Repurposing the Anticancer Drug KP46 to Beat the CRAB out of Resistance: Towards an Orally Active Ga-Based Antiplantonic and Antibiofilm Agent
Abstract
1. Introduction
2. Results and Discussion
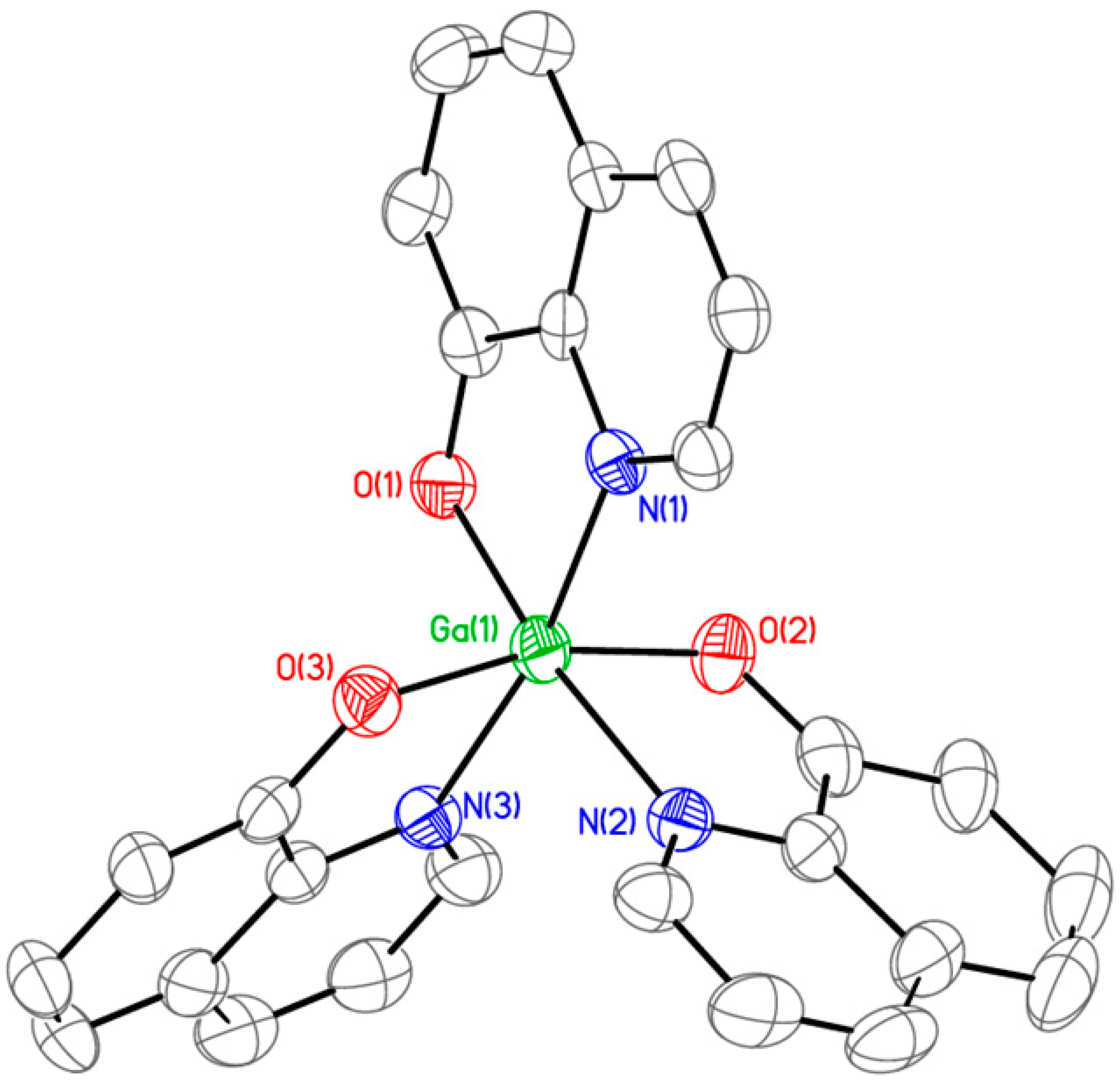
2.1. Synthesis and Characterization of KP46
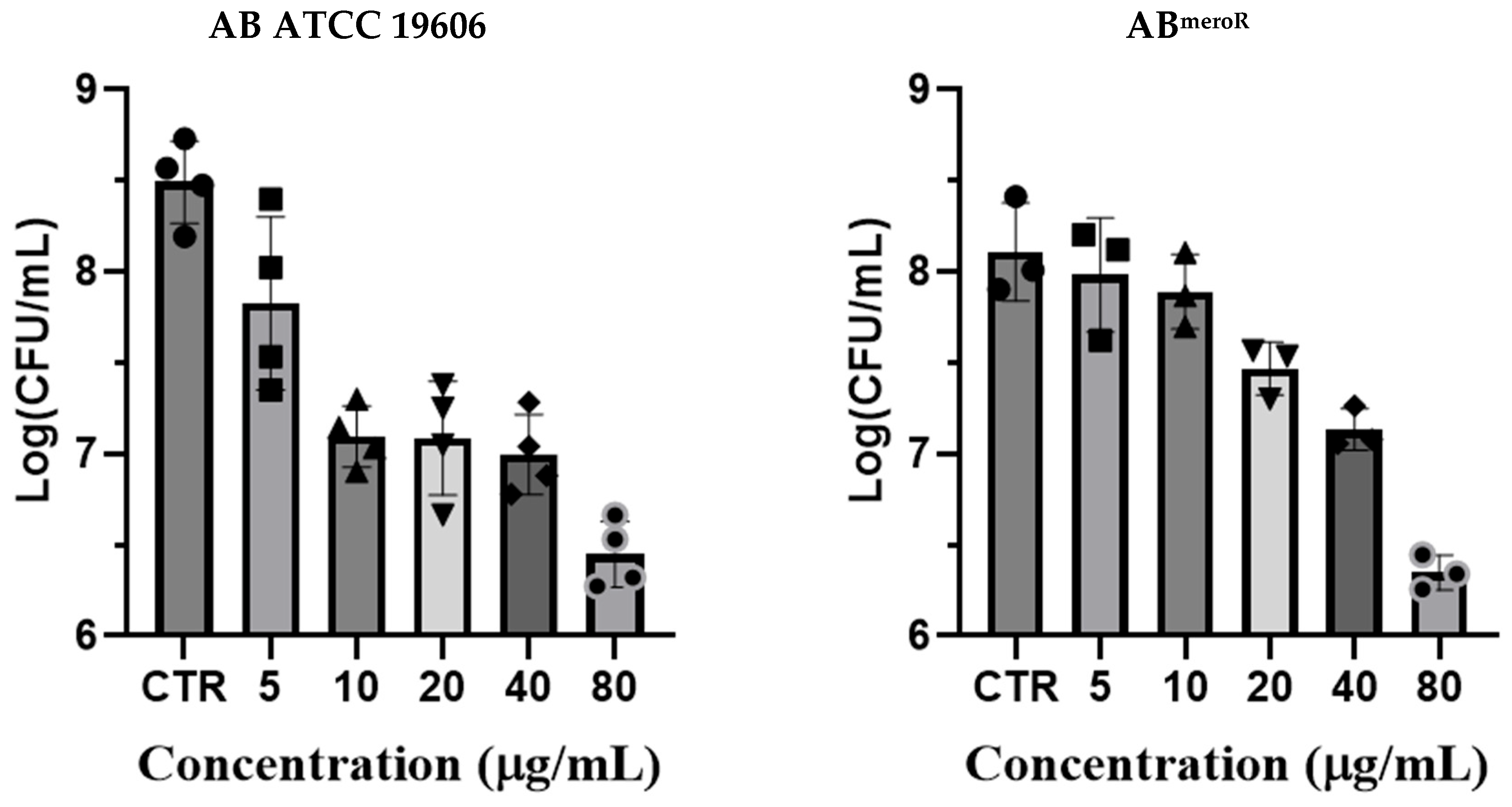
2.2. Antibacterial Activity of KP46 Against Different Strains of AB Bacteria
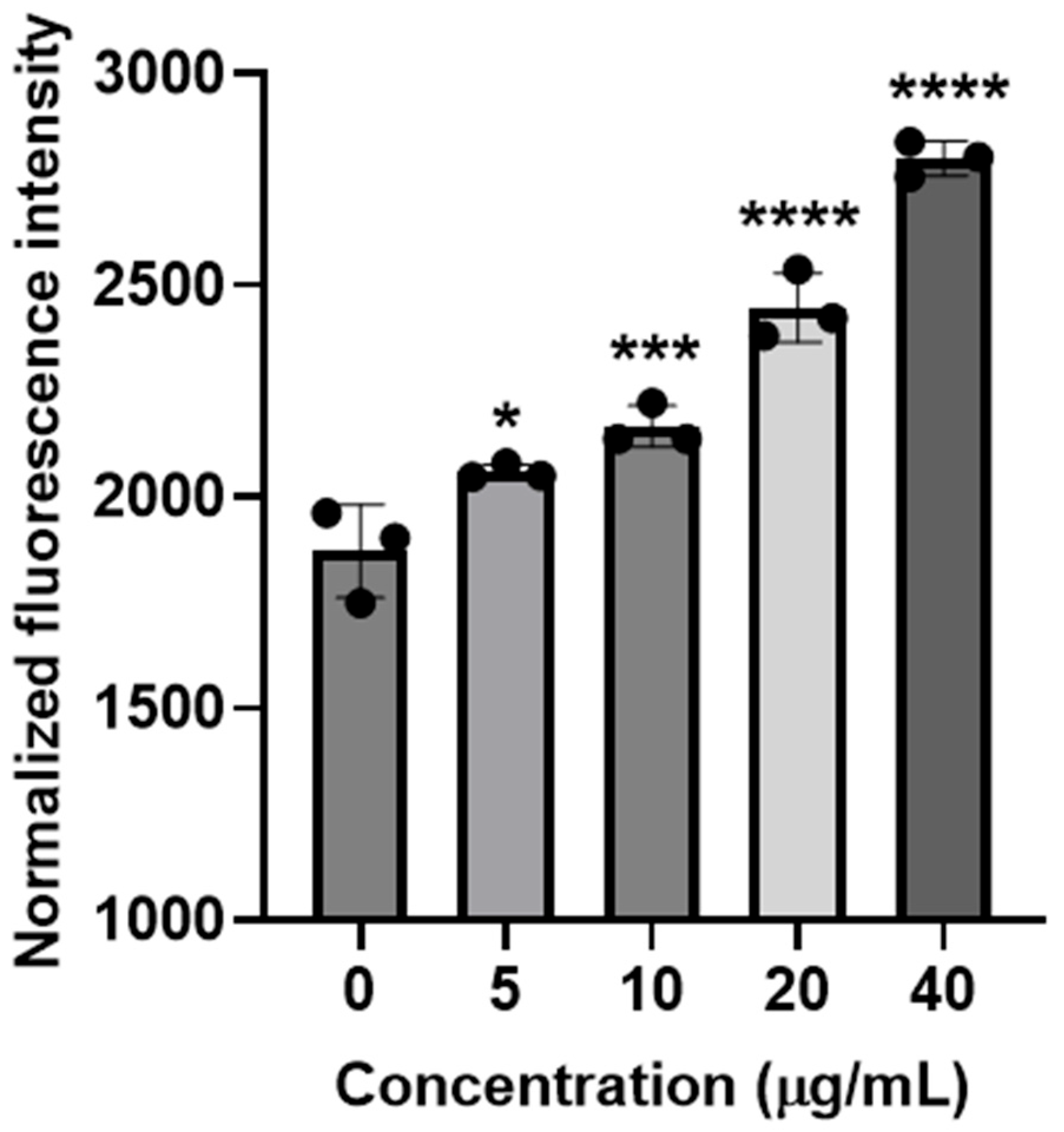
2.3. Measurements of Intracellular ROS Generation
