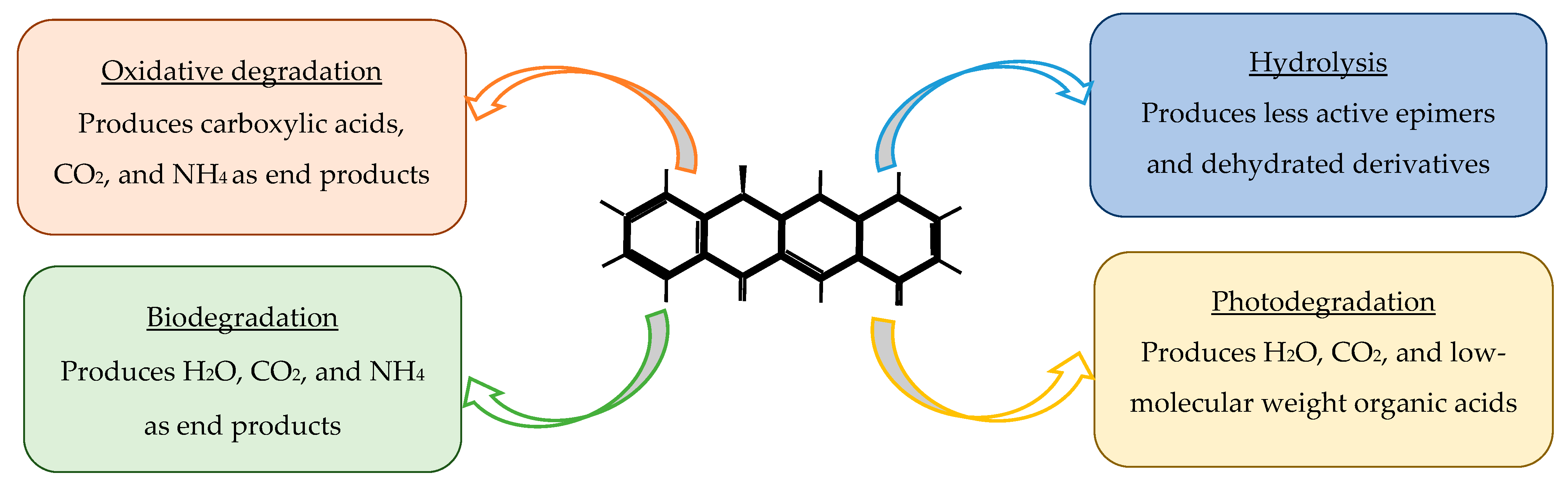1. Introduction
Tetracyclines are among the oldest groups of antibiotics, characterized by a broad spectrum of activity effective against both Gram-positive and Gram-negative bacteria, as well as
Chlamydia,
Legionella,
Rickettsia, and
Mycoplasma species [
1]. They have wide-ranging applications in human and veterinary medicine, agriculture, and aquaculture, accounting for approximately 10–12% of the global antimicrobial market [
2]. The use of tetracyclines in human medicine is less common than in veterinary practice due to the availability of newer antibiotic classes. However, tetracyclines remain extensively used in veterinary medicine for the treatment of zoonotic infections and as growth promoters, as they are inexpensive, well absorbed, and generally associated with few adverse effects [
3]. The widespread global use of tetracyclines has led to their pervasive distribution in the environment, resulting in several negative ecological and public health consequences.
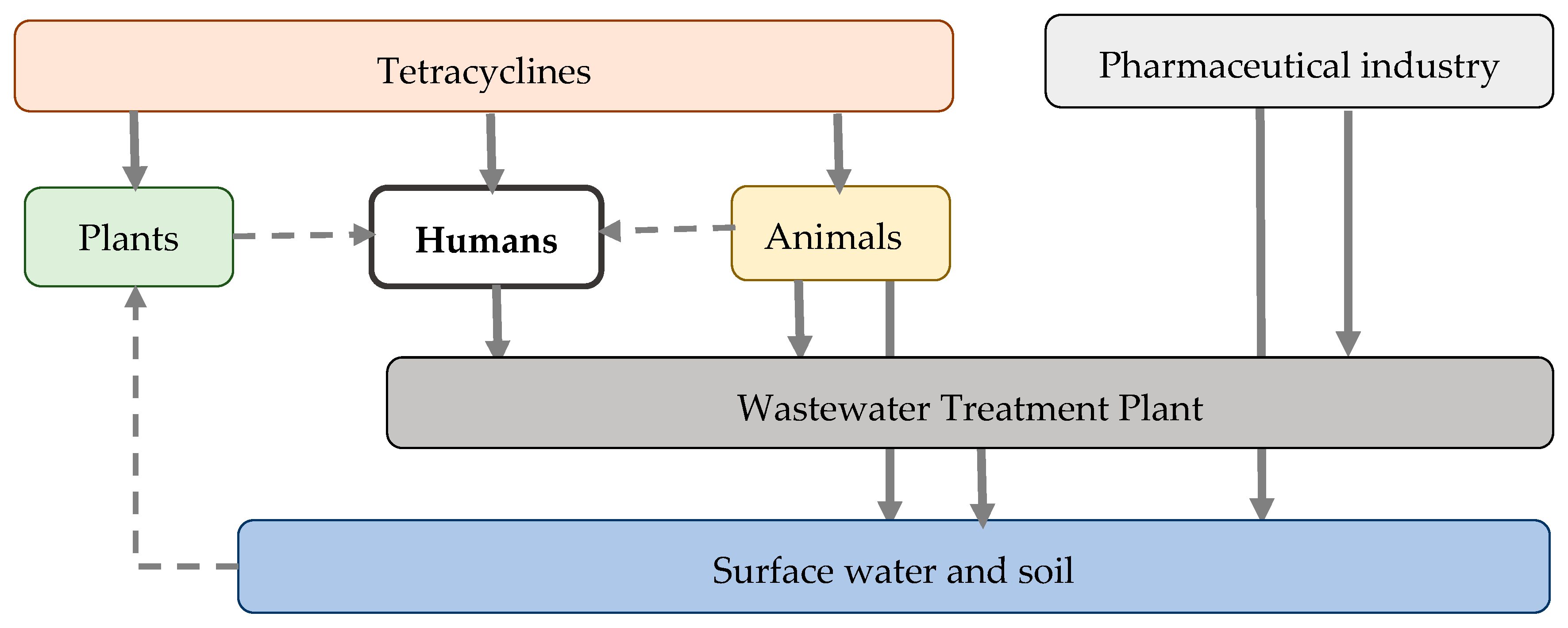
The ecological consequences of tetracycline occurrence in the environment include disruption of terrestrial and aquatic microbiota, which affects organic matter decomposition and plant growth processes [
4]. Tetracyclines can also exert toxic effects on non-target organisms such as algae, invertebrates, and fish, leading to growth inhibition and reproductive toxicity [
5]. Moreover, tetracyclines are capable of bioaccumulation, potentially resulting in biomagnification through the food chain and chronic exposure in higher organisms, including humans [
6]. In addition to their toxic effects, tetracyclines contribute to the development and dissemination of antimicrobial resistance (AMR), posing significant ecological and public health risks.
AMR refers to the ability of microorganisms to survive exposure to antimicrobial agents that would normally inhibit their growth or cause cell death [
7]. The overuse and misuse of antimicrobial agents are major drivers of AMR development [
8]. In particular, the extensive application of tetracyclines in human and veterinary medicine, agriculture, and aquaculture results in the persistence of tetracycline residues in soil, surface water, and sediments. These residues exert continuous selective pressure, promoting the maintenance and dissemination of tetracycline-resistant microorganisms [
9]. Consequently, the environment acts as both a reservoir and a conduit for AMR spread, facilitating the potential reintroduction of resistant bacteria into human and animal populations through water, food, and direct contact pathways [
10]. To address the growing problem of tetracycline resistance, a range of strategies has been proposed. Antimicrobial stewardship (AMS) practices promote the rational use of tetracyclines in human and animal health sectors [
11], while a variety of degradation techniques have been developed to remove tetracyclines already present in the environment [
12].
