1. Introduction
Biofilm-associated infections are a well-known and serious complication in present-day implant surgery, with approximately 80% of implant-related infections linked to bacterial biofilms [
1]. Among these, the
Staphylococcus aureus is the most prevalent pathogen, responsible for 30% of all biofilm-associated implant infections [
2,
3].
Biofilm is a fixed matrix containing exopolysaccharides, fibrins and lipoproteins in which microbial communities reside, and it has the ability to irreversibly attach to implants surfaces [
4]. Biofilm formation is a complex process involving an attachment phase, microcolony formation phase, and maturation and dispersion phase [
5]. Biofilm can exhibit tolerance to the host immune system by the inhibition of phagocytosis, opsonization avoidance and inactivation of the complement system. The formation of mature biofilm, with high bacterial density, is associated with the chronicity of implant infections [
6]. Moreover, in chronic biofilm-associated implant infections, persister cells can regrow and cause infection relapse after antibiotic therapy is discontinued [
7]. Older biofilms are more tolerant to antibiotics, with minimal inhibitory concentrations 10 to 1000 times higher than those for planktonic bacteria [
8].
Moreover, in these biofilms, mutations are induced by spontaneous, antibiotic- or stress-induced mutagenesis. Owing to the increased number of bacteria that can survive within the biofilm, this layer serves as a reservoir for resistant bacterial clones, which paves the way for the development of antimicrobial resistance [
9]. Given the challenges posed by biofilm and the growing threat of antimicrobial resistance, new bactericidal strategies must be developed.
Radioimmunotherapy (RIT) is the application of antibodies labeled with radionuclides to provide targeted radioactive therapy. This type of treatment is already a known and an applied treatment option in oncology and could be used to treat biofilm-associated implant infections, in case a specific antibody is used that targets the biofilm [
10]. Teichoic acids are surface polymers found on the bacterial cell wall of most Gram-positive bacteria, such as the
S. aureus. If bound to a peptidoglycan, it is called wall teichoic acid (WTA), a glycopolymer also expressed in the extracellular matrix of biofilm [
11,
12]. WTA has been demonstrated to be an excellent target for 4497-IgG1 antibody (anti-β-GlcNAc WTA) in vitro and in vivo and can labeled with alpha or beta radioisotopes to create targeted radioimmunotherapy for Gram-positive bacterial infections [
13]. Alpha and Beta radiation have tissue penetration depth of 85 µm and 1–2 mm, respectively [
14,
15,
16]. In combination with pathogen-specific antibodies, cytotoxic radiation can be delivered at the infection site, resulting in bacterial cell death through single- or double-strand DNA breaks. Additionally, oxidative stress can contribute to bacterial death through radiation-induced reactive oxygen species formation [
17]. In vitro antimicrobial efficacy against the
S. aureus with the 4497-IgG1 antibodies labeled with Bismuth-213 (alpha-radiation, half-life of 45.6 min), Actinium-225 (alpha-radiation, half-life 9.9 days) and Lutetium-177 (beta-radiation, half-life 6.7 days), has been documented [
18,
19]. Likewise, in vitro and in vivo antifungal efficacy against
Cryptococcus neoformans fungi with 18B7-IgG1 labeled with Bismuth-213 and Rhenium-188 (beta-radiation, half-life of 16.9 h) has been reported [
20,
21].
In this preliminary study, we present a proof of principle for the application of radionuclides for treatment of a biofilm associated infection of an intramedullary implant. Therefore, we used Wistar Han rats with a surgically placed femur implant colonized with a maturated S. aureus biofilm, mimicking a chronic implant infection. Radionuclides Actinium-225 and Lutetium-177 were used with and without the antibody against S. aureus peptidoglycan β-GlcNAc WTA, which is also highly enclosed within the biofilm. We herewith tested if the application of different doses of radioimmunotherapy exerts a relevant antimicrobial effect on the implant-associated infection and used SPECT/CT and gamma counting to determine specific targeting and biodistribution of the radionuclides. Lastly, we conducted a short-term tolerance assessment of the radioimmunotherapy.
3. Discussion
There is substantial evidence that alpha-radiation and beta-radiation can eradicate bacteria and fungi in vitro and in vivo [
18,
21]. Including our previous results with the same 4497-antibody on
S. aureus bacteria, displaying 99% and 99.99% killing when labeled with Actinium-225 and Lutetium-177 radionuclide, respectively [
19].
However, no in vivo radioimmunotherapy treatments for surgical subjects with biofilm-associated implant infections have been attempted. This study is the first to apply radioimmunotherapy (RIT) and unbound radionuclide treatment in Wistar Han rats with an intramedullary femur implant infected with three-day matured S. aureus biofilm. This study was designed as an exploratory in vivo proof-of-concept investigation to evaluate the feasibility and biodistribution of pathogen-specific radioimmunotherapy.