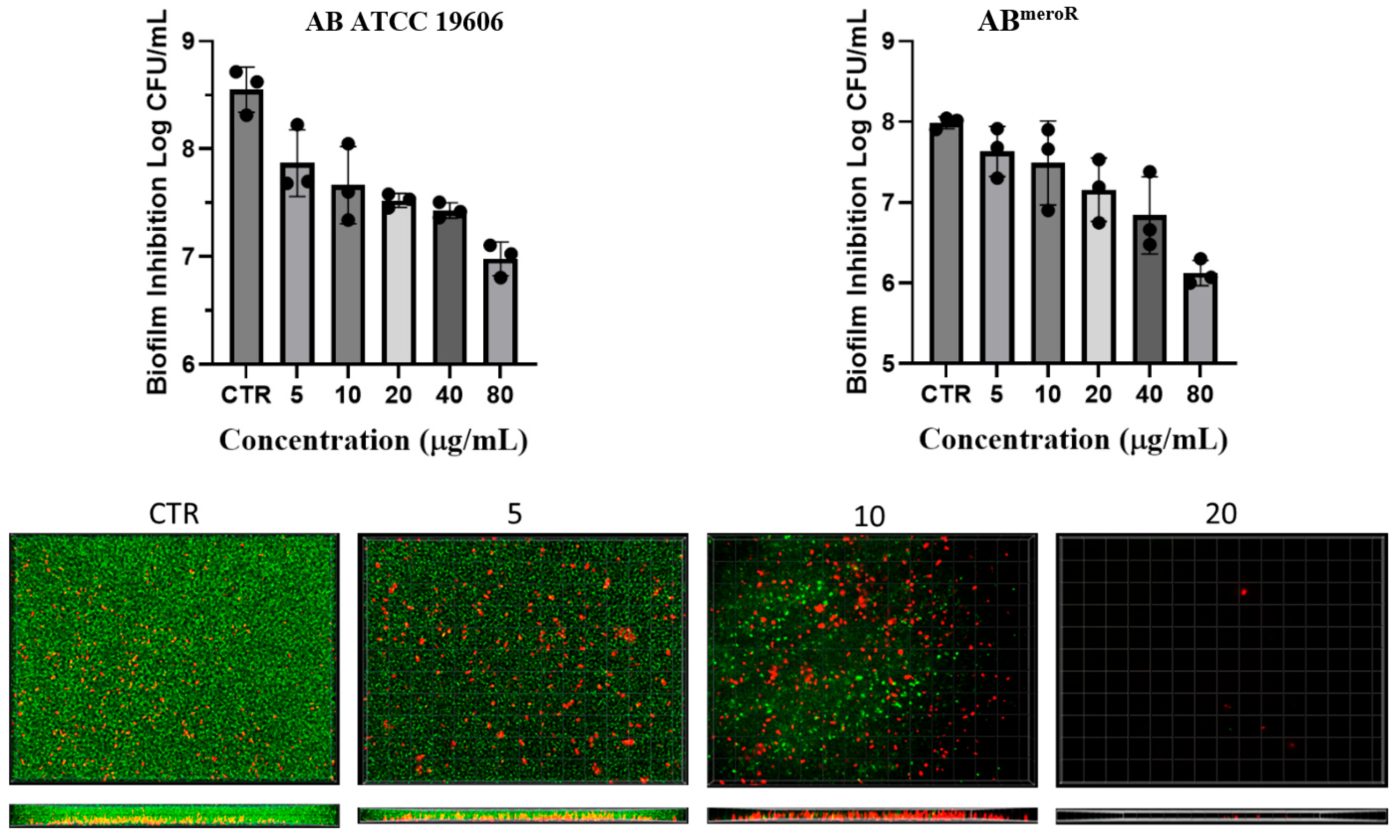
2.4. Investigation of Anti-Biofilm Activity of KP46
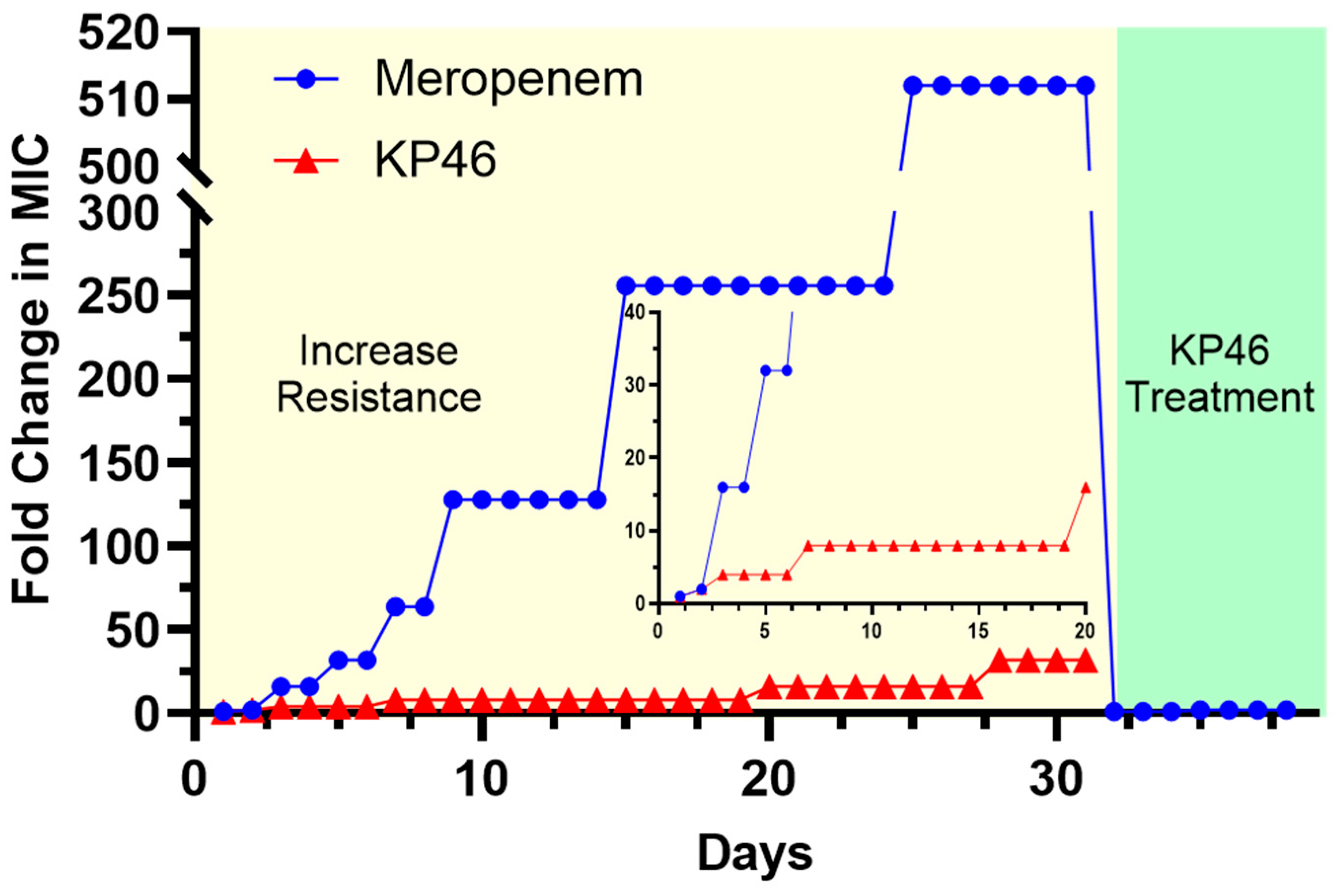
2.5. Evaluation of Ga Resistance Development
3. Materials and Methods
3.1. Synthesis of KP46 (i.e., Tris(8-Quinolinolato)Gallium(III))
3.2. Single-Crystal X-Ray Diffraction Analysis
3.3. Evaluation of Minimum Inhibitory Concentrations (MICs)
3.4. Colony-Forming Unit (CFU) Reduction Assays
3.5. Determination of Intracellular ROS Generation