Although numerous studies have examined individual aspects of tetracycline use, environmental fate, or resistance, most existing reviews address these topics in isolation. There remains a lack of an updated, integrated synthesis that simultaneously examines global consumption of tetracyclines across major sectors; their persistence in terrestrial and aquatic environments; the mechanisms of resistance; and the full spectrum of mitigation strategies, including AMS interventions and emerging degradation approaches, within a unified One Health framework [
13]. The innovation of this narrative review lies in its integrative scope. By synthesizing evidence from human and veterinary medicine, agriculture, aquaculture, environmental pollution, and public health, it provides a state-of-the-art, cross-sectoral overview. Through this holistic perspective, the review aims to support the development of evidence-based interventions to mitigate the environmental and public health impacts associated with tetracycline use.
2. Tetracycline Class of Antibiotics
Tetracyclines are a class of antibiotics originally derived from bacteria of the
Streptomyces genus. Chlortetracycline, oxytetracycline, tetracycline, and demeclocycline constitute the first generation of tetracyclines. The first antibiotic in this class, chlortetracycline, was isolated from
Streptomyces aureofaciens in 1945 by Benjamin Minge Duggar at Lederle Laboratories. Oxytetracycline was the second to be discovered, isolated from
Streptomyces rimosus in 1949 by a research team at Charles Pfizer & Co., including John and Alexander Finlay and Frank E. Hegeman. Tetracycline was subsequently developed as a semisynthetic hydrogenated derivative of chlortetracycline by Lloyd Conover at Pfizer and approved for clinical use in 1953. Demeclocycline was later isolated in 1957 by Jerry Robert Daniel McCormick and colleagues from a mutant strain of
Streptomyces aureofaciens [
14]. The second-generation tetracyclines—such as minocycline, doxycycline, lymecycline, rolitetracycline, and methacycline—emerged during the 1960s and 1970s and were developed to improve pharmacokinetic profiles and spectrum of activity [
15]. The third generation, known as glycylcyclines (e.g., tigecycline, omadacycline, sarecycline, and eravacycline), consists of fully synthetic analogues developed in the 2000s and 2010s to overcome increasing bacterial resistance [
16]. Despite structural modifications across generations, all tetracyclines share a characteristic core structure.
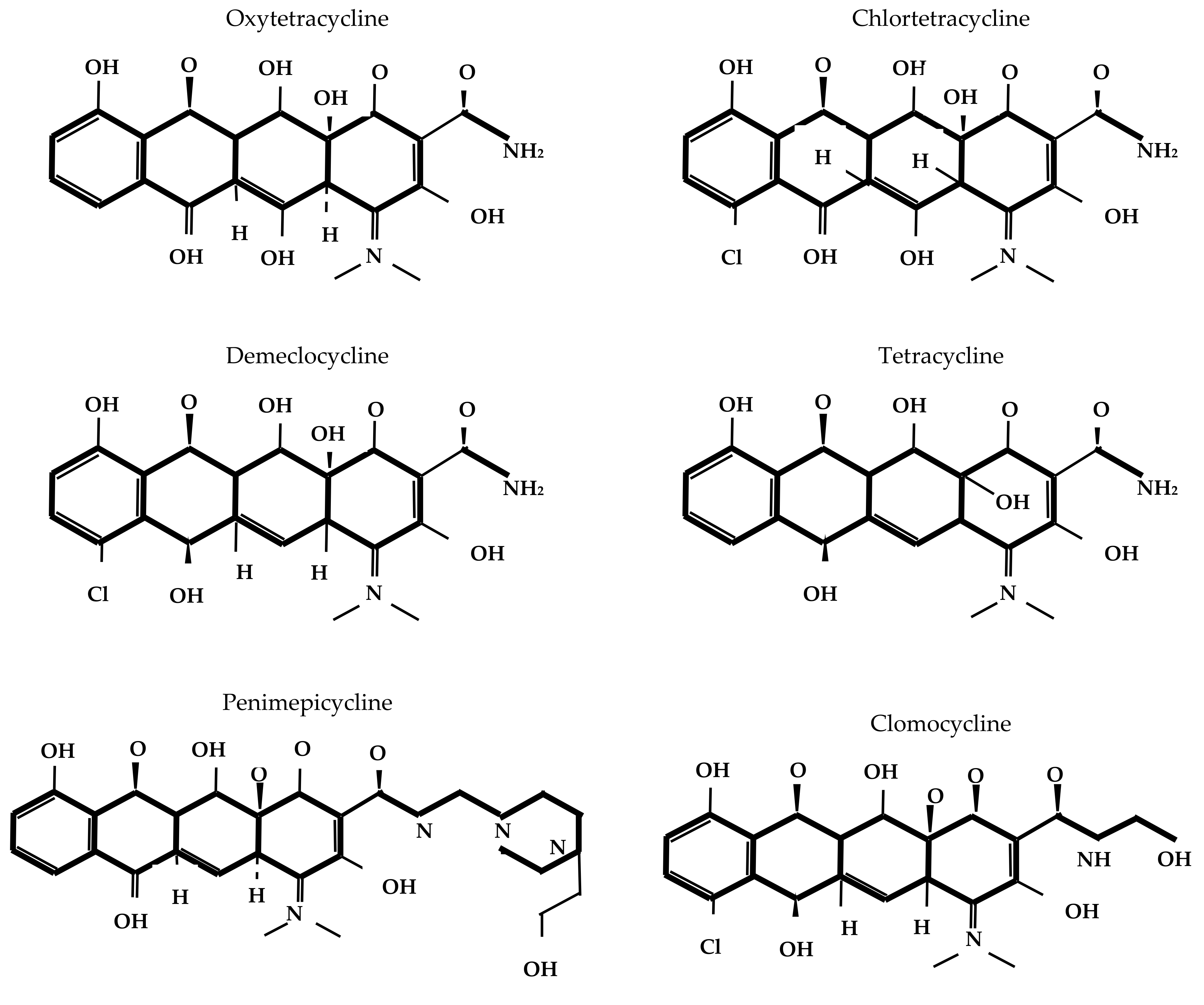
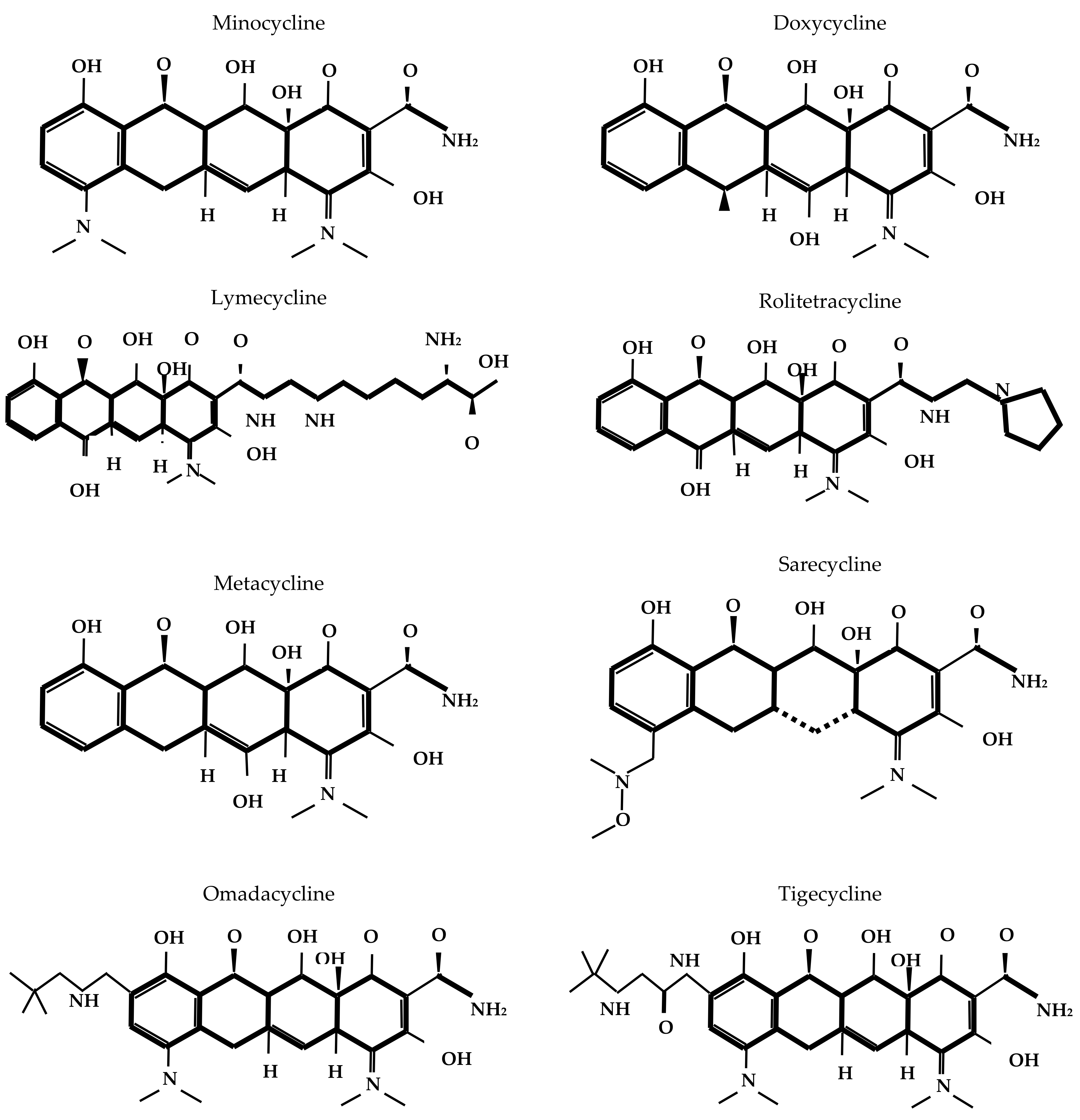
2.1. Chemical Structure and Mechanism of Action
The core structure of tetracycline molecules consists of four linearly fused six-membered rings (conventionally labeled A, B, C, and D), forming the characteristic naphthacene ring system. In this scaffold, ring A typically bears a dimethylamino group at position C4; rings B and C contain key keto-enol tautomeric functionalities; and ring D carries hydroxyl or other substituents, depending on the specific derivative. Structural variations at positions C5, C6, C7, and C9 contribute to the pharmacokinetic, antimicrobial, and resistance profiles of different tetracycline compounds [
17].
Figure 1 depicts the chemical structure of different tetracyclines.
Tetracyclines possess bacteriostatic activity, and their mode of antibacterial action involves the inhibition of protein synthesis at the bacterial ribosome. Specifically, tetracyclines bind reversibly to the 16S rRNA component of the 30S ribosomal subunit in susceptible bacteria, distorting the A-site and blocking the attachment of aminoacyl-tRNA. This interference disrupts the codon–anticodon pairing and blocks the addition of new amino acids to the elongating polypeptide chain. As a result, protein synthesis is arrested at the elongation stage, leading to a bacteriostatic effect [
18]. However, it was demonstrated that tetracyclines may exhibit bactericidal properties at high concentrations, potentially due to their ability to disrupt the functional integrity of cytoplasmic membrane [