Due to the dormant character of the biofilm, sufficient infection development was ensured by allowing a three-day incubation period before administering the RIT and the unbound radionuclide treatment. Our results indicate that targeted alpha-radiation (
225Ac-4497) has a combined small antimicrobial effect on the implant and surrounding bone, whereas beta-radiation (
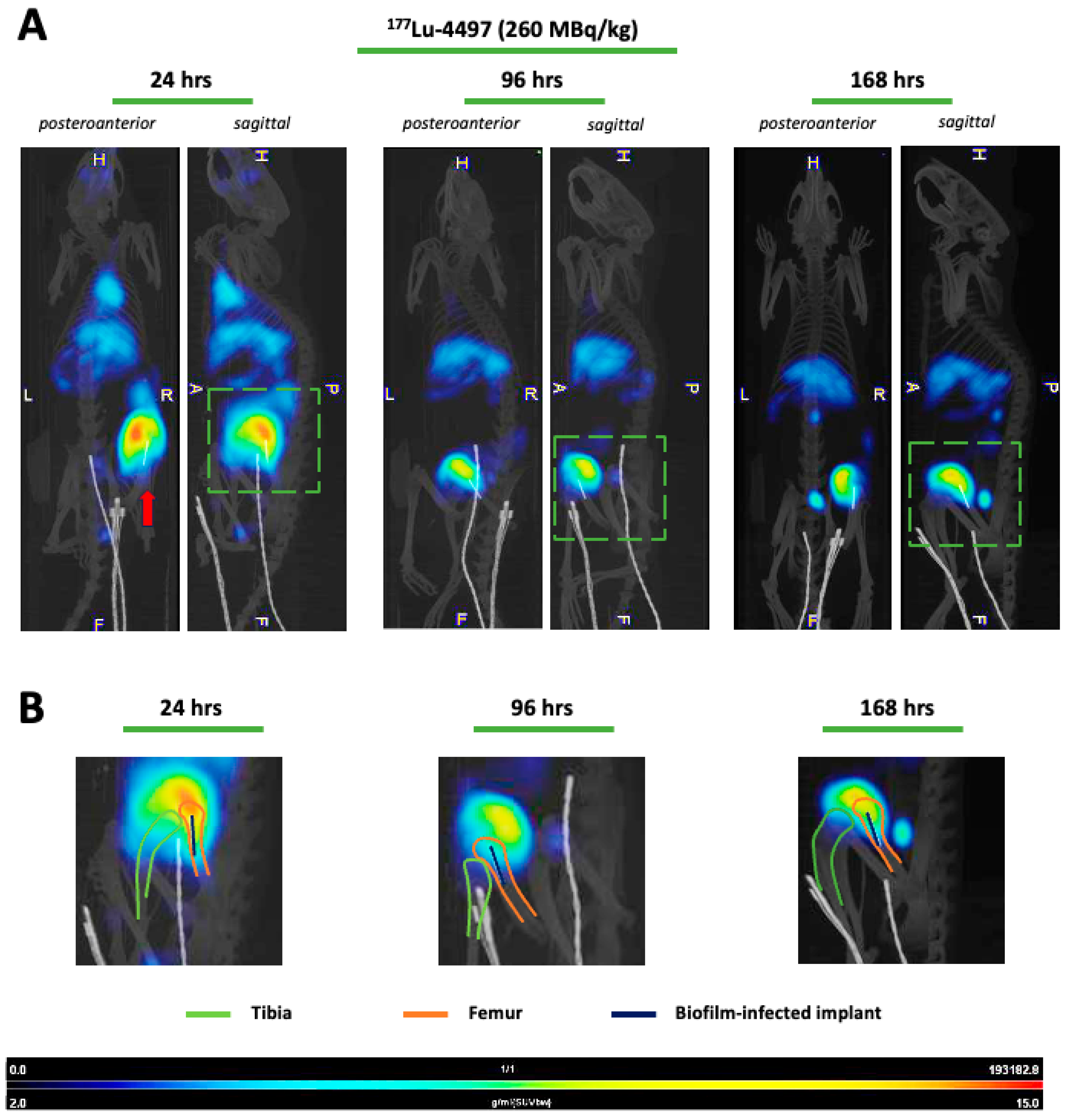
177Lu-4497) had a similar effect on the implant but almost no antimicrobial efficacy on the surrounding bone after the seven-day follow-up period. A significant observation in this study was the shift in RIT accumulation to the joint and articular capsule of the infected leg as a consequence of new infection onset in the joint (
Figure 3). For effective radioimmunotherapy, precise targeting is essential, as is defining an antimicrobial dose that is both therapeutically effective and minimally toxic. Given that alpha radiation has a tissue penetration depth of only 47–85 µm, whereas beta radiation penetrates approximately 1–2 mm, achieving accurate radionuclide localization is critical [
15,
16,
17]. Any redistribution of the radiopharmaceutical could therefore alter the treatment effect. The observed redistribution might have resulted in reduced radiation exposure to the deeper-positioned infected implant and bone. As a consequence, we did not report the full potential of the antimicrobial effect of RIT on a biofilm-associated implant infection. The antimicrobial effects on the implant and surrounding bone were minimal (ranging from a 45–93% reduction) and did not achieve the desired bactericidal effects, which typically requires a 99.9% reduction (a 3-log reduction) [
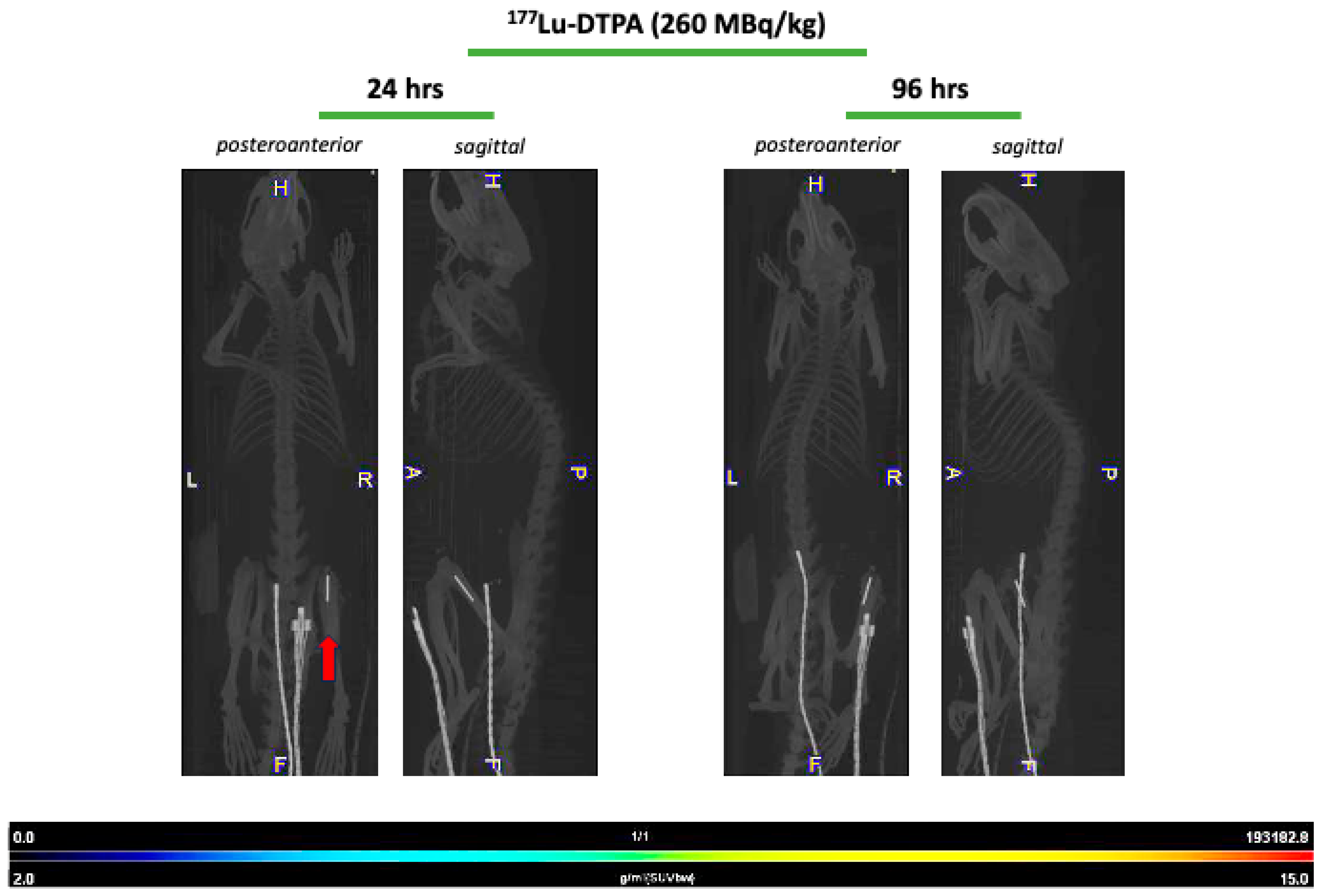
22]. Initially, it was hypothesized that unbound radionuclide treatment would serve as a negative control, leading to rapid clearance of the radioisotope, and consequently, highlighting the targeting efficiency of the 4497-antibody. This was observed during treatment with unbound Lutetium-177, which showed no uptake throughout the study duration (
Figure 4). No SPECT/CT analyses of
225Ac-4497 were performed due to the low gamma emission of Actinium-225, which makes sufficient imaging challenging. However, since the same 4497-antibody was used, a similar biodistribution was expected. Surprisingly, the unbound radionuclide treatment group using Actinium-225 also performed in the same range as RIT, achieving an 80% CFU reduction on the implant. Even more unexpectedly, this treatment group demonstrated a 98% CFU reduction in the surrounding bone, approaching nearly a 1-log reduction (99%). This finding was supported by the comparable ex vivo biodistribution accumulation of the
225Ac-DTPA and
225Ac-4497 in the femoral bone surrounding the implant (
Figure 2A) and of the prematurely terminated Wistar Han rat (
Figure A3). Additionally, this femoral accumulation was further corroborated by the observed leukopenia of the
225Ac-DTPA treatment group (
Figure 5A). It is important to note that, in this preliminary investigation,
225Ac-DTPA was utilized primarily as a reference control to define baseline biodistribution patterns and quantify non-specific tissue accumulation, rather than as a comparator for therapeutic efficacy.