3.6. In Vitro Evaluations of Resistance Development
3.7. Investigation of Antibiofilm Activity of KP46
3.8. SYTO 9/Propidium Iodide Live–Dead Staining
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- .Jamal, S.; Al Atrouni, A.; Rafei, R.; Dabboussi, F.; Hamze, M.; Osman, M. Molecular mechanisms of antimicrobial resistance in Acinetobacter baumannii, with a special focus on its epidemiology in Lebanon. J. Glob. Antimicrob. Resist. 2018, 15, 154–163. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D. Insights into Acinetobacter baumannii: A Review of Microbiological, Virulence, and Resistance Traits in a Threatening Nosocomial Pathogen. Antibiotics 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the critically ill: Complex infections get complicated. Front. Microbiol. 2023, 14, 1196774. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.A.; Suda, K.J.; Poggensee, L.; Vivo, A.; Wirth, M.; Wilson, G.; Evans, M.; Evans, C.T. Epidemiology and clinical outcomes associated with extensively drug-resistant (XDR) Acinetobacter in US Veterans’ Affairs (VA) medical centers. Infect. Control Hosp. Epidemiol. 2021, 42, 305–310. [Google Scholar] [CrossRef]
- WHO. WHO Updates List of Drug-Resistant Bacteria Most Threatening to Human Health. Available online: https://www.who.int/news/item/17-05-2024-who-updates-list-of-drug-resistant-bacteria-most-threatening-to-human-health (accessed on 7 August 2025).