19]. The binding of tetracyclines to the bacterial ribosome is reversible, which accounts for their primarily bacteriostatic nature and relatively modest post-antibiotic effect [
17].
Intracellular accumulation of tetracyclines is essential for their antibacterial efficacy and underlies their selective toxicity. Tetracyclines typically enter the periplasm of bacteria via energy-independent diffusion of a tetracycline–Mg
2+ complex through outer membrane porins [
20]. Subsequently, the Donnan potential aids dissociation of the tetracycline–Mg
2+ complex, resulting in an uncharged, lipophilic tetracycline molecule that can diffuse across the cytoplasmic membrane [
21]. Moreover, energy-dependent uptake occurs via the proton motive force and ATP hydrolysis, contributing to active transport across the cytoplasmic membrane [
17]. Inside the cell, tetracyclines undergo repeated chelation with Mg
2+ ions, stabilizing their interaction with the ribosomal target [
16]. It has to be noted that tetracycline molecules rely partially on bacterial-specific transport systems that are absent or significantly less active in mammalian cells, which explains their selective inhibition of bacterial protein synthesis with relatively low cytotoxicity in host tissues [
22].
Beyond their antibacterial effects, certain tetracyclines, particularly doxycycline and minocycline, demonstrate non-antibiotic properties, such as anti-inflammatory activity. These effects have expanded their therapeutic applications to include conditions like acne vulgaris, rosacea, periodontitis, rheumatoid arthritis, and inflammatory bowel disease [
23]. Moreover, tetracyclines such as doxycycline, minocycline, and tigecycline have shown anticancer potential through mechanisms involving mitochondrial dysfunction, inhibition of matrix metalloproteinases, and apoptosis induction [
24]. In addition to their antibacterial and anti-inflammatory roles, doxycycline and minocycline have also been investigated for their potential therapeutic effects in neuropsychiatric conditions, including major depressive disorder [
25] and neurodegenerative diseases such as Alzheimer’s disease [
26].
Tetracycline-class antibiotics are classified based on their elimination half-life (EHL) into short-acting, intermediate-acting, and long-acting agents. Short-acting tetracyclines, such as tetracycline, oxytetracycline, and chlortetracycline, have an EHL of approximately 6 to 8 h. Intermediate-acting agents include demethylchlortetracycline, lymecycline, rolitetracycline, clomocycline, penimepicycline, and methacycline, with an EHL of 12 to 15 h. In contrast, long-acting tetracyclines—such as doxycycline, minocycline, sarecycline, eravacycline, omadacycline, and tigecycline—have an EHL exceeding 16 h [
17]. The EHL of tetracyclines depends on their pharmacokinetic characteristics, including absorption, distribution, metabolism, and excretion pathways. Short- and intermediate-acting tetracyclines are primarily excreted via the kidneys, whereas long-acting tetracyclines are predominantly excreted through the gastrointestinal tract [
14].
2.2. Spectrum of Antibacterial Activity and Indications for Use
Tetracyclines are broad-spectrum antibiotics, effective against a wide range of aerobic and anaerobic, Gram-positive and Gram-negative bacteria (
Table 1). In addition to the listed bacteria, new and rare pathogens occasionally found to be sensitive to tetracyclines [
27].
Beyond bacterial pathogens, tetracyclines are also effective in infections caused by some protozoa and parasites, like
Entamoeba histolytica and
Plasmodium spp., and in bacterial infections closely related to treponemal diseases, such as yaws caused by
Treponema pallidum subspecies
pertenue [
28].
Tetracyclines possess a broad antimicrobial spectrum and exhibit extensive tissue distribution, which makes them effective in treating both systemic and localized infections caused by susceptible organisms. Although tetracyclines penetrate nearly all body tissues, the highest concentrations are typically observed in the bile, liver, spleen, kidneys, lungs, and bone [
29]. Notably, doxycycline and minocycline have been shown to cross the blood–brain barrier, allowing for potential use in central nervous system infections [
17].