Actinides, such as the radionuclide Actinium-225, have been shown to have a high affinity for bone glycoproteins [
23,
24]. Similar to lanthanides, Actinium ions can substitute for calcium in the hydroxyapatite matrix because of their similar ionic radii and chemical properties [
25,
26]. This can occur particularly in cases of increased bone turnover, such as after bone implant surgery or during a local bone infection.
Presumably, three potential mechanisms may explain this unexpected accumulation. First, the instability of the
225Ac-DTPA complex could result in the release of free
225Ac
3+, which may then accumulate in the infected femur (
Figure 2A). This phenomenon was already observed in a mouse model [
27]. Second, the intact
225Ac-DTPA complex itself may have organ-specific affinity. Davis et al. (1999) reported that Actinium-225 bound to a CHX-DTPA complex preferentially accumulates in the liver and bone compared to other organs five days post-injection [
28]. Consistent with this, increased liver uptake of
225Ac-DTPA was also observed in the present study (
Figure 2A). Third, a combination of both free isotope release and organ-specific uptake of the intact complex may contribute to the observed distribution.
As a result of its bone-targeting properties, the 225Ac-DTPA treatment localized to the entire femoral bone near the biofilm-infected implant, leading to the greatest observed reduction in CFU. Interestingly, despite the use of a limited small-animal sample size, the results suggest in vivo evidence of modest antimicrobial efficacy through radiation. Further research is needed to elucidate the in vivo stability of conjugating molecules with Actinium-225 and the mechanisms underlying Actinium-225 deposition in bone for its potential future application in biofilm-associated implant infections in larger animal studies.
The biodistribution of both
225Ac-4497 and
177Lu-4497 treatment groups shows greater uptake in the liver and spleen, which is expected due to the fenestrated capillaries with large paracellular pores and sinusoids to facilitate convective transport of the radionuclide-antibody conjugate [
29]. As observed from the SPECT/CT analyses (
Figure 3A), there is substantial accumulation of
177Lu-4497 (260 MBq/kg) at the right infected leg. However, it seems that the highest uptake occurs in the knee joint and articular capsule, as was confirmed with biodistribution analysis and SPECT/CT analyses (
Figure 2B and
Figure 3B). It can be confidently stated that during implantation, the joint and the surgical tract became infected with the same
S. aureus AH4802 bacteria, resulting in the accumulation of the radionuclide-antibody conjugate in these locations. Given the fact that this distally located tissue is likely to be highly vascularized, the bacteria in the arthritic tissue likely take away a large portion of the