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm Formation in Acinetobacter baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar]
- Lee, H.W.; Koh, Y.M.; Kim, J.; Lee, J.C.; Lee, Y.S.; Cho, Y.H.; Kim, S.I. Ability of Acinetobacter baumannii to Form Biofilm and Adhere to Epithelial Cell Surfaces. Clin. Microbiol. Infect. 2008, 14, 49–54. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Actis, L.A. Regulation of Acinetobacter baumannii Biofilm Formation. Future Microbiol. 2009, 4, 273–278. [Google Scholar] [CrossRef]
- Kubin, C.J.; Garzia, C.; Uhlemann, A.-C. Acinetobacter baumannii Treatment Strategies: A Review of Therapeutic Challenges and Considerations. Antimicrob. Agents Chemother. 2025, 69, e0106324. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, F.M.; Akinlade, E.A.; Yusuf-Omoloye, N.A.; Ajigbewu, O.H.; Dare, A.P.; Wahab, A.A.; Oyedara, O.O.; Isiaka, H.S.; Usamat, A.O. Carbapenem-Resistance in Acinetobacter baumannii: Prevalence, Antibiotic Resistance Profile and Carbapenemase Genes in Clinical and Hospital Environmental Strains. BMC Infect. Dis. 2025, 25, 786. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Review on Antimicrobial Resistance; Wellcome Trust and HM Government: London, UK, 2016; Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 7 August 2025).
- Luepke, K.H.; Suda, K.J.; Boucher, H.; Russo, R.L.; Bonney, M.W.; Hunt, T.D.; Mohr, J.F. Past, Present, and Future of Antibacterial Economics: Increasing Bacterial Resistance and Fewer New Antibiotics. Pharmacotherapy 2017, 37, 71–84. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; WHO Press: Geneva, Switzerland, 2022. [Google Scholar]
- Enyedy, É.A.; Dömötör, O.; Bali, K.; Hetényi, A.; Tuccinardi, T.; Keppler, B.K. Interaction of the Anticancer Gallium(III) Complexes of 8-Hydroxyquinoline and Maltol with Human Serum Proteins. J. Biol. Inorg. Chem. 2015, 20, 77–88. [Google Scholar] [CrossRef]
- Jain, S.; Jain, N.K.; Pitre, K.S. Bio-Inorganic Studies on the Fe(II) Sparfloxacin Complex. Met.-Based Drugs 2002, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chitambar, C.R. Gallium-containing anticancer compounds. Future Med Chem. 2012, 4, 1257–1272. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The Transition Metal Gallium Disrupts P. aeruginosa Iron Metabolism and Has Antimicrobial and Antibiofilm Activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Banin, E.; Lozinski, A.; Brady, K.M.; Berenshtein, E.; Butterfield, P.W.; Moshe, M.; Chevion, M.; Greenberg, E.P.; Banin, E. The Potential of Desferrioxamine-Gallium as an Anti-Pseudomonas Therapeutic Agent. Proc. Natl. Acad. Sci. USA 2008, 105, 16761–16766. [Google Scholar] [CrossRef]
- Baldoni, D.; Steinhuber, A.; Zimmerli, W.; Trampuz, A. In Vitro Activity of Gallium Maltolate against Staphylococci in Logarithmic, Stationary, and Biofilm Growth Phases: Comparison of Conventional and Calorimetric Susceptibility Testing Methods. Antimicrob. Agents Chemother. 2010, 54, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.C.; Imperi, F.; Minandri, F.; Visca, P. In Vitro and In Vivo Antimicrobial Activities of Gallium Nitrate against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 5961–5970. [Google Scholar] [CrossRef]
