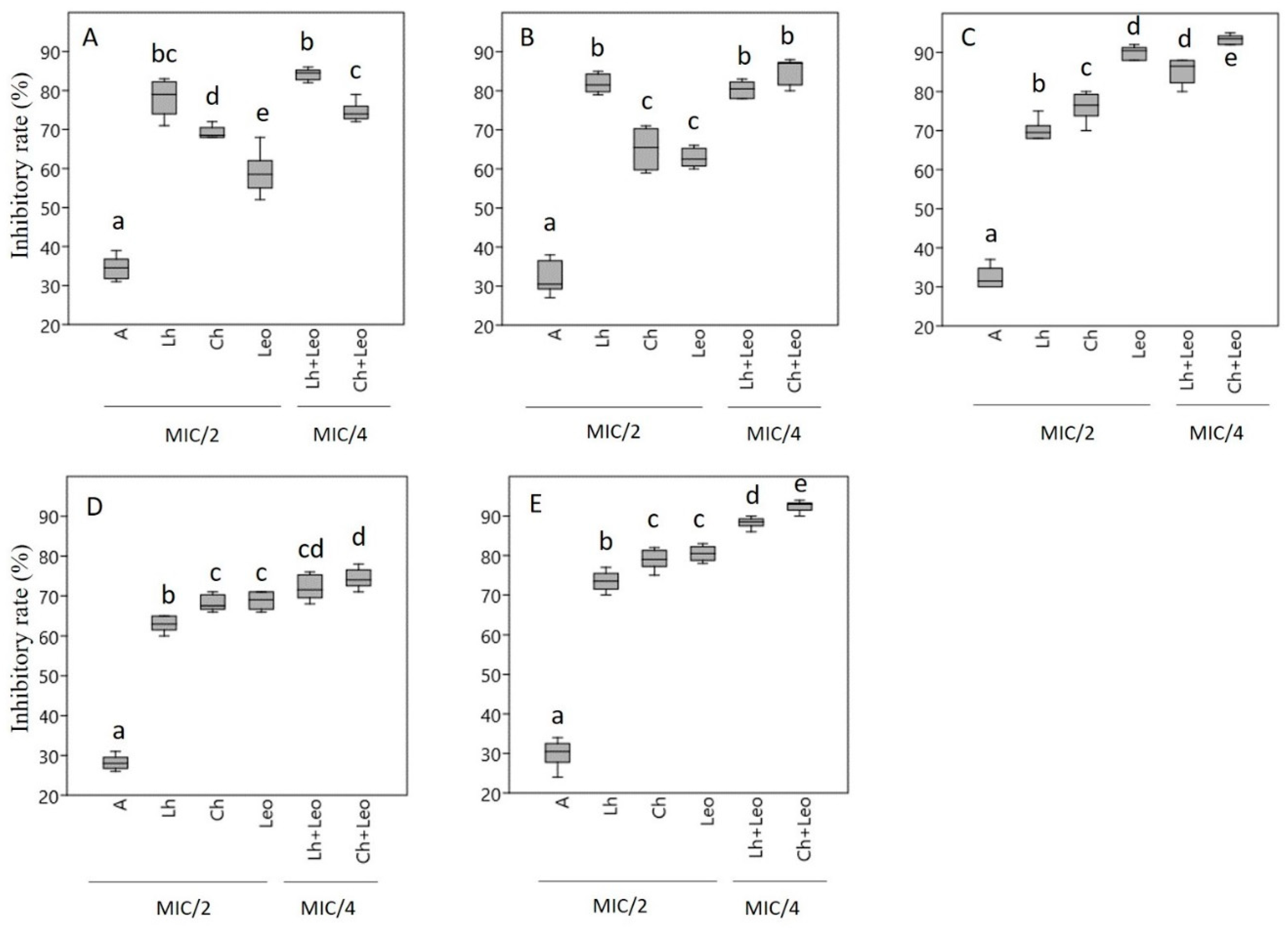
2.4. Biofilm Eradication Activity
2.5. Checkerboard Assay
2.6. Membrane Degradation Assay
3. Discussion
4. Materials and Methods
4.1. Honey and Essential Oil Samples
4.2. Melissopalynological Analysis
4.3. Physicochemical Parameters of Honey
4.4. Bacterial Strains
4.5. Determination of Antibiotic Sensitivity of Test Bacteria
4.6. Determination of Minimum Inhibitory Concentration (MIC)
4.7. Biofilm Eradication Assay
4.8. Checkerboard Assay
4.9. Membrane Degradation Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webber, M.A.; Piddock, L.J. The importance of efflux pumps in bacterial antibiotic resistance. J. Antimicrob. Chemother. 2003, 51, 9–11. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Moura de Sousa, J.; Lourenço, M.; Gordo, I. Horizontal gene transfer among host-associated microbes. Cell Host Microbe 2023, 31, 513–527. [Google Scholar] [CrossRef]
- D’Aquila, P.; De Rango, F.; Paparazzo, E.; Passarino, G.; Bellizzi, D. Epigenetic-Based Regulation of Transcriptome in Escherichia coli Adaptive Antibiotic Resistance. Microbiol. Spectr. 2023, 11, e0458322. [Google Scholar] [CrossRef]
- López, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, L.; Mei, A.; Xu, Y.; Ruan, X.; Wang, W.; Shao, J.; Yang, D.; Dong, X. Recent Nanotechnologies to Overcome the Bacterial Biofilm Matrix Barriers. Small 2023, 19, e2206220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bera, H.; Wang, H.; Wang, J.; Guo, Y.; Shi, C.; Cun, D.; Moser, C.; Høiby, N.; Yang, M. Combination and nanotechnology based pharmaceutical strategies for combating respiratory bacterial biofilm infections. Int. J. Pharm. 2022, 616, 121507. [Google Scholar] [CrossRef]
- Kragh, K.N.; Tolker-Nielsen, T.; Lichtenberg, M. The non-attached biofilm aggregate. Commun. Biol. 2023, 6, 898. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, H.; De Paepe, A.S.; Lambert, E.; Van Belleghem, J.D.; Cools, P.; Van Simaey, L.; Deschaght, P.; Vaneechoutte, M.; Dhooge, I. Haemophilus influenzae biofilm formation in chronic otitis media with effusion. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Clark, J.; Julien, B.; Islam, F.; Roos, K.; Grimwood, K.; Little, P.; Del Mar, C.B. Probiotics for preventing acute otitis media in children. Cochrane Database Syst. Rev. 2019, 6, CD012941. [Google Scholar] [CrossRef] [PubMed]
- Gaddey, H.L.; Wright, M.T.; Nelson, T.N. Otitis Media: Rapid Evidence Review. Am. Fam. Physician 2019, 100, 350–356. [Google Scholar]
- Danishyar, A.; Ashurst, J.V. Acute Otitis Media; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Paramasivan, S.; Bassiouni, A.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B.; et al. The international sinonasal microbiome study: A multicentre, multinational characterization of sinonasal bacterial ecology. Allergy 2020, 75, 2037–2049. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Kim, N.S.; Lee, D.S. Comparison of different extraction methods for the analysis of fragrances from Lavandula species by gas chromatography–mass spectrometry. J. Chromatogr. A 2002, 982, 31–47. [Google Scholar] [CrossRef]
- Pandur, E.; Balatinácz, A.; Micalizzi, G.; Mondello, L.; Horváth, A.; Sipos, K.; Horváth, G. Anti-inflammatory effect of lavender (Lavandula angustifolia Mill.) essential oil prepared during different plant phenophases on THP-1 macrophages. BMC Complement. Med. Ther. 2021, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Koulivand, P.H.; Ghadiri, M.K.; Gorji, A. Lavender and the nervous system. Evid. -Based Complement. Altern. Med. 2013, 2013, 681304. [Google Scholar] [CrossRef]