177Lu-4497 radionuclide-antibody conjugate. Unlike the application of RIT in tumor models, postoperative surgical effects and bacterial dispersion leading to new infection onset are factors that influence the biodistribution and may have affected the treatment efficacy of RIT in this model. The development of infection within the close-by joint and surgical tract creates a competitive environment in which radiolabeled antibodies may preferentially bind to the most accessible site of infection.
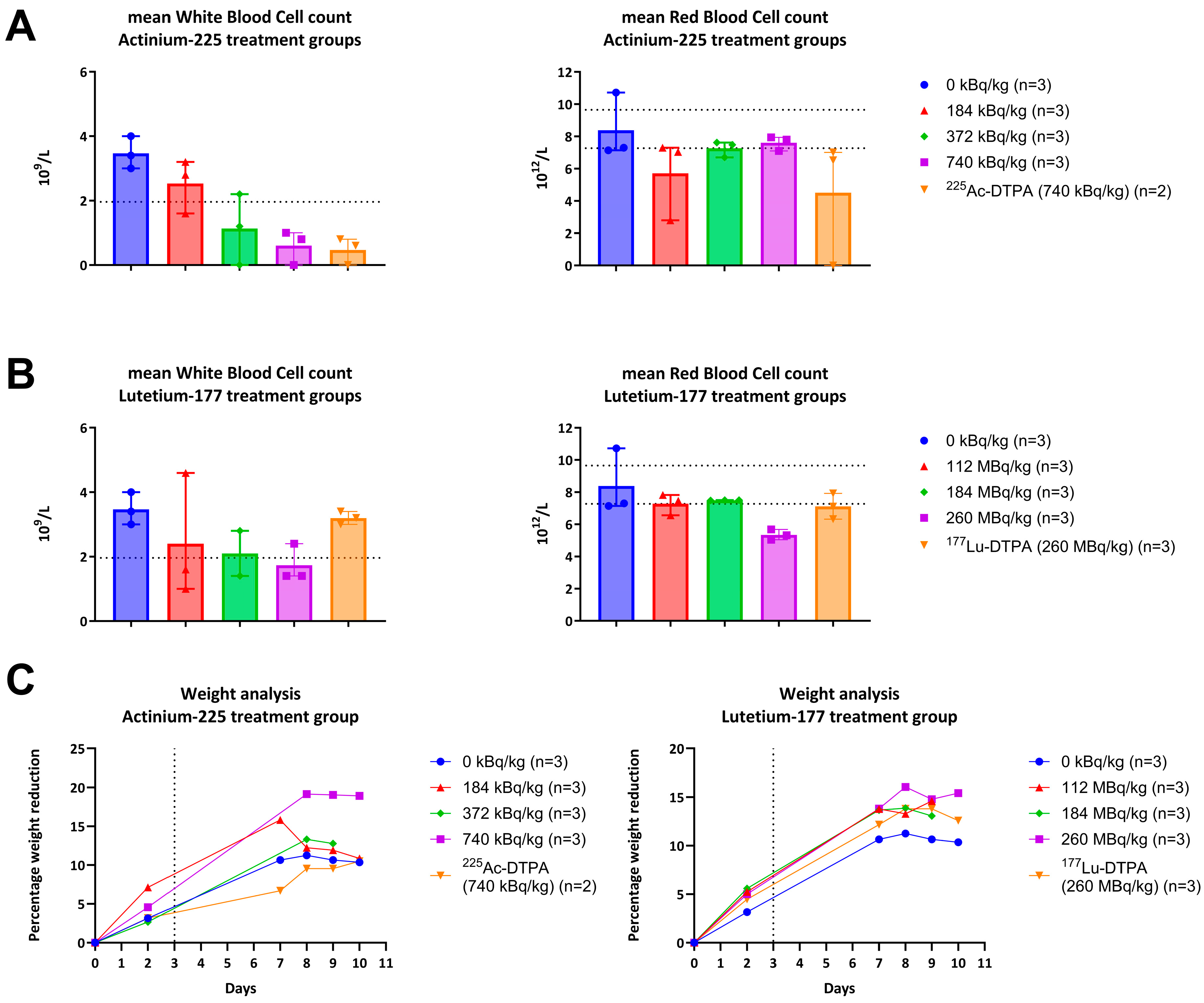
Regarding RIT with Actinium-225 and Lutetium-177, the bacterial reduction across all treatment groups was approximately within the same range for the implant and the surrounding bone. However, the results demonstrate that
225Ac-4497 (740 kBq/kg) treatment group performed best of all RIT treatments with 78% and 93% CFU reduction on the implant and surrounding bone despite the accumulation shift and even under the challenge of low WBC count (
Figure 5A). Combined with the unbound Actinium-225 treatment group, this possibly suggests that alpha radiation exerts a greater initial antimicrobial effect in vivo compared to beta-radiation at the doses used.
Single- and double DNA strand breakage, as well as the formation of ROS within the bacteria, are both key mechanisms underlying the application of radioimmunotherapy for infections [
18,
19]. Besides these intended effects, ionizing particles can also have a negative impact on physiological systems, including parts of the immune system. The decay of Actinium-225 releases four alpha-particles with energies ranging from 5.8 to 8.4 MeV [
17]. Due to this high linear energy transfer, a dose-dependent negative trend was observed in the white blood cell counts, compromising the host’s first line of defense within the innate immune system against the induced biofilm-associated implant infection. As the linear energy transfer of Lutetium-177 (0.497 MeV) is approximately tenfold lower than for Actinium-225, the treatment and systemic effects are expected to be milder [
14,
15]. Despite these mild antibacterial effects, a dose-dependent decrease in the white blood cell count was observed, similar to the
225Ac-4497 treatment groups (
Figure 5A). There is evidence suggesting that these effects are transient and typically return to baseline over time [
30,
31]. Based on initial data, the
177Lu-4497 treatment group exhibited a dose-dependent increase in ALT and AST, unlike the
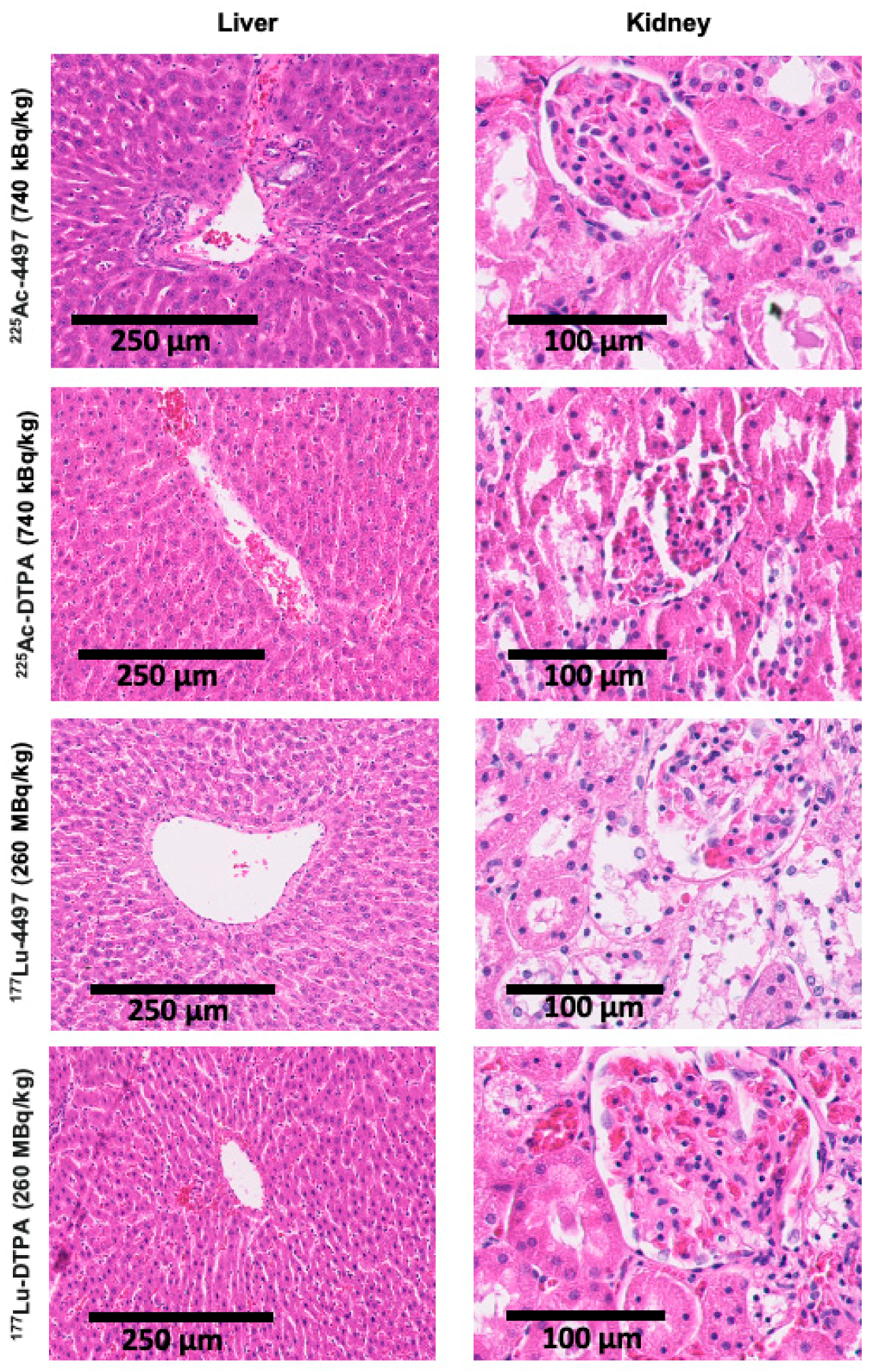
225Ac-4497 treatment group. This suggests that short-term tolerability for beta-radiation is achieved at 112 MBq/kg for Wistar Han rats. Because increases in aminotransferases can occur before visible tissue changes, no significant histopathological alterations were observed in the present study. In contrast, alpha-radiation treatment did not lead to increased liver or kidney function markers, suggesting that the maximum administered dose in the present study was still well tolerated after one week. No weight loss exceeding 20% was observed in this study. While some of the weight loss may be related to radiation exposure, it appears to be limited, especially considering that the rats also underwent major surgery with a biofilm-infected implant, which can all induce stress responses and associated appetite suppression.