- Olakanmi, O.; Kesavalu, B.; Pasula, R.; Abdalla, M.Y.; Schlesinger, L.S.; Britigan, B.E. Gallium Nitrate Is Efficacious in Murine Models of Tuberculosis and Inhibits Key Bacterial Fe-Dependent Enzymes. Antimicrob. Agents Chemother. 2013, 57, 6074–6080. [Google Scholar] [CrossRef]
- Hijazi, S.; Visaggio, D.; Pirolo, M.; Frangipani, E.; Bernstein, L.; Visca, P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018, 8, 316. [Google Scholar] [CrossRef]
- Goss, C.H.; Kaneko, Y.; Khuu, L.; Anderson, G.D.; Ravishankar, S.; Aitken, M.L.; Lechtzin, N.; Zhou, G.; Czyz, D.M.; McLean, K.; et al. Gallium Disrupts Bacterial Iron Metabolism and Has Therapeutic Effects in Mice and Humans with Lung Infections. Sci. Transl. Med. 2018, 10, eaat7520. [Google Scholar] [CrossRef]
- Wang, Y.; Han, B.; Xie, Y.; Wang, H.; Wang, R.; Xia, W.; Li, H.; Sun, H. Combination of Gallium(III) with Acetate for Combating Antibiotic Resistant Pseudomonas aeruginosa. Chem. Sci. 2019, 10, 6099–6106. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.R.; Britigan, B.E.; Narayanasamy, P. Dual Inhibition of Klebsiella pneumoniae and Pseudomonas aeruginosa Iron Metabolism Using Gallium Porphyrin and Gallium Nitrate. ACS Infect. Dis. 2019, 5, 1559–1569. [Google Scholar] [CrossRef]
- Pandey, A.; Savino, C.; Ahn, S.H.; Yang, Z.; Van Lanen, S.G.; Boros, E. Theranostic Gallium Siderophore Ciprofloxacin Conjugate with Broad Spectrum Antibiotic Potency. J. Med. Chem. 2019, 62, 9947–9960. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, J.; Benin, B.M.; Yu, B.; Bunge, S.D.; Abeydeera, N.; Huang, S.D.; Kim, M.H. Lipophilic Ga Complex with Broad-Spectrum Antimicrobial Activity and the Ability to Overcome Gallium Resistance in Both Pseudomonas aeruginosa and Staphylococcus aureus. J. Med. Chem. 2021, 64, 9381–9388. [Google Scholar] [CrossRef]
- Scott, Z.W.; Choi, S.R.; Britigan, B.E.; Narayanasamy, P. Development of Gallium(III) as an Antimicrobial Drug Targeting Pathophysiologic Iron Metabolism of Human Pathogens. ACS Infect. Dis. 2023, 9, 716–738. [Google Scholar] [CrossRef]
- Green, M.A.; Welch, M.J. Gallium radiopharmaceutical chemistry. Int. J. Radiat. Appl. Instrum. B Nucl. Med. Biol. 1989, 16, 435–443, 445–448. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Lettenbauer, J.; Wilkinson, D.L.; Müller, G.; Kumberger, O. Modellsysteme für die Gallium-Extraktion, I. Struktur und Moleküldynamik von Aluminium- und Galliumtris(oxinaten). Z. Naturforschung B 1991, 46, 901–911. [Google Scholar] [CrossRef]
- Bankovskii, Y.; Bel’skii, V.K.; Pech, L.; Ashaks, Y. Molecular and crystal structure of the 8-hydroxyquinolinates of antimony, thallium, indium, cobalt, and gallium. Russ. J. Inorg. Chem. 1993, 38, 1988. (In Russian) [Google Scholar]
- Wang, Y.; Zhang, W.; Li, Y.; Ye, L.; Yang, G. X-ray crystal structure of gallium tris-(8-hydroxyquinoline): Intermolecular π–π stacking interactions in the solid state. Chem. Mater. 1999, 11, 530–532. [Google Scholar] [CrossRef]