- Keitel, U.; Schilling, E.; Knappe, D.; Al-Mekhlafi, M.; Petersen, F.; Hoffmann, R.; Hauschildt, S. Effect of antimicrobial peptide from Apis mellifera hemolymph and its optimized version Api88 on biological activities of human monocytes and mast cells. Innate Immun. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, L.; Yang, H.; Liu, S.; Huang, C. Effect of apigenin on surface associated characteristics and adherence of Streptococcus mutans. Dent. Mater. J. 2020, 39, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Vakili, O.; Ahmadi, N.; Soltani Fard, E.; Mousavi, P.; Khalvati, B.; Maleksabet, A.; Savardashtaki, A.; Taheri-Anganeh, M.; Movahedpour, A. Glucose oxidase: Applications, sources, and recombinant production. Biotechnol. Appl. Biochem. 2021, 69, 939–950. [Google Scholar] [CrossRef]
- Balázs, V.L.; Nagy-Radványi, L.; Filep, R.; Kerekes, E.; Kocsis, B.; Kocsis, M.; Farkas, Á. In vitro antibacterial and antibiofilm activity of Hungarian honeys against respiratory tract bacteria. Foods 2021, 10, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Á.; Balázs, V.L.; Kőszegi, T.; Csepregi, R.; Kerekes, E.; Horváth, G.; Szabó, P.; Gaál, K.; Kocsis, M. Antibacterial and Biofilm Degradation Effects of Hungarian Honeys Linked with Botanical Origin, Antioxidant Capacity and Mineral Content. Front. Nutr. 2022, 9, 953470. [Google Scholar] [CrossRef] [PubMed]
- Sakač, M.; Jovanov, P.; Marić, A.; Četojević-Simin, D.; Novaković, A.; Plavšić, D.; Škrobot, D.; Kovač, R. Antioxidative, Antibacterial and Antiproliferative Properties of Honey Types from the Western Balkans. Antioxidants 2022, 11, 1120. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Sousa, J.C.; Silva, A.C.; Melo, L.D.; Sillankorva, S. Chestnut Honey and Bacteriophage Application to Control Pseudomonas aeruginosa and Escherichia coli Biofilms: Evaluation in an ex vivo Wound Model. Front. Microbiol. 2018, 9, 1725. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Teixeira-Santos, R.; Rodrigues, A.G.; Pina-Vaz, C.; Fresno, J.M.; Tornadijo, M.E. Antibacterial action mechanisms of honey: Physiological Effects of Avocado, Chestnut, and Polyfloral Honey upon Staphylococcus aureus and Escherichia coli. Molecules 2020, 25, 1252. [Google Scholar] [CrossRef] [PubMed]
- Cumhur, A.; Hakan, A.; Tuğrul, Y.; Ahmet, T.; Nuray, T.D. Determination of the superior quality properties of randomly selected chestnut honey samples from the Sinop region. Spectrosc. Lett. 2023, 56, 353–363. [Google Scholar] [CrossRef]
- Qiao, J.; Chen, L.; Kong, L.; Dong, J.; Zhou, Z.; Zhang, H. Characteristic Components and Authenticity Evaluation of Rape, Acacia, and Linden Honey. J. Agric. Food Chem. 2020, 68, 9776–9788. [Google Scholar] [CrossRef]
- Farkas, Á.; Horváth, G.; Kuzma, M.; Mayer, M.; Kocsis, M. Phenolic compounds in Hungarian acacia, linden, milkweed and goldenrod honeys. Curr. Res. Food Sci. 2023, 6, 100526. [Google Scholar] [CrossRef]