Animal experiments involving biofilms are complex. In an in vivo experiment with biofilms, one must account for the dynamic flow of antimicrobials, hypoxic conditions, and time-dependent effects. Additionally, the in vivo environment can trigger adaptive survival mechanisms in the biofilm, which may alter its metabolic rate, replication rate, or surface protein expression, potentially modifying antimicrobial effects [
32].
In this study, a clinical setting was simulated with an intramedullary femur implant in which a chronic infection was mimicked by introducing a three-day maturated biofilm-associated infection. The small sample size (n = 3 per group) limits our ability to draw firm conclusions for each individual group. However, the combined data shows a modest antimicrobial effect with Actinium-225. Whether Actininium-225 should be used with or without an antibody targeting the bacteria and their biofilm may depend on the intended application. Hypothetically, radioisotopes known to accumulate in bone (without the conjugation to an antibody) could be applied for osteomyelitis treatment and should be further explored. Despite the limitations regarding radiochemical stability and small animal sample size, this study provides the first in vivo indication that RIT can localize and affect biofilm-associated implant infections, warranting further preclinical optimization.
Our infection model, in which an implant infection is induced on the same day of surgery, accompanied postoperative healing processes, limits our ability to truly replicate a chronic infection. Nonetheless, by inducing an implant infection solely with a mature biofilm, the challenges of biofilm-associated implant infections, such as inhibition of phagocytosis, opsonization avoidance, and inactivation of the complement system, are being mimicked, unlike with planktonic bacterial infections [
7]. It would be valuable to pursue future research with alpha-emitting radionuclides with shorter half-life, such as Bismuth-212/213 or Lead-212, which would increase radiation emission per unit of time. This approach could potentially better account for the in vivo bacterial replication rate, which has been reported to be in the range of 1–3 h in humans [
33]. In oncology, radioimmunotherapy (RIT) is often administered fractionally to enhance therapeutic success [
34]. In this study, however, we used a single injection approach. Future RIT applications in surgical implant infection models could explore multi-injection regimens. Moreover, applying RIT in this surgical infection model introduces complexities in biodistribution, as the onset of bacterial arthritis may have competed with the implant infection site for antibody binding. Interestingly, co-injecting unlabeled antibodies could potentially increase the systemic circulation of radiolabeled antibodies by reducing splenic antibody uptake. This strategy may enhance radiolabeled antibody accumulation at the target site, thereby improving treatment efficacy [
35,
36,
37]. Additionally, intra-articular injection of the radiolabeled antibodies could result in higher local concentrations at the implant location, which might improve the treatment effects as well. After identifying an in vivo stable radionuclide–antibody complex and determining the optimal administration method, long-term toxicity assessments of liver and kidney function and larger animal studies should be conducted to evaluate its clinical potential.
Importantly, at this early stage, as we seek to establish evidence for the antimicrobial potential of targeted radiation therapy, combining antibiotics with radioimmunotherapy may prove highly valuable for treating biofilm-associated implant infections. Ionizing radiation is not hindered by the physical barrier of the biofilm or the low metabolic rate of biofilm-embedded (dormant) bacteria. Radiation could disrupt the biofilm, potentially increasing bacterial sensitivity to antibiotics and leading to a synergistic effect. If such a combination therapy could lower the minimal inhibitory concentration (MIC) of the antibiotic required for biofilm eradication, we would be one step closer to minimizing the burden of biofilm-associated implant infections and antimicrobial resistance.
4. Materials and Methods
4.1. Study Design
A biofilm-associated infection with a femoral implant was induced in 27 Wistar Han Rats. The antimicrobial effects of three different doses of Actinium-225-labeled antibodies (
225Ac-4497) and Lutetium-177-labeled antibodies (
177Lu-4497), as well as unlabeled
225Ac and
177Lu (conjugated to DTPA), were evaluated after seven days of follow-up. A saline injection (0 kBq/kg) served as the control intervention. Primary study outcomes included antimicrobial efficacy on the implant and surrounding bone, determined by bacterial count CFU. Secondary study outcomes involved short-term systemic effects (blood analysis) and tissue response (histology). Additionally, specific targeting of the biofilm and biodistribution was assessed during follow-up using SPECT/CT analyses (
177Lu-4497 only) and ex vivo via gamma-count analyses (both isotopes). The in vivo experiments were approved by the Animal Research Ethics Board of the University of Saskatchewan, Canada (protocol AUP20230036). All experiments were performed in accordance with institutional guidelines and regulations, and with the ARRIVE guidelines for reporting animal research [
38].
4.2. Antibody Conjugation
Antibodies were made according to protocol as described in detail by de Vor et al. [
13]. In short, the Mut (H+Y) HuIgG1-antiWTA-4497 antibody (4497-antibody) was produced by cloning the human constant regions into pcDNA3.4 vectors harboring the variable heavy (VH) and light (VL) chain sequences. These sequences, which were codon-optimized and contained KOZAK and HAVT20 signal peptides, were originally derived from B cells of patients infected with
S. aureus. After transfection into EXPI293F cells, IgG1 antibodies were recovered using HiTrap protein A columns from the supernatant within 4–5 days. Following dialysis in PBS and filter sterilization, the antibodies were examined for aggregation and stored at a concentration of 7.68 mg/mL at 4 °C.
Conjugation of the 4497-IgG1 antibody to the bifunctional chelator S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (p-SCN-Bn-DOTA, Macrocyclics
TM, Plano, TX, USA) was performed by exchanging the antibody’s storage buffer with NaHCO
3 and Na
2CO
3 buffer solution that had been passed through a chelex-100 cation exchange resin to remove any advantageous metals (chelexed). A 0.5 mL 30 kDa molecular weight cutoff Amicon microconcentrator (Millipore, Burlington, MA, USA) was used to ensure complete exchange of the storage buffer; this process was repeated 10 times at 4 °C. A 10-fold molar excess of p-SCN-Bn-DOTA was prepared in NaHCO
3 and Na
2CO
3 buffer solution immediately prior to use and then added to the antibody solution. This reaction mixture was incubated at 37 °C for 1.5 h under agitation. The 4497-DOTA mixture was then exchanged into a 0.15 M ammonium acetate buffer (chelexed) using a 30 kDa molecular weight cutoff Amicon microconcentrator, followed by centrifugation (10×
g at 4 °C) [
39].