In veterinary medicine, tetracyclines are used not only for therapeutic purposes but also as growth promoters, often added to animal feed at subtherapeutic levels. This practice enhances feed efficiency and weight gain, primarily by suppressing subclinical infections and reducing immune stress in intensive breeding environments. It may also improve intestinal health and nutrient utilization indirectly through modulation of gut microbiota composition. However, the use of tetracyclines in animals for growth promotion is increasingly restricted due to concerns over associated resistance [
30]. Additionally, tetracyclines are employed in aquaculture to manage infections in commercially farmed fish, in agriculture to treat bacterial diseases in trees and plants, and in insect farming to prevent and treat infections in bees and other economically important insects [
17].
Despite their initially broad spectrum of antimicrobial activity, the effectiveness of tetracyclines in the prevention and treatment of infections has been significantly compromised by the emergence and spread of resistance. This resistance is largely attributed to the widespread use of tetracyclines in both human and veterinary medicine. Understanding the patterns of tetracycline consumption is essential for guiding AMS strategies.
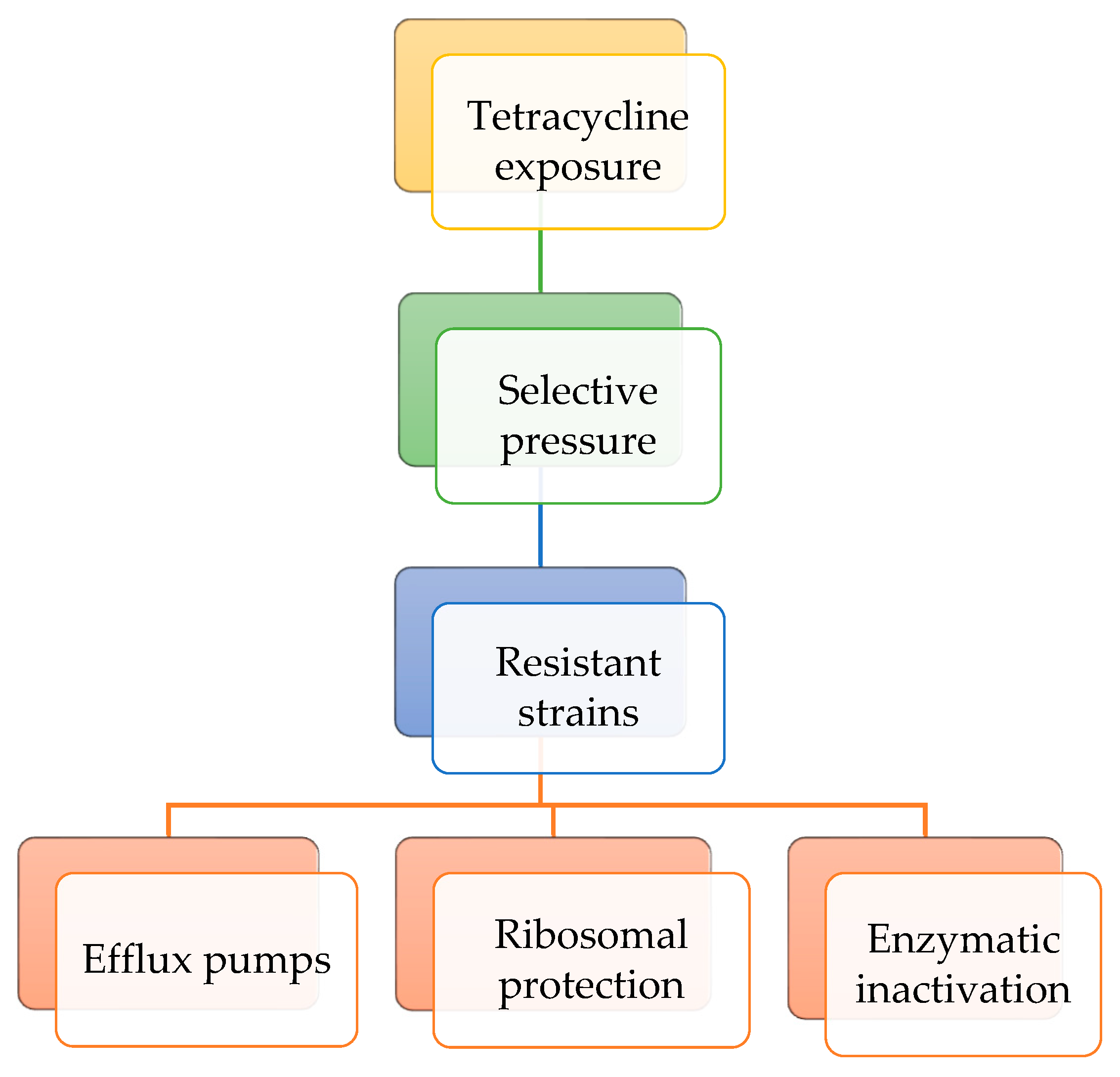
5. Resistance Mechanisms and Patterns
When bacterial populations are exposed to tetracyclines at sub-inhibitory concentrations over extended time periods, selective pressure can enrich resistant strains or induce genetic changes that enhance survival. Bacteria exhibit resistance to tetracyclines through three main mechanisms. In the efflux pump mechanism, membrane proteins actively expel tetracyclines from the bacterial cell, reducing their intracellular concentration. In the ribosomal protection level, specific proteins bind to the 30S ribosomal subunit, preventing tetracycline from binding and, in some cases, displacing the antibiotic if it is already bound. Regarding enzymatic inactivation mechanism, enzymes such as tetracycline monooxygenases chemically modify tetracyclines, rendering them inactive. These resistance mechanisms may coexist within the same bacterial strain [
18].
Figure 5 presents the resistance mechanisms to tetracycline class of antibiotics.
Resistance determinants can arise through both intrinsic and extrinsic pathways. Intrinsic resistance is naturally occurring, often chromosomally encoded, and transmitted vertically from parent to daughter cells during replication. In contrast, acquired resistance arises when bacteria obtain new genetic material via horizontal gene transfer, most commonly through plasmids, but also via mobile genetic elements such as transposons and integrons [
93]. A major environmental source of resistance genes is resistant gut microbiota. When resistant bacteria are excreted into the environment, they can transfer their genes to other bacteria via horizontal gene transfer. Studies have demonstrated that applying pig manure to the environment increases the abundance of antibiotic resistance genes more than cow manure, whereas the application of chicken manure leads to the highest levels of resistance genes among these livestock manures [
100]. In aquatic environments, resistance genes are disseminated primarily through agricultural runoff and wastewater discharge [
93].
More than 40 different tetracycline resistance genes (
tet genes) have been identified in Gram-positive and Gram-negative bacteria.
Tet(A),
tet(B), and
tet(D) are commonly reported in Gram-negative bacteria and encode efflux pumps, while
tet(K) and
tet(L) are more frequent in Gram-positive bacteria [
29].
Tet(M),
tet(O),
tet(Q), and
tet(W) encode ribosomal protection proteins, whereas certain Gram-negative bacteria possess the
tet(X) gene, which mediates enzymatic inactivation of tetracyclines [
29]. Efflux pumps generally confer resistance to the older tetracyclines but are less effective against doxycycline, minocycline, and largely ineffective against third-generation agents such as tigecycline. Ribosomal protection proteins provide broader resistance, though activity against newer tetracyclines is reduced. By contrast,
tet(X) and its variants are capable of inactivating nearly all tetracyclines, including tigecycline [
29].
Humans may acquire resistant bacteria and their associated genes through direct contact with contaminated environments, consumption of food products derived from plants, animals, or fish exposed to antimicrobials, or through selective pressure from direct exposure to tetracyclines [
101]. Similar to animals, the human gut microbiome can act as a reservoir, where commensal bacteria harbor and exchange resistance determinants that may subsequently transfer to pathogenic species. However, unlike animals, the contribution of the human gut microbiome to the dissemination of resistance genes into the environment is comparatively limited [
101]. Nevertheless, investigation of the distribution of
tet genes in different human populations worldwide remains as a matter of interest.