- Skadiņš, I.; Labsvārds, K.D.; Grava, A.; Amirian, J.; Tomsone, L.E.; Ruško, J.; Viksna, A.; Bandere, D.; Brangule, A. Antimicrobial and Antibiofilm Properties of Latvian Honey against Causative Agents of Wound Infections. Antibiotics 2023, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Alissandrakis, E.; Tarantilis, P.A.; Pappas, C.; Harizanis, P.C.; Polissiou, M. Investigation of Organic Extractives from Unifloral Chestnut (Castanea sativa L.) and Eucalyptus (Eucalyptus globulus Labill.) Honeys and Flowers to Identification of Botanical Marker Compounds. LWT-Food Sci. Technol. 2011, 44, 1042–1051. [Google Scholar] [CrossRef]
- Mayda, N.; Ozok, A.; Sorkun, K. Some Characteristic Properties of Chestnut and Rhododendron Honeys in Turkey. J. Biol. Chem. 2018, 1, 135–145. [Google Scholar] [CrossRef]
- Turska, M.; Paluszkiewicz, P.; Turski, W.A.; Parada-Tuska, J. A Review of the Health Benefits of Food Enriched with Kynurenic Acid. Nutrients 2022, 14, 4182. [Google Scholar] [CrossRef] [PubMed]
- Assaggaf, H.M.; Naceiri, M.H.; Rajab, B.S.; Attar, A.A.; Hamed, M.; Sheikh, R.A.; Omari, N.E.; Menyiy, N.E.; Belmehdi, O.; Mahmud, S.; et al. Singular and Combined Effects of Essential Oil and Honey of Eucalyptus Globulus on Anti-Inflammatory, Antioxidant, Dermatoprotective, and Antimicrobial Properties: In Vitro and In Vivo Findings. Molecules 2022, 27, 5121. [Google Scholar] [CrossRef]
- Almasaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Millezi, A.F.; Piccoli, R.H.; Oliveira, J.M.; Pereira, M.O. Anti-biofilm and Antibacterial Effect of Essential Oils and Their Major Compounds. J. Essent. Oil Bear. Plants 2016, 19, 624–631. [Google Scholar] [CrossRef]
- Oddo, L.P.; Piro, R.; Bruneau, É.; Guyot-Declerck, C.; Ivanov, T.; Piskulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H.; et al. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35, 38–81. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Pezantes-Orellana, C.; German, B.F.; De la Cruz, C.M.; Montalvo, J.L.; Orellana-Manzano, A. Essential oils: A systematic review on revolutionizing health, nutrition, and omics for optimal well-being. Front. Med. 2024, 16, 1337785. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Park, S.M.; Yu, H.; Seo, G.; Kim, H.; Kim, S.A.; Rhee, M. Recent Advances in the Application of Antibacterial Complexes Using Essential Oils. Molecules 2020, 25, 1752. [Google Scholar] [CrossRef]
- Plesiat, P.; Nikaido, H. Outer membranes of Gram-negative bacteria are permeable to steroid probes. Mol. Microbiol. 1992, 6, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Prevention of drug access to bacterial targets: Permeability barriers and active efflux. Science 1994, 264, 382–388. [Google Scholar] [CrossRef]
- Huang, M.Y.; Liao, M.H.; Wang, Y.K.; Huang, Y.S.; Wen, H.C. Effect of lavender essential oil on LPS-stimulated inflammation. Am. J. Chin. Med. 2012, 40, 845–859. [Google Scholar] [CrossRef]
- Todorova, D.; Yavorov, N.; Lasheva, V.; Damyanova, S.; Kostova, I. Lavender Essential Oil as Antibacterial Treatment for Packaging Paper. Coatings 2023, 13, 32. [Google Scholar] [CrossRef]
- Kollef, M.H.; Torres, A.; Shorr, A.F.; Martin-Loeches, I.; Micek, S.T. Nosocomial Infection. Crit. Care Med. 2021, 49, 169–187. [Google Scholar] [CrossRef]
- Sousa, S.A.; Feliciano, J.R.; Pita, T.; Soeiro, C.F.; Mendes, B.L.; Alves, L.G.; Leitão, J.H. Bacterial Nosocomial Infections: Multidrug Resistance as a Trigger for the Development of Novel Antimicrobials. Antibiotics 2021, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Shivshankar, P.; Collum, S.; Bi, W. Haemophilus influenzae is associated with fibrotic phenotype of COVID-19 and idiopathic pulmonary fibrosis. ERJ Open Res. 2022, 8, 236. [Google Scholar] [CrossRef]