4.3. Radiolabeling with Actinium-225 and Lutetium-177
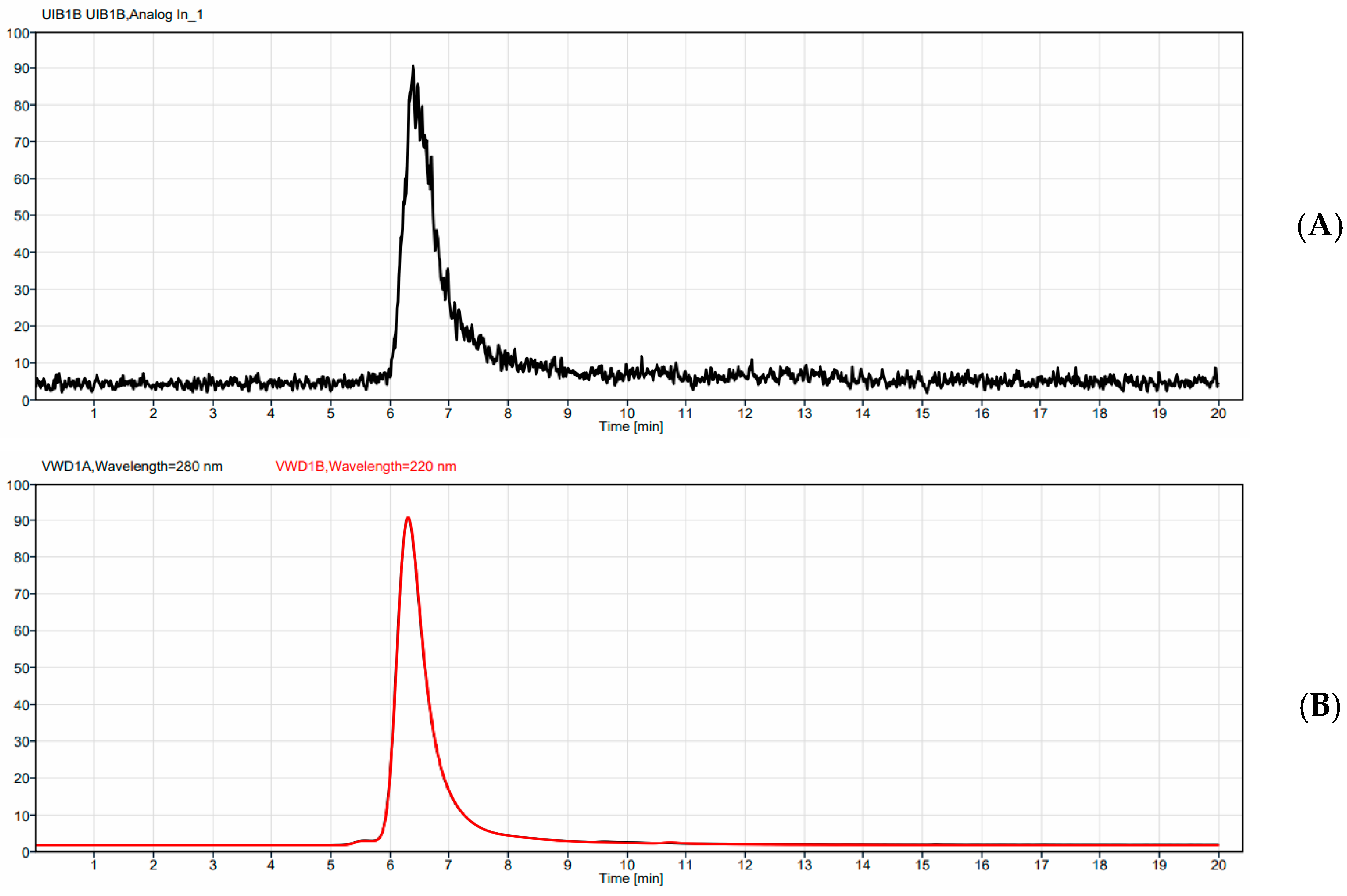
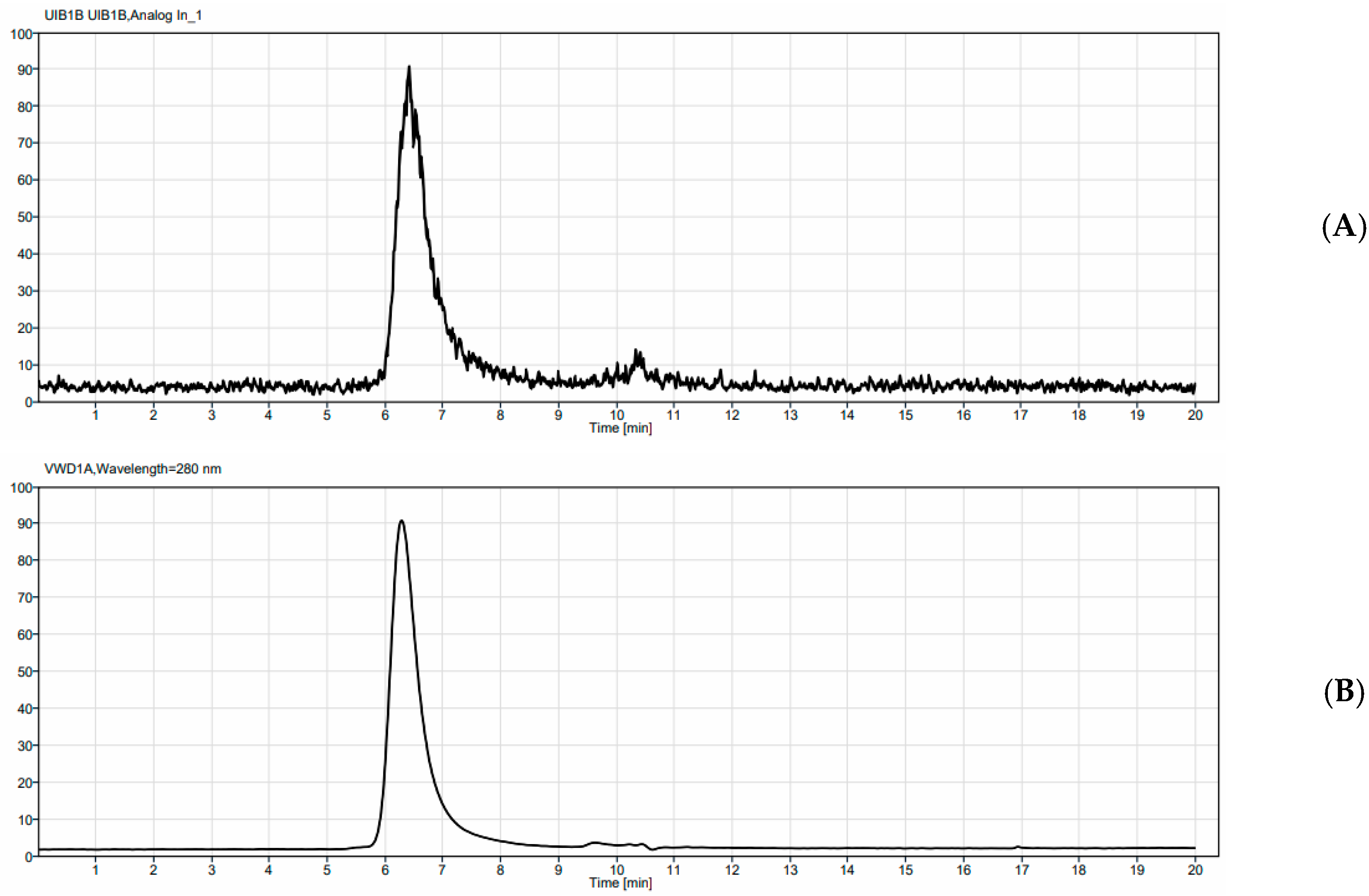
0.037 MBq of 225Ac and 0.37 MBq of 177Lu per µg of 4497-DOTA conjugate were radiolabeled at 37 °C for 1 h (the pH was maintained at 7 throughout the radiolabeling process). Generally, for 225Ac (Oakridge National Labs, Oak Ridge, TN, USA) labeling, the desired amount of 225AcCl3 (37 mBq), dissolved in 0.01 M HCl, was added to a minimum volume of 0.15 M ammonium acetate, giving a 50 µL final volume. To this solution, 100 µg of antibody (47.6 µL) was added, and the solution was heated at 37 °C for 1 h. After, 3 µL of 5 mM DTPA solution was added to bind any free 225AcCl3. For 177Lu (McMaster University, Hamilton, ON, Canada), labeling was performed in a similar manner using 555 mBq of 177LuCl3 in 50 µL of 0.15 M ammonium acetate solution. 1.5 mg of 4497-DOTA conjugate (714 µL) was added to the radiometal and heated at 37 °C for 1 h. After, 3 µL of 5 mM EDTA solution was added to bind any free 177LuCl3. Silica gel strips (10 cm length, Agilent Technologies Inc., Santa Clara, CA, USA) were used to measure the radiopurity of the radiolabeled 4497-DOTA conjugate by instant thin layer chromatography (iTLC) with 0.5 mM EDTA as mobile phase, approximately 30 to 60 min before injection. The strips were cut in half, and each part was measured using a gamma counter (2470 Wizard2 Gamma counter, PerkinElmer, Waltham, MA, USA). The gamma counter was calibrated for either the Actinium-225 or Lutetium-177 emission spectrum, and only emissions within this range were counted in counts per minute (CPM). The CPM of the radiolabeled antibody was divided by the total sum of CPM from both strips and multiplied by 100 to calculate radiopurity.
Samples of each radio-conjugate were then run on an Agilent HPLC (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a UV detector monitoring at UV = 280, a BioScan radioactivity detector (Eckert & Ziegler, Valencia, CA, USA), and a TSKgel SuperSw2000, 4.6 mm I.D. X 30 cm, 4 µm SEC column (TOSOH Bioscience, Tokyo, Japan). An isocratic method was run for 20 min with a 150 mM Sodium Phosphate buffer, pH 7, as the eluant.
A stability study was carried out by taking purified 225Ac-4497 and diluting it in 90% human serum or 90% PBS and storing it at 37 °C. Aliquots were removed at 24, 48, 72, and 168 h and added to a 5 mM DTPA solution. Samples were then allowed to sit for 5 min before being run on iTLC strips as previously described.