Several studies have reported the prevalence of
tet genes and associated resistance to tetracyclines in both clinical and non-clinical settings across Europe over the past five years. Overall, the diversity of
tet genes and the frequency of tetracycline resistance are higher in clinical settings, largely due to the more intensive and frequent exposure of patient populations to antimicrobial therapies compared with the general population. The
tet(A) and
tet(B) genes are among the most commonly detected in a variety of pathogens [
102,
103,
104,
105,
106,
107,
108,
109,
110,
111]. In some instances, resistance to tetracycline may reach 100%, as shown in a study from Italy that characterized the genetic and phenotypic features of
Streptococcus suis isolated from cerebrospinal fluid [
108]. By contrast, a study in Bulgaria examining
Enterococcus faecalis isolated from breast milk in a non-clinical setting, that is, from healthy mothers outside of hospitals or healthcare facilities, reported a resistance rate of 22% [
109]. Furthermore, a study from Spain documented resistance to tigecycline in
Klebsiella pneumoniae and
Pseudomonas aeruginosa strains, with prevalence rates of 10.8% and 36.3%, respectively [
103].
In the Americas, the
tet(O),
tet(A), and
tet(M) genes are the most frequently reported in isolates obtained from both clinical and non-clinical settings [
112,
113,
114,
115,
116,
117,
118,
119]. Similar to observations in Europe, isolates from patients often exhibited higher rates of tetracycline resistance, in some cases reaching 100%, such as in
Campylobacter species isolated in Honduras [
115]. Although resistance rates in isolates from healthy individuals were generally lower, they remained substantial; for instance, a study from Panama reported a 30% resistance rate with tetracycline in
Escherichia coli [
119].
Tet(A) and
tet(B) genes were the most frequently reported in studies from Asia; however, several studies also documented the presence of
tet(X) [
120,
121,
122,
123,
124,
125,
126,
127,
128,
129,
130,
131,
132]. The
tet(X) gene, a plasmid-associated determinant, confers resistance to all generations of tetracycline-class antibiotics. A study from China confirmed this by reporting 100% resistance to tigecycline, tetracycline, doxycycline, minocycline, eravacycline, and omadacycline in
Klebsiella pneumoniae isolates from patients [
120]. In contrast, a study from Kuwait identified
tet(X) in
Enterobacteriaceae species but found lower resistance rates to tigecycline, ranging from 0.8% to 4.1% [
131]. Consistent with findings from other continents, resistance to tetracyclines in non-clinical settings was generally lower than in clinical isolates, though still considerable; for instance, a study from Lebanon reported a 42% resistance rate in
Escherichia coli [
133].
Australia and New Zealand are high-income countries with well-established AMS systems that promote the prudent use of antibiotics in both veterinary and human medicine. Reported rates of tetracycline resistance in clinical settings in Australia were 11.1% for
Campylobacter jejuni and 2.4% for
Campylobacter coli [
134]. In New Zealand, the rates of tetracycline resistance in clinical isolates were higher, ranging from 33% to 100% in
Shigella species [
135].
Tet(A) and
tet(B) genes were also the most frequently identified in isolates obtained from both clinical and non-clinical settings across countries of the African continent [
136,
137,
138,
139,
140,
141,
142,
143,
144,
145,
146,
147]. These countries report some of the highest levels of tetracycline resistance, particularly in non-clinical settings. For example, a study from Nigeria found 83% tetracycline resistance in
Escherichia coli isolated from healthy poultry farm workers [
139], while a study from Ethiopia reported resistance rates ranging from 92% to 100% in rural communities [
141]. In contrast, the rates of tetracycline resistance were generally lower in urban populations, with 22.6% of urban dwellers in Ghana carrying tetracycline-resistant isolates [
142], whereas higher rates were observed in disadvantaged urban communities in Kenya, where 50% of residents harbored resistant bacteria [
143]. The differences in tetracycline resistance rates between rural and urban populations in Africa may be attributed to varying levels of antimicrobial use in agriculture, differences in sanitation and hygiene, and disparities in access to healthcare AMS.
Table 4 provides an overview of studies reporting tetracycline resistance rates and
tet genes isolated from humans worldwide from 2019 onward.
The global distribution of tet genes and the regional variation in tetracycline resistance underline the multifactorial nature of antimicrobial resistance dynamics. Several strategies can be considered to address the growing challenge of tetracycline resistance. These include the implementation of AMS programs in both human and veterinary medicine, the promotion of rational tetracycline use in agriculture, and efforts to reduce environmental contamination with these compounds. The following section provides an overview of AMS approaches relevant to the control of tetracycline resistance.
7. Degradation Strategies
AMS strategies help reduce tetracycline consumption; however, they do not address the persistence of tetracyclines already present in the environment. These compounds tend to accumulate in soil and aquatic systems, where they continue to exert selective pressure on microbial communities, thereby promoting AMR. Although tetracyclines are relatively stable under environmental conditions, various physicochemical degradation techniques have been developed to exploit their specific structural and chemical characteristics [
18].
Tetracycline degradation occurs through several distinct mechanisms, each involving characteristic structural transformations of the tetracycline scaffold [
79]. Hydrolytic degradation primarily affects the amide and lactone functional groups, leading to cleavage of the C–N and C–O bonds and formation of less active ring-opened products [
92]. Photodegradation is initiated by light-induced excitation of the conjugated aromatic system, promoting tautomerization, hydroxylation, demethylation, and oxidative cleavage of the dimethylamino group at C4, ultimately generating a series of epimers, anhydro-derivatives, and smaller aromatic fragments [
79]. Oxidative degradation, including advanced oxidation processes, typically proceeds through hydroxyl radicals and other reactive oxygen species (ROS) that attack the phenolic D-ring, causing hydroxylation, dealkylation, and fragmentation of the polycyclic structure [
93]. Biodegradation involves microbial enzymatic pathways—such as deamination, decarboxylation, and ring-cleavage reactions—producing simpler organic acids, alcohols, and CO
2 [
92]. Together, these mechanisms reduce tetracycline bioactivity and influence their persistence, mobility, and ecotoxicity in the environment.
Tetracycline metabolites, produced through environmental degradation, can exhibit toxicity and affect aquatic organisms. For example, they have been shown to impair the growth and photosynthesis of freshwater algae [
167]. Although sometimes less active than the parent compound, these metabolites persist in water and soil, posing ecological risks by impacting microbial communities and higher trophic levels [
168]. Thus, the environmental effects of tetracyclines should consider both the parent drugs and their potentially toxic metabolites.
7.1. Degradation of Tetracyclines via Hydrolysis, Photodegradation, and Oxidative Degradation
Degradation of tetracyclines through hydrolysis is significantly influenced by environmental factors such as pH and temperature. Extreme pH values and elevated temperatures accelerate the process. For instance, the hydrolysis rate has been reported to increase by 2.22- to 2.74-fold with every 10 °C rise in temperature [
169]. Conversely, lower ambient temperatures markedly reduce the rate of hydrolysis; at 4 °C, more than 90% of tetracycline remained unchanged after 48 h [
170]. In addition to temperature, pH strongly affects the hydrolysis rate, with alkaline conditions promoting hydrolysis more efficiently. For example, the rate of oxytetracycline hydrolysis was 72.7% at pH 6.91, but only 10.5% at pH 3.09 [
171]. Furthermore, the presence of certain metal ions, such as calcium and magnesium, can influence hydrolysis kinetics, as tetracyclines are known to form chelate complexes with these cations [
172]. The primary hydrolysis products are epimers and anhydro-tetracyclines [
169]. Several of these transformation products retain significant biological activity and, in some cases, exhibit greater toxicity than the parent compound. For example, 4-epitetracycline and anhydro-tetracycline have been shown to demonstrate enhanced cytotoxic effects and may contribute to gastrointestinal irritation and nephrotoxicity. Thus, although hydrolysis can reduce the concentration of the parent compound, it does not necessarily eliminate ecological or toxicological risks due to the persistence and biological activity of the resulting degradation products [
173].