- Cavanagh, H.M.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, M.; Jovanović, K.K.; Marković, T.; Marković, D.; Gligorijević, N.; Radulović, S.; Soković, M. Chemical composition, antimicrobial, and cytotoxic properties of five Lamiaceae essential oils. Ind. Crops Prod. 2014, 61, 225–232. [Google Scholar] [CrossRef]
- de Rapper, S.; Viljoen, A.; van Vuuren, S. The In Vitro Antimicrobial Effects of Lavandula angustifolia Essential Oil in Combination with Conventional Antimicrobial Agents. Evid. Based Complement. Altern. Med. 2016, 2016, 2752739. [Google Scholar] [CrossRef]
- Mourão, A.; Serrano, I.; Cunha, E.; Tavares, L.; Lourenco, A.; Oliveira, M. In vitro efficacy of lavender oil, otological gel and gentamicin to eradicate biofilm produced by Pseudomonas aeruginosa. Vet. Dermatol. 2024, 35, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Végh, A.; Bencsik, T.; Molnár, P.; Böszörményi, A.; Lemberkovics, É.; Kovács, K.; Kocsis, B.; Horváth, G. Composition and antipseudomonal effect of essential oils isolated from different lavender species. Nat. Prod. Commun. 2012, 7, 1393–1396. [Google Scholar] [CrossRef]
- Donadu, M.; Usai, D.; Pinna, A.; Porcu, T.; Mazzarello, V.; Fiamma, M.; Marchetti, M.; Cannas, S.; Delogu, G.; Zanetti, S.; et al. In vitro activity of hybrid lavender essential oils against multidrug resistant strains of Pseudomonas aeruginosa. J. Infect. Dev. Ctries. 2018, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Irie, Y.; Borlee, B.R.; O’Connor, J.R.; Hill, P.J.; Harwood, C.S.; Wozniak, D.J.; Parsek, M.R. Self-produced exopolysaccharide is a signal that stimulates biofilm formation in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 2012, 109, 20632–20636. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Byrd, M.S.; Sergeant, S.; Azad, A.K.; Parsek, M.R.; McPhail, L.; Schlesinger, L.S.; Wozniak, D.J. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell. Microbiol. 2012, 14, 95–106. [Google Scholar] [CrossRef]
- Jennings, L.K.; Storek, K.M.; Ledvina, H.E.; Coulon, C.; Marmont, L.S.; Sadovskaya, I.; Secor, P.R.; Tseng, B.S.; Scian, M.; Filloux, A.; et al. Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 2015, 112, 11353–11358. [Google Scholar] [CrossRef] [PubMed]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef]
- Yap, P.S.X.; Krishnan, T.; Yiap, B.C.; Hu, C.P.; Chan, K.-G.; Lim, S.H.E. Membrane disruption and anti-quorum sensing effects of synergistic interaction between Lavandula angustifolia (lavender oil) in combination with antibiotic against plasmid-conferred multi-drug-resistant Escherichia coli. J. Appl. Microbiol. 2014, 116, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Gismondi, A.; Di Marco, G.; Redi, E.L.; Ferrucci, L.; Cantonetti, M.; Canini, A. The antimicrobial activity of Lavandula angustifolia Mill. essential oil against Staphylococcus species in a hospital environment. J. Herb. Med. 2021, 26, 100426. [Google Scholar] [CrossRef]
- Leigh-de Rapper, S.; van Vuuren, S.F. Odoriferous Therapy: A Review Identifying Essential Oils against Pathogens of the Respiratory Tract. Chem. Biodivers. 2020, 17, 6. [Google Scholar] [CrossRef]