Evaluation of non-targeted radiation effects was performed with the unbound radionuclide treatment groups. Diethylenetriaminepentaacetic acid (DTPA) was used as a chelator for the unbound Actinium-225 and Lutetium-177 radionuclides [
40]. Due to the multiple binding sites of this chelating molecule, it facilitates the formation of stable negatively charged in vivo complexes with trivalent metal ions such as Actinium-225 (Ac
3+) and Lutetium-177 (Lu
3+), thereby preventing the hydrolysis of free radiometals and facilitating their excretion through the kidneys (
Figure 8). The hydrophilic DTPA was chosen over the more hydrophobic DOTA chelating molecule to reduce unwanted accumulation of the unbound Actinium-225 and Lutetium-177 ions. For the unbound radionuclide treatment groups, the administered radioactive dose was matched with the highest dose of the
225Ac-4497 (740 kBq/kg) and
177Lu-4497 (260 MBq/kg) radioimmunotherapy treatment groups.
4.4. Biofilm Cultures on Implants
In these experiments, the
S. aureus USA300 LAC (AH4802) strain was used [
41]. The bacteria were cultured at 37 °C overnight on tryptic soy broth (TSB). The overnight culture was then re-inoculated into fresh TSB medium and incubated for 3 h to allow logarithmic growth before use.
The implants were made of a titanium alloy (medical grade 23, ELI, Ti6AI4V) with a length of 10 mm and a width of 0.8 mm. The implants were produced at the Additive Manufacturing Lab (TU Delft, Delft, The Netherlands) using a selective laser melting machine (SLM-125, Realizer, Frankfurt, Germany). Grooves were incorporated into the implants to improve the attachment of biofilm upon insertion and the implants were autoclaved prior to culturing biofilm on the surface of these titanium implants. To further facilitate bacterial binding, 250 μL of human fibronectin (Sigma, Saint Louis, MO, USA) at a concentration of 20 μg/mL in carbonate–bicarbonate buffer was incubated with the implants overnight at 4 °C. An overnight culture with S. aureus bacteria was diluted to an OD600 of 1 and then diluted 1:10 in fresh biofilm growth medium (TSB containing 0.5% w/v glucose and 3% w/v NaCl). Subsequently, 500 μL of this suspension was transferred to the implant and incubated statically for 72 h at 37 °C. Every 24 h, 250 μL of the medium was replaced with fresh biofilm growth medium. Prior to implantation, the implants with three-day-old biofilms were washed three times to remove any planktonic bacteria.