Similar to hydrolysis, the photodegradation of tetracyclines is affected by pH, temperature, light intensity, wavelength, and the presence of photosensitizers. In general, alkaline conditions enhance photodegradation due to increased formation of ROS and photoactive anionic forms of tetracyclines, while acidic conditions tend to slow the process because of reduced light absorption and the predominance of less reactive protonated species [
76]. For example, the photocatalytic oxidation efficiency of tetracycline increased from 75.8% to 86.3% when pH rose from 4 to 10 [
174]. The optimal pH range for photocatalytic degradation is typically 8–9, whereas strongly alkaline conditions (pH 11) may inhibit the process [
175]. Temperature also affects photodegradation: higher temperatures enhance molecular mobility and reaction kinetics, leading to faster degradation, although its effect is secondary to that of light intensity, wavelength, or pH. For example, studies have shown that tetracycline photodegradation proceeds more rapidly at 60–80 °C than at 45 °C [
176]. The degradation rate also depends on the wavelength and intensity of light. Shorter wavelengths promote faster photodegradation due to higher photon energy, while greater irradiance increases the number of photoexcited electrons and holes, thereby generating more ROS and accelerating degradation [
177]. Moreover, environmental photosensitizers such as humic substances and nitrogen oxides can further enhance photodegradation by facilitating ROS production [
178]. The photodegradation of tetracyclines can lead to the formation of numerous transformation products, including epimers, iso-tetracyclines, anhydro-derivatives, and various hydroxylated and ring-cleaved intermediates. Several of these photoproducts exhibit equal or greater toxicity compared with the parent compound. For example, anhydro- and iso-tetracycline derivatives have been associated with increased phototoxicity, enhanced cytotoxic effects, and potential hepatotoxicity. Moreover, some photoproducts also retain antimicrobial activity, contributing to sustained selective pressure in aquatic environments [
92].
Ozone, hypochlorous acid, hydrogen peroxide, peroxymonosulfate, and persulfate are commonly used oxidants for the oxidative degradation of tetracyclines. Similar to hydrolysis and photodegradation, the efficiency of oxidative degradation depends on environmental factors such as pH, initial tetracycline concentration, and the presence of interfering substances. Lower pH generally enhances oxidation, with the highest degradation rate constant observed at pH 4. As pH increases, the rate decreases, reaching its minimum at pH 12, which is approximately 1/38 of that observed at pH 4 [
179]. Regarding the influence of initial tetracycline concentration, the maximum degradation rate constant was reported at 5 mg/L, while further increases in concentration led to progressively slower degradation, with the rate constant at 100 mg/L reduced to about 1/76 of that at 5 mg/L [
179]. The presence of natural organic matter such as humic acids and other dissolved organic compounds can either enhance or inhibit oxidative degradation. For example, in a peroxymonosulfate/LaCoO
3 system, tetracycline degradation reached 90% within 30 min in the absence of coexisting substances, while low concentrations of humic acid slightly accelerated the process [
180]. Conversely, other studies have shown that humic acid can inhibit tetracycline degradation by scavenging reactive radicals and reducing intermediate oxidation products back to their original state [
181]. Several oxidative transformation products of tetracyclines (including anhydro- and epimeric derivatives) have been shown to retain antimicrobial activity and may induce oxidative stress in algae and other biota [
92]. For example, UV/PS-generated by-products inhibited algal growth and triggered antioxidant enzyme responses in
Chlorella [
182].
In current environmental and engineering contexts, photodegradation primarily governs the fate of tetracyclines in surface environments, whereas oxidative processes are employed in wastewater treatment systems for rapid and near-complete removal. At the same time, hydrolysis remains a slower, natural attenuation pathway that contributes mainly to partial degradation [
92]. In recent years, biodegradation approaches, using bacteria, fungi, and enzyme-based systems, have been proposed as environmentally sustainable alternatives and represent an active area of ongoing research.
7.2. Biodegradation of Tetracyclines
Biodegradation of tetracyclines is an area of active investigation, particularly as a sustainable alternative to physicochemical degradation techniques. It can proceed through two main pathways: (1) biotransformation, in which the antibiotic molecule is structurally modified or detoxified through enzymatic reactions, and (2) mineralization, involving the complete breakdown of the compound into inorganic end products such as carbon dioxide, water, and mineral salts [
183]. Biodegradation may be carried out by a single microbial strain, commonly belonging to the
Proteobacteria,
Actinobacteria, or
Firmicutes phyla, or by microbial consortia that coexist in environments such as soil, activated sludge, or livestock manure [
184]. The use of mixed microbial communities often enhances the degradation efficiency because of the synergistic interactions between species capable of attacking different structural moieties of tetracycline molecules. For example, studies have shown that co-cultures outperform monocultures in tetracycline/oxytetracycline biodegradation, likely due to complementary enzymatic activities [
185]. Fungi, like yeasts, are also capable for tetracycline degradation [
186].
Similar to physicochemical degradation techniques, the biodegradation of tetracyclines is also influenced by environmental factors such as temperature, pH, and the presence of other substances. For instance, the degradation half-life of oxytetracycline decreases with increasing pH (from 5.5 to 7.4) [
187]. Likewise, oxytetracycline degradation is more pronounced at higher temperatures (23.0 °C and 31.2 °C) compared with lower temperatures (1.8 °C) [
187]. Elevated temperatures, such as those occurring during composting, can further enhance the degradation of residual tetracyclines. For example, the degradation rate of tetracyclines in swine manure increased with rising temperature and reached its maximum at 55 °C, likely due to a combined effect of biodegradation and thermal degradation [
188]. When tetracyclines are degraded by yeasts, temperature also plays a critical role, with the highest biodegradation efficiency (85.1%) observed at 35 °C, followed by 40 °C and 30 °C [
186].
The presence of other substances, such as easily biodegradable organic matter or catalysts, can enhance tetracycline biodegradation or affect the overall removal efficiency. Co-existing organic matter, including simple carbon sources like glucose or acetate, may stimulate tetracycline removal by promoting the growth and metabolic activity of degrading microorganisms. Additionally, humic substances and natural organic matter can facilitate the sorption of tetracyclines, thereby modifying their bioavailability and degradation kinetics [
189]. In soil environments, earthworms have been shown to accelerate tetracycline degradation by increasing soil pH and dissolved organic carbon concentrations [
190]. Catalytic materials, such as biochar, metal oxides, and clay minerals, can also influence biodegradation by enhancing adsorption processes that concentrate the antibiotic and create nutrient-rich microenvironments favorable for microbial activity [
191]. However, excessive levels of co-existing organic compounds or metals may inhibit enzymatic activity or induce microbial competition, ultimately reducing the overall degradation efficiency of tetracyclines [
192]. Studies have reported that certain biodegradation intermediates can inhibit algal growth, induce oxidative stress in aquatic microorganisms, and alter microbial community composition due to their residual bioactivity [
193]. Consequently, biologically active metabolites of tetracycline biodegradation may persist in the environment, maintaining ecological and toxicological risks.
Nevertheless, it should be noted that despite substantial progress in developing various tetracycline degradation techniques, most approaches remain confined to laboratory-scale studies and have limited translation into real-world environmental or engineering applications. Hydrolysis, photodegradation, and biological degradation occur naturally and contribute significantly to tetracycline degradation under environmental conditions [
194]. In contrast, oxidative degradation methods face practical challenges, including high operational costs, complex process optimization, and limited scalability [
195]. These limitations highlight the need for further research aimed at optimizing such technologies for field implementation and assessing their long-term environmental performance.
Figure 6 provides an overview of the currently available tetracycline degradation techniques.