- Luca, L.; Pauliuc, D.; Oroian, M. Honey microbiota, methods for determining the microbiological composition and the antimicrobial effect of honey—A review. Food Chem. 2024, 23, 101524. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Hatmal, M.M.; Sattar, K.; Ahmad, S.; Mustafa, M.Z.; Bittencourt, M.D.C.; Mohamud, R. Antiviral and immunomodulatory effects of phytochemicals from honey against COVID-19: Potential mechanisms of action and future directions. Molecules 2020, 25, 5017. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Bobis, O.; Bonta, V.; Acaroz, U.; Shah, S.R.A.; Istanbullugil, F.R.; Arslan Acaroz, D. General nutritional profile of bee products and their potential antiviral properties against mammalian viruses. Nutrients 2022, 14, 3579. [Google Scholar] [CrossRef] [PubMed]
- Farkasovska, J.; Bugarova, V.; Godocikova, J.; Majtan, V.; Majtan, J. The role of hydrogen peroxide in the antibacterial activity of different floral honeys. Eur. Food Res. Technol. 2019, 245, 2739–2744. [Google Scholar] [CrossRef]
- Sawicki, T.; Starowicz, M.; Kłębukowska, L.; Hanus, P. The profile of polyphenolic compounds, contents of total phenolics and flavonoids, and antioxidant and antimicrobial properties of bee products. Molecules 2022, 27, 1301. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayaghi, A.M.; Al-Kabsi, A.M.; Abduh, M.S.; Saghir, S.A.M.; Alshawsh, M.A. Antibacterial Mechanism of Action of Two Types of Honey against Escherichia coli through Interfering with Bacterial Membrane Permeability, Inhibiting Proteins, and Inducing Bacterial DNA Damage. Antibiotics 2022, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simoes, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Tao, H.; Chang, Y.-N. Characterization of antioxidant activity and analysis of phenolic acids and flavonoids in linden honey. Food Sci. Technol. 2022, 42, e76621. [Google Scholar] [CrossRef]
- Kim, J.; Uy, N.P.; Kim, D.; Lee, S. Analysis of Phenolic Acid Content and Antioxidant Activity of Chestnut Honey from Different Regions of Korea. Natl. Prod. Sci. 2023, 29, 127–131. [Google Scholar] [CrossRef]
- Anand, S.; Deighton, M.; Livanos, G.; Morrison, P.D.; Pang, E.C.K.; Mantri, N. Antimicrobial Activity of Agastache Honey and Characterization of Its Bioactive Compounds in Comparison with Important Commercial Honeys. Front. Microbiol. 2019, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Bogomolnaya, L.M.; Andrews, K.D.; Talamantes, M.; Maple, A.; Ragoza, Y.; Vazquez-Torres, A.; Andrews, H. Polymenis The ABC-type efflux pump MacAB protects Salmonella enterica serovar typhimurium from oxidative stress. mBio 2013, 4, e00630-13. [Google Scholar] [CrossRef] [PubMed]
- Khataybeh, B.; Jaradat, Z.; Ababneh, Q. Anti-bacterial, anti-biofilm and anti-quorum sensing activities of honey: A review. J. Ethnopharmacol. 2023, 317, 116830. [Google Scholar] [CrossRef]
- Nishino, K.; Yamasaki, S.; Nakashima, R.; Zwama, M.; Hayashi-Nishino, M. Function and inhibitory mechanisms of multidrug efflux pumps. Front. Microbiol. 2021, 12, 737288. [Google Scholar] [CrossRef] [PubMed]
- Babin, B.M.; Atangcho, L.; van Eldijk, M.B.; Sweredoski, M.J.; Moradian, A.; Hess, S.; Tolker-Nielsen, T.; Newman, D.K.; Tirrell, D.A. Selective proteomic analysis of antibiotic-tolerant cellular subpopulations in pseudomonas aeruginosa biofilms. mBio 2017, 8, e01593-17. [Google Scholar] [CrossRef]
- Zimmermann, S.; Klinger-Strobel, M.; Bohnert, J.A.; Wendler, S.; Rödel, J.; Pletz, M.W.; Löffler, B.; Tuchscherr, L. Clinically approved drugs inhibit the Staphylococcus aureus multidrug norA efflux pump and reduce biofilm formation. Front. Microbiol. 2019, 10, 2762. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K. Can flavonoids from honey alter multidrug resistance? Med. Hypotheses 2011, 76, 535–537. [Google Scholar] [CrossRef]