4.5. Biofilm-Associated Implant Infection and Radioimmunotherapy
Twenty-seven male Wistar Han rats (Charles River laboratories, Montreal, QC, Cananda) of approximately 13 weeks old were operated to insert one femoral rod implant into the intramedullary canal of the right femur. Three days after the surgical procedure, radioimmunotherapy was administered intravenously via the tail.
In the Actinium-225 study, twelve Wistar Han rats were treated with the 4497-antibody radiolabeled with Actinium-225 (225Ac-4497). The doses per treatment group were 184 kBq/kg (n = 3), 372 kBq/kg (n = 3) and 740 kBq/kg (n = 3), respectively. Three rats received DTPA-bound Actinium-225 (225Ac-DTPA treatment group) as an unbound radionuclide treatment with an activity of 740 kBq/kg (n = 3).
In the Lutetium-177 study, twelve Wistar Han rats were treated with the 4497-antibody radiolabeled with Lutetium-177 (177Lu-4497). The doses per treatment group were 112 MBq/kg (n = 3), 184 MBq/kg (n = 3) and 260 MBq/kg (n = 3), respectively. Three rats received DTPA-bound Lutetium-177 (177Lu-DTPA treatment group) as an unbound radionuclide treatment with an activity of 260 MBq/kg (n = 3). Additionally, three rats were treated with saline, serving as a control group (0 kBq/kg).
The doses used for the Actinium-225 and Lutetium-177 treatment groups were guided by general insights from prior research within our group and studies involving radiolabeled antibodies. All rats were followed for seven days after radioimmunotherapy administration prior to termination. Food and water were provided ad libitum. Only in the Lutetium-177 study, SPECT/CT analyses were performed at 24 h, 96 h and 168 h post-injection for the 177Lu-4497 (260 MBq/kg) treatment group and 177Lu-DTPA treatment group (260 MBq/kg).
4.6. Implant Surgery
In short, all Wistar Han rats were anesthetized with isoflurane (induction dose: 5% and maintenance dose: 2–3%). Prior to the surgery, 2 mL NaCl 0.9%, slow-release buprenorphine (0.05 mg/kg) and meloxicam (2 mg/kg) were administered subcutaneously. After a straight midline incision on the right knee, the patella was repositioned to the medial side exposing the femoral condyles. The entrance to the medullary canal was established using a drill with a length of 1 cm and a diameter of 1 mm, creating an insertion hole between the two femoral condyles. The biofilm-infected implant was inserted, and the opening was then closed with bone wax (SMI). Absorbable sutures were used to reposition the patella back to anatomical position and to close the inner tissues. Non-absorbable sutures were used to close the skin. Slow-release buprenorphine (0.05 mg/kg) was administered every other day, and meloxicam (2 mg/kg) was given daily for three days post-surgery for pain management.
4.7. CFU Count Assessment
Prior to biofilm maturation, three randomly chosen autoclaved implants and the biofilm growth medium were evaluated for bacterial growth to ensure no introduction of other pathogens than the S. aureus USA300 LAC (AH4802) pathogen. Additionally, prior to implantation of the biofilm-infected implants, five randomly chosen implants were assessed for CFU after three days of biofilm maturation to evaluate initial bacterial load upon insertion. After seven days of follow-up, the biofilm-infected femur with implant was collected from all animals, and subsequently stored in ice until assessment. Before the CFU assessment, the femurs were rinsed with sterile PBS. Aseptically, the implant was separated from the bone and stored in 1 mL of sterile PBS. The surrounding femoral bone was cut into pieces and placed in 20 mL sterile PBS. A Kinematica Polytron® (PT 10), Kinematica AG, Luzern, Switzerland) was used to homogenize the bone. Both implant and homogenized bone were sonicated for 10 min. Ten-fold serial dilutions were cultured on Colombia Blood Agar plates in duplicate. CFU was counted after overnight incubation in a 37 °C stove. The total number of bacteria in the bone and on the implant was calculated by multiplying the CFU/mL by the total volume of the sample.
4.8. Ex Vivo Biodistribution Assessment
Assessment of the biodistribution after seven days of follow-up was performed by removal of the organs and the implants with the surrounding femur immediately after euthanizing the rats with an overdose of isoflurane. Only in the Lutetium-177 study (177Lu-4497 and 177Lu-DTPA treatment groups), the articular capsule was also collected for ex vivo biodistribution assessment, as accumulation was observed due to new infection onset in the joint, with SPECT analyses. All samples were weighed. Radioactivity was measured with a gamma counter. Prior to the injection of interventions, a standard was created with one-tenth of the injected dose of each treatment group and was subsequently kept in 0.15 M ammonium acetate. These standards were counted in a gamma-counter at the same time as the collected organs and implants with the surrounding femur, allowing for decay correction. The standards were diluted by 10-fold for the 225Ac samples and 100-fold for the 177Lu samples to keep the standards in the linear range for the detector. The percentage of injected dose per gram (%ID/g) organs and implant was calculated by the following formula with Z representing the dilution factor (Z = 10 for 225Ac and Z = 100 for 177Lu):
4.9. Short-Term Toxicity Assessment
Blood collection was performed by heart puncture and stored in tubes with Lithium Heparin (BD microtainer). Blood plasma was obtained by centrifuging the samples for 4 min at 10 G. Subsequently, all blood plasma samples were stored at −80 °C until no detectable radioactivity was measured prior to the liver panel analysis (creatinine and urea) and kidney panel analysis (AST and ALT). White blood cell count (WBC) and red blood cell count (RBC) were assessed directly after termination using a Beckman-Coulter Ac.T differential hematology analyzer (Beckman Coulter Inc., Brea, CA, USA).
4.10. Histological Processing
Histopathological analysis of radiation damage to the liver and kidney were investigated by fixing the organs in 4% w/v formaldehyde. Subsequently, the samples were dehydrated in an ethanol series. Thereafter, the samples were embedded in paraffin and cut into 6 µm thick slices using a sawing microtome (Leica, Nussloch, Germany). The slices were stained with hematoxylin and eosin (H&E). assessed the slices for organ damage. The kidney samples were evaluated for renal architecture, glomerular, and tubulointerstitial changes. The liver samples were evaluated for liver architecture, fibrosis, and hepatocellular changes.
4.11. Statistical Analysis
The aim of this study was to evaluate an in vivo antimicrobial effect of targeted radiation in a dose-dependent setting and to perform a tolerance assessment for Wistar Han rats. An arbitrary sample size of three was chosen per treatment group. Due to the small sample size, no statistical tests were performed on the antimicrobial efficacy. The percentage change in bacterial count (CFU) compared to the control treatment group was calculated and reported. The mean and range were reported in graphs using GraphPad Prism 7.4. An unpaired
t-test was performed to assess differences in the ex vivo biodistribution assessment. All reported hematological parameters, kidney panel results (creatinine and urea), and liver panel results (AST and ALT) were compared to the clinical lab parameters provided by Charles River Laboratories [
42].