7.3. Additional Removal and Treatment Technologies for Tetracyclines
In addition to chemical and biological degradation processes, various treatment technologies have been developed to remove tetracyclines from aquatic and terrestrial environments. Adsorption is one of the most widely applied approaches, using materials such as activated carbon, biochar, clays, metal–organic frameworks, and graphene-based materials, which can efficiently bind tetracyclines through electrostatic interactions, π–π stacking, and surface complexation. These materials offer an efficient, often low-cost, and environmentally friendly method for treating wastewater contaminated with antibiotics [
196].
Membrane filtration techniques like nanofiltration and reverse osmosis are highly effective at removing contaminants but face challenges with membrane fouling and high energy consumption. While reverse osmosis can provide very high removal efficiencies, nanofiltration may be a more energy-efficient alternative for certain applications [
197]. Constructed wetlands and biofiltration systems are increasingly used to sustainably remove tetracycline from water by using natural processes like plant–microbe interactions and substrate adsorption. These systems act as a low-energy option that uses a combination of biological breakdown by microbes, physical removal through filtration, and chemical processes like adsorption and precipitation to eliminate tetracyclines [
198]. These removal technologies complement degradation pathways and form part of an integrated strategy for mitigating tetracycline contamination in the environment.
Table 8 summarizes the advantages and disadvantages of different degradation methods.
In addition to efficiency and environmental considerations, economic and energy requirements are important factors when evaluating tetracycline degradation methods. Chemical oxidation tends to be energy-intensive and incurs higher operational costs due to the need for reagents, catalysts, and specialized equipment [
199]. Photodegradation, particularly when using high-intensity light sources, also increases energy demand and associated operational costs [
200]. Hydrolysis is relatively low-cost and passive, making it energy-efficient, but it is limited by long reaction times and strong dependence on environmental conditions [
173]. Adsorption processes are generally economical and energy-efficient; however, the need for regeneration or replacement of adsorbents contributes to long-term costs [
196]. Similarly, biodegradation is typically low-energy and cost-effective, but degradation rates are slow and may require large treatment areas [
183]. Overall, among these methods, biodegradation and adsorption are more feasible for large-scale, low-cost applications, whereas oxidative and photochemical treatments are better suited for the rapid degradation of highly concentrated effluents, albeit at higher economic and energy costs [
93].
8. Future Directions
The findings summarized in this review indicate two major directions for future work: research and policy development. Tetracyclines remain essential for human and veterinary medicine, as well as for agricultural and aquacultural applications; therefore, their preservation is critical in addressing AMR within this antibiotic class. In addition to the ongoing search for novel tetracycline derivatives, a recommendation applicable to other antibiotic classes as well [
201], the prudent use of existing tetracycline agents must be ensured through the implementation of coordinated and evidence-based strategies.
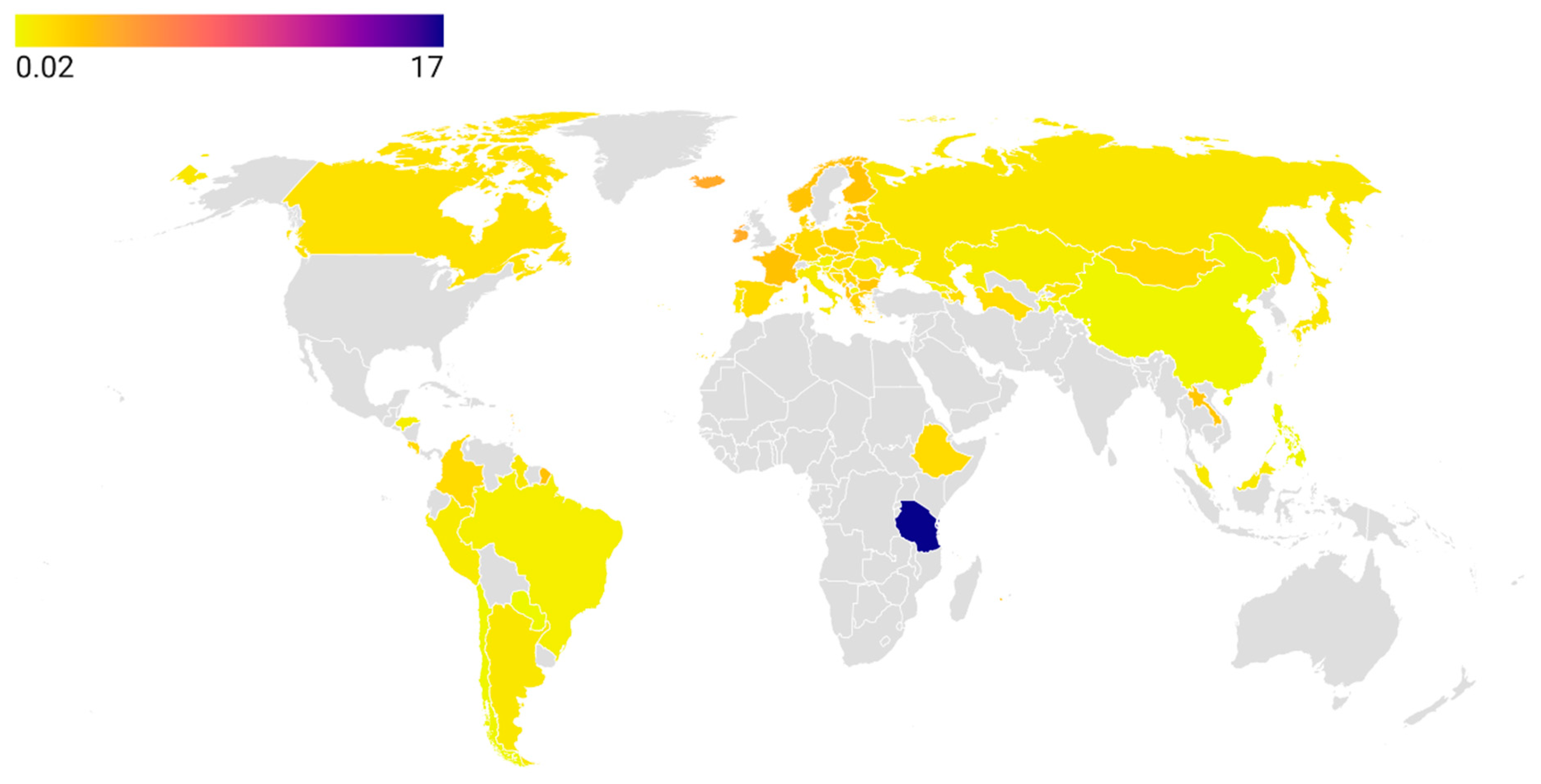
In the context of human medicine, many countries and territories remain underinvestigated with respect to tetracycline consumption during the period from 2019 to the present. Although the ECDC [
33] and the WHO Regional Offices for Europe [
34] and the Western Pacific [
35] collect antibiotic consumption data and publish annual reports for their member states, such systematic monitoring does not extend to other parts of the world. The African continent remains the largest blind spot on the global map of tetracycline consumption, despite Tanzania reporting the highest global rate (17.0 DDD in 2019) [
39]. The reasons for this exceptionally high figure require further investigation, and the reported consumption in Tanzania’s human healthcare sector should be independently validated. A possible explanation may relate to the high prevalence of zoonotic infections such as
Brucella spp., for which tetracyclines are classified as critically important antibiotics [
202]. Establishing robust and reliable national surveillance systems for antibiotic consumption is essential to support the effective implementation of AMS strategies [
203].