- Preda, V.G.; Sandulescu, O. Communication is the key: Biofilms, quorum sensing, formation and prevention. Discoveries 2019, 7, e100. [Google Scholar] [CrossRef]
- Truchado, P.; Gil-Izquierdo, A.; Tomás-Barberán, F.; Allende, A. Inhibition by chestnut honey of N-Acyl-L-homoserine lactones and biofilm formation in Erwinia carotovora, Yersinia enterocolitica, and Aeromonas hydrophila. J. Agric. Food Chem. 2009, 57, 11186–11193. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; Coppola, R. Quorum sensing and phytochemicals. Int. J. Mol. Sci. 2013, 14, 12607–12619. [Google Scholar] [CrossRef] [PubMed]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum vulgare L. Essential Oil. Evid.-Based Complement. Altern. Med. 2018, 7842583, 14. [Google Scholar] [CrossRef] [PubMed]
- Qasem, A.; Assaggaf, H.; Montesano, D.; Khalil, Z.; Al-Mijalli, S.H.; Baaboua, A.E.; El Omari, N.; El Menyiy, N.; Bakrim, S.; Sheikh, R.A.; et al. Determination of Chemical Compounds and Investigation of Biological Properties of Matricaria chamomilla Essential Oils, Honey, and Their Mixture. Molecules 2022, 27, 5850. [Google Scholar] [CrossRef]
- Katekar, V.P.; Rao, A.B.; Sardeshpande, V.R. A hydrodistillation-based essential oils extraction: A quest for the most effective and cleaner technology. Sustain. Chem. Pharm. 2023, 36, 101270. [Google Scholar] [CrossRef]
- Balázs, V.L.; Bordás, B.; Nagy-Radványi, L.; Ormai, E.; Kocsis, M.; Kocsis, B.; Farkas, Á.; Micalizzi, G.; Mondello, L.; Bencsik-Kerekes, E.; et al. Efficacy of lavender essential oil against respiratory tract bacteria is influenced by harvesting time. Sci. Rep. 2025; under review. [Google Scholar]
- Nagy-Radványi, L.; Balázs, V.L.; Kocsis, B.; Csikós, E.; Ángyán, V.D.; Szabó, P.; Biró, V.; Kocsis, M.; Farkas, Á. Antibacterial activity of Hungarian varietal honeys against respiratory pathogens as a function of storage time. Sci. Rep. 2024, 14, 10200. [Google Scholar] [CrossRef]
- Von Der Ohe, W.; Oddo, L.P.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized Methods of Melissopalynology. Apidologie 2004, 35, 18–25. [Google Scholar] [CrossRef]
- TCapture V4.3.0.605. 2022. Available online: https://www.tucsen.com/uploads/TCapture4.3.0.605.zip (accessed on 20 October 2022).
- Codex STAN 12-1981; Revised Codex Standard for Honey. Codex Alimentarius Commission: Rome, Italy, 2001.
- Bogdanov, S.; Lüllmann, C.; Martin, P. Harmonised methods of the European Honey Commission. Apidologie 1997, extra issue, 1–59. [Google Scholar]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Hindler, J.A.; Jorgensen, J.H. Susceptibility test methods: Fastidious bacteria. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Ed.; ASM Press: Washington, DC, USA, 2011; pp. 1180–1187. [Google Scholar]
- Nagy-Radványi, L.; Ormai, E.; Koloh, R.; Ángyán, V.D.; Kocsis, B.; Bencsik-Kerekes, E.; Szabó, P.; Csikós, E.; Farkas, Á.; Horváth, G.; et al. Biofilm Inhibition Activity of Fennel Honey, Fennel Essential Oil and Their Combination. Microorganisms 2024, 12, 2309. [Google Scholar] [CrossRef]
- CLSI Document M 2-A10; Performance Standards for Antimicrobial Disk Susceptibility Tests. 10th ed, Approved Standard. CLSI (Clinical and Laboratory Standards Institute): Wayne, PA, USA, 2009.