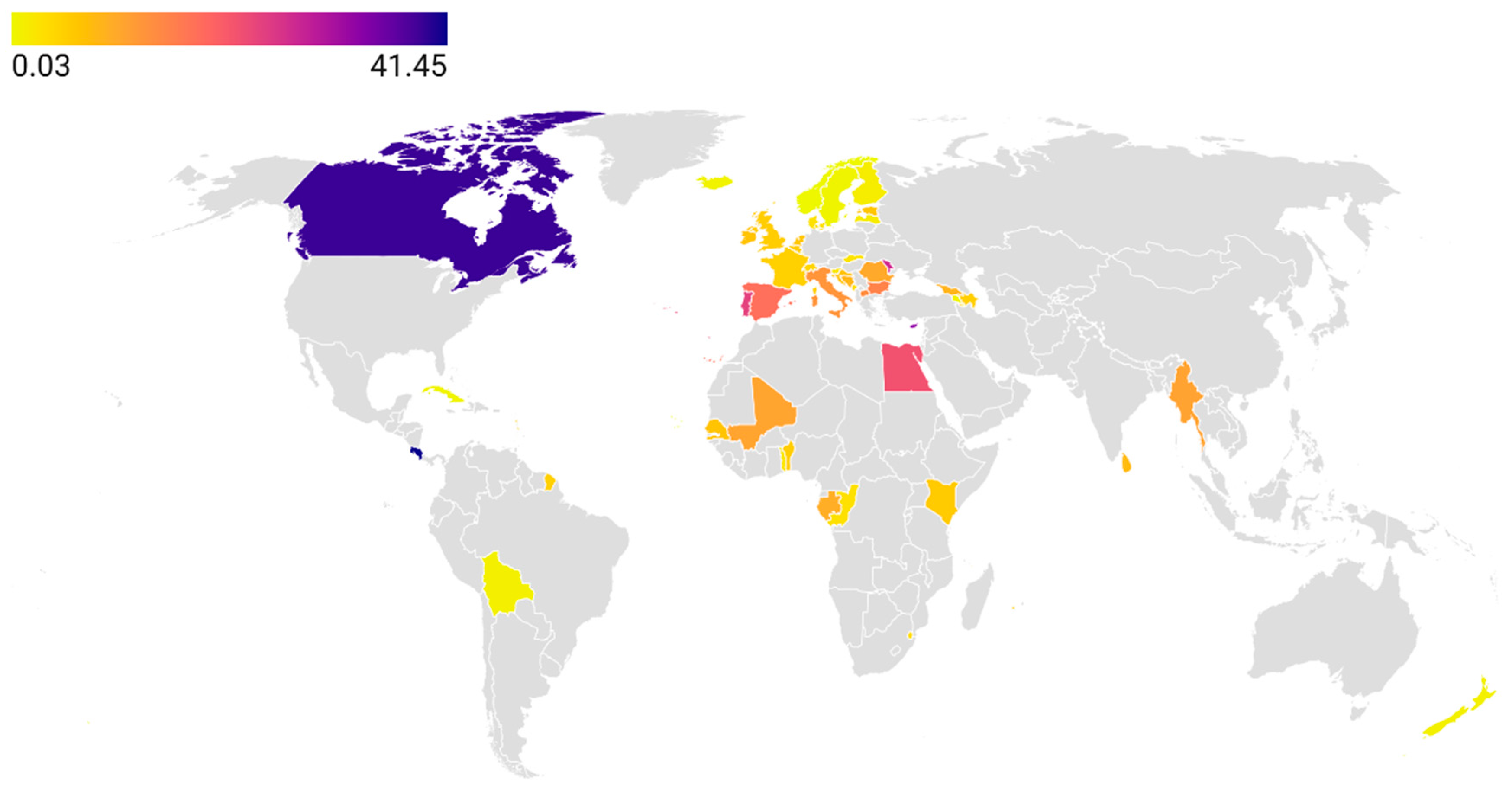
Even less is known about tetracycline consumption in the animal health sector, despite this antibiotic class being the most widely used in veterinary medicine. Although 85 countries are members of the WOAH, an even smaller number report data on tetracycline use to the ANIMUSE platform [
44]. While available evidence suggests that Canada has among the highest reported tetracycline consumption rates, this finding should be interpreted with caution due to the limited availability of data from most countries in Asia, Africa, and Latin America. Given that the use of tetracyclines for growth promotion is now banned in many regions, further research is needed to quantify their therapeutic use and to assess national policies promoting their rational use in the animal health sector [
204]. In agriculture and aquaculture, no international organization systematically collects data on tetracycline consumption, leaving the true scale of the problem largely unknown. Therefore, establishing reliable consumption estimates represents a critical first step toward developing evidence-based policy interventions in these sectors [
205]. This aligns with the One Health framework and contribute directly to achieving global targets under the WHO AMR Action Plan and the UN Sustainable Development Goals on health and clean water.
Significant knowledge gaps remain regarding the environmental occurrence of tetracyclines over the past five years, as no international agency systematically collects such data. The information available in the EMPODAT database [
60] is largely outdated, with most records on tetracycline pollution referring to earlier time periods. Another limitation is the predominant focus of existing studies on tetracycline concentrations and removal efficiency in WWTPs. However, it is equally important to assess tetracycline levels in aquatic environments, particularly in water bodies used as sources for human and animal consumption [
206]. Furthermore, data on tetracycline contamination in terrestrial ecosystems are scarce, with most reports limited to manure and soils surrounding livestock farms. Understanding the extent of tetracycline pollution in other terrestrial environments, especially those used for animal feeding and crop production, is essential for comprehensive environmental risk assessment [
207].
Although numerous studies have reported the prevalence of tetracycline resistance and the distribution of various
tet genes in humans, most of these investigations are based on clinical samples collected from patients with diverse infections. This makes it challenging to determine the extent to which observed resistance patterns are attributable to prior environmental exposure to tetracyclines or to recent antibiotic therapy [
18]. Therefore, additional studies assessing the prevalence of resistance to different tetracycline-class antibiotics, as well as
tet genes, in healthy populations are needed. Of particular importance is the surveillance of resistance to tigecycline, minocycline, omadacycline, and eravacycline, four tetracyclines classified within the “Reserve” group [
153], along with the monitoring of
tet(X) genes involved in horizontal gene transfer that can confer resistance to these agents [
208]. Such surveillance should be conducted at the country or subregional level, as resistance patterns and the dissemination of
tet genes tend to vary geographically due to differences in antibiotic usage practices and local microbial ecology [
209].
Among the currently available degradation techniques, oxidative processes, particularly advanced oxidation processes, show considerable promise, as they are capable of degrading tetracyclines into smaller and less toxic molecules within relatively short timeframes [
210]. Despite their high efficiency, large-scale implementation of oxidative degradation technologies remains limited due to high operational costs, the need for precise chemical dosing, and the complexity of maintaining optimal reaction conditions in real-world environmental settings [
211]. Future translational research should aim to optimize these methods for broader environmental applications by incorporating low-cost catalysts, renewable energy sources, and hybrid treatment systems that integrate oxidation with biological or adsorption-based processes [
212]. In particular, a focus needs to be made on developing green, energy-efficient degradation methods, such as solar-driven photocatalysis and biochar-assisted hybrid systems, to reduce environmental impact and operational costs [
213]. At the agricultural level, particular emphasis should be placed on developing scalable manure treatment and management strategies, including advanced approaches such as composting with optimized microbial consortia, anaerobic digestion, and integrated thermal or oxidative treatment technologies [
214].
Moving forward, a multidisciplinary approach integrating microbiology, environmental science, and health policy is essential to address the complex challenges posed by tetracyclines. Linking antibiotic consumption data with environmental monitoring and resistance surveillance will enable the development of predictive models to assess AMR risks. In parallel, the translation of laboratory-based degradation technologies into field-scale applications requires joint efforts from chemists, engineers, and policymakers to ensure economic feasibility and environmental sustainability. Establishing international platforms for data sharing and harmonized surveillance will be key to guiding evidence-based interventions and promoting the responsible use of tetracyclines across sectors.