- CLSI Document M 100-S20; Performance Standards for Antimicrobial Susceptibility Testing; 19th Informational Supplement. CLSI (Clinical and Laboratory Standards Institute): Wayne, PA, USA, 2010.
- Patel, J.B.; Tenover, F.C.; Turnidge, J.D.; Jorgensen, J.H. Susceptibility test methods: Dilution and disk diffusion methods. In Manual of Clinical Microbiology, 10th ed.; Versalovic, J., Ed.; ASM Press: Washington, DC, USA, 2011; Volume 1, pp. 1122–1143. [Google Scholar]
- CLSI Document M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Balázs, V.L.; Nagy-Radványi, L.; Bencsik-Kerekes, E.; Koloh, R.; Szabó, D.; Kocsis, B.; Kocsis, M.; Farkas, Á. Antibacterial and Antibiofilm Effect of Unifloral Honeys against Bacteria Isolated from Chronic Wound Infections. Microorganisms 2023, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm formatting and anti-quorum sensing activity of selected essential oils and their main components on food related microorganisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, S.; Zhang, C.; Liu, Y.; Ma, L.; Zhang, X. Effects of sub-minimum inhibitory concentrations of lemon essential oil on the acid tolerance and biofilm formation of Streptococcus mutans. Arch. Oral Biol. 2018, 87, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Bennis, S.; Chami, F.; Chami, N.; Bouchikhi, T.; Remmal, A. Surface Alteration of Saccharomyces Cerevisiae Induced by Thymol and Eugenol. Lett. Appl. Microbiol. 2004, 38, 454–458. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]

| Pollen Type—Relative Frequency (%) | |||||
|---|---|---|---|---|---|
| Honey Sample | Tilia spp. | Robinia pseudoacacia | Brassica napus | Castanea sativa | Other |
| Linden | 36.0 | 25.7 | 20.4 | 6.2 | 11.7 |
| Chestnut | 0.2 | 4.3 | 1.4 | 93.1 | 1.0 |
| Honey Type, Plant Name | Sensory Characteristics (Color, Odor, and Consistency) | ABS450–720 (mAU) | Electrical Conductivity (mS/cm) | pH |
|---|---|---|---|---|
| Linden, Tilia spp. | Light amber, strong odor, semisolid, fine, granulated | 107 ± 1.4 | 0.528 ± 0.01 | 4.1 ± 0.01 |
| Chestnut, Castanea sativa | Dark amber, intense odor, semisolid, fine, granulated | 215.3 ± 1.4 | 0.598 ± 0.01 | 4.7 ± 0.01 |
| Antibiotics | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Amikacin | - | R | S | S | S |
| Ampicillin | R | R | S | R | R |
| Amoxicillin/ clavulanic acid | - | S | S | R | R |
| Ciprofloxacin | R | S | S | S | S |
| Ceftazidim | - | S | S | - | - |
| Eritromicin | - | - | R | - | S |
| Gentamicin | S | S | S | S | S |
| Imipenem | S | S | S | S | S |
| Levofloxacin | - | - | R | - | - |
| Oxacillin | R | - | - | - | - |
| Penicillin | R | - | - | - | - |
| Piperacillin/ tazobactam | - | S | S | - | - |
| Vankomicin | S | - | - | - | - |
| MIC Values (mg/mL) | |||||
|---|---|---|---|---|---|
| S. pneumoniae | H. influenzae | H. parainfluenzae | M. catarrhalis | P. aeruginosa | |
| Linden honey | 142.86 | 111.11 | 111.11 | 142.86 | 176.47 |
| Chestnut honey | 142.86 | 111.11 | 111.11 | 111.11 | 176.47 |
| Lavender essential oil | 0.31 | 0.63 | 0.63 | 1.25 | 2.50 |
| Antibiotics | 0.0008 | 0.0031 | 0.0016 | 0.0002 | 0.0063 |
| Bacterial Strains | Test Samples | Combination MIC |
|---|---|---|
| P. aeruginosa | Leo | 0.3125 |
| Lh | 88.235 | |
| Leo | 0.3125 | |
| Ch | 44.1175 | |
| S. pneumoniae | Leo | 0.0775 |
| Lh | 71.43 | |
| Leo | 0.0775 | |
| Ch | 35.715 |
| Bacterial Strains | Test Samples | FICI | Combination Effect |
|---|---|---|---|
| P. aeruginosa | Leo | 0.625 | additive effect |
| Lh | |||
| Leo | 0.375 | synergistic effect | |
| Ch | |||
| S. pneumoniae | Leo | 0.75 | additive effect |
| Lh | |||
| Leo | 0.5 | synergistic effect | |
| Ch |
| DNA Release from Bacterial Cells (%) | |||
|---|---|---|---|
| Samples | Concentrations | P. aeruginosa | S. pneumoniae |
| Chestnut honey (Ch) | MIC/2 | 11.7 ± 1.2 | 14.3 ± 1.3 |
| MIC | 26.9 ± 2.3 | 31.6 ± 1.7 | |
| MIC × 2 | 60.7 ± 2.5 | 66.3 ± 2.3 | |
| MIC × 4 | 100 | 100 | |
| Lavender essential oil (Leo) | MIC/2 | 15.6 ± 1.8 | 18.1 ± 1.3 |
| MIC | 36.5 ± 2.2 | 42.2 ± 2.1 | |
| MIC × 2 | 67.1 ± 2.5 | 68.4 ± 2.3 | |
| MIC × 4 | 100 | 100 | |
| Combination | MIC Ch + MIC Leo (1:1 ratio) | 67.9 ± 2.8 | 69.8 ± 3.0 |
| DNA Release from Bacterial Cells (%) | |||
|---|---|---|---|
| Samples | Time (min) | P. aeruginosa | S. pneumoniae |
| Chestnut honey (Ch) conc.: MIC × 2 | 0 | 0 | 0 |
| 20 | 34.4 ± 2.1 | 39.2 ± 2.2 | |
| 40 | 55.2 ± 2.3 | 58.9 ± 2.1 | |
| 60 | 60.7 ± 2.5 | 66.3 ± 2.3 | |
| 90 | 66.6 ± 2.2 | 68.0 ± 2.6 | |
| Lavender essential oil (Leo) conc.: MIC × 2 | 0 | 0 | 0 |
| 20 | 40.3 ± 2.2 | 49.1 ± 2.0 | |
| 40 | 62.8 ± 2.5 | 64.4 ± 2.6 | |
| 60 | 67.1 ± 2.5 | 68.4 ± 2.3 | |
| 90 | 69.2 ± 2.3 | 70.5 ± 2.8 | |
| Combination conc.: MIC Ch + MIC Leo (1:1 ratio) | 0 | 0 | 0 |
| 20 | 46.2 ± 2.4 | 53.6 ± 1.9 | |
| 40 | 63.7 ± 2.2 | 68.0 ± 2.1 | |
| 60 | 67.9 ± 2.8 | 69.8 ± 3.0 | |
| 90 | 70.9 ± 2.6 | 71.2 ± 2.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ángyán, V.D.; Balázs, V.L.; Kocsis, M.; Kocsis, B.; Horváth, G.; Farkas, Á.; Nagy-Radványi, L. Synergistic Antibiofilm Effects of Chestnut and Linden Honey with Lavender Essential Oil Against Multidrug-Resistant Otitis Media Pathogens. Antibiotics 2025, 14, 146. https://doi.org/10.3390/antibiotics14020146
Ángyán VD, Balázs VL, Kocsis M, Kocsis B, Horváth G, Farkas Á, Nagy-Radványi L. Synergistic Antibiofilm Effects of Chestnut and Linden Honey with Lavender Essential Oil Against Multidrug-Resistant Otitis Media Pathogens. Antibiotics. 2025; 14(2):146. https://doi.org/10.3390/antibiotics14020146
Chicago/Turabian StyleÁngyán, Virág D., Viktória L. Balázs, Marianna Kocsis, Béla Kocsis, Györgyi Horváth, Ágnes Farkas, and Lilla Nagy-Radványi. 2025. "Synergistic Antibiofilm Effects of Chestnut and Linden Honey with Lavender Essential Oil Against Multidrug-Resistant Otitis Media Pathogens" Antibiotics 14, no. 2: 146. https://doi.org/10.3390/antibiotics14020146
APA StyleÁngyán, V. D., Balázs, V. L., Kocsis, M., Kocsis, B., Horváth, G., Farkas, Á., & Nagy-Radványi, L. (2025). Synergistic Antibiofilm Effects of Chestnut and Linden Honey with Lavender Essential Oil Against Multidrug-Resistant Otitis Media Pathogens. Antibiotics, 14(2), 146. https://doi.org/10.3390/antibiotics14020146