- Rajeswaran, M.; Jarikov, V.V. Tris(quinolin-8-olato)gallium(III). Acta Crystallogr. Struct. Rep. Online 2004, 60, m217–m218. [Google Scholar] [CrossRef]
- CLSI. Modification of Antimicrobial Susceptibility Testing Methods—Technical Guidance; Clinical and Laboratory Standards Institute: Wayne, NY, USA, 2024. [Google Scholar]
- Collery, P.; Domingo, J.L.; Keppler, B.K. Preclinical toxicology and tissue gallium distribution of a novel antitumour gallium compound: Tris (8-quinolinolato) gallium (III). Anticancer Res. 1996, 16, 687–691. [Google Scholar]
- Kubista, B.; Schoefl, T.; Mayr, L.; van Schoonhoven, S.; Heffeter, P.; Windhager, R.; Keppler, B.K.; Berger, W. Distinct activity of the bone-targeted gallium compound KP46 against osteosarcoma cells-synergism with autophagy inhibition. J. Exp. Clin. Cancer Res. 2017, 36, 52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- APEX6, Version 2024.9-0. Program for Data Collection on Area Detectors. Bruker AXS Inc.: Madison, WI, USA, 2024.
- SAINT, Version 6.45A. Program for Data Reduction and Integration of X-Ray Diffraction Data. Bruker AXS Inc.: Madison, WI, USA, 2003.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71 Pt 1, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Mudarmah, K.; Abeydeera, N.; Chen, G.; Jogadi, W.; Krause, J.A.; Budzik, J.M.; Huang, S.D. Synthesis, Structural Characterization, and Antimicrobial Activity of Zn(cloxyquin)2: Towards Harnessing Zinc Intoxication and Immune Response Restoration to Combat Staphylococcus aureus and Mycobacterium tuberculosis. Dalton Trans. 2025, 54, 9975–9983. [Google Scholar] [CrossRef] [PubMed]
- Werth, B.J.; Abdelraouf, K.; Wencewicz, T.A.; Moehle, N.; Nicolau, D.P. Evolution of Cefiderocol Resistance by Serial Passage. JAC-Antimicrob. Resist. 2022, 4, dlac011. [Google Scholar] [CrossRef]
| Bacterial Strains | KP46 | Meropenem | |||
|---|---|---|---|---|---|
| µM | µg/mL | µM | µg/mL | ||
| Lab Strains | ATCC 19606 | 20 | 10 | 2.5 | 1 |
| ABmeroR | 40 | 20 | 80 | 32 | |
| Clinic isolate Strains | PR 328 | 10 | 5 | 80 | 128 |
| PR 336 | 20 | 10 | 80 | 128 | |
| PR 347 | 20 | 10 | 40 | 128 | |
| PR 352 | 10 | 5 | >80 | >128 | |
| PR 376 | 10 | 5 | >80 | >128 | |
| PR 315 | 20 | 10 | 10 | 32 | |
| PR 380 | 10 | 5 | >80 | >128 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Whitley, L.; Tong, X.; Bunge, S.D.; Kim, M.-H.; Shin, W.S.; Huang, S.D. Repurposing the Anticancer Drug KP46 to Beat the CRAB out of Resistance: Towards an Orally Active Ga-Based Antiplantonic and Antibiofilm Agent. Antibiotics 2025, 14, 1175. https://doi.org/10.3390/antibiotics14121175
Chen G, Whitley L, Tong X, Bunge SD, Kim M-H, Shin WS, Huang SD. Repurposing the Anticancer Drug KP46 to Beat the CRAB out of Resistance: Towards an Orally Active Ga-Based Antiplantonic and Antibiofilm Agent. Antibiotics. 2025; 14(12):1175. https://doi.org/10.3390/antibiotics14121175
Chicago/Turabian StyleChen, Guanyu, LeDarius Whitley, Xiaogang Tong, Scott D. Bunge, Min-Ho Kim, Woo Shik Shin, and Songping D. Huang. 2025. "Repurposing the Anticancer Drug KP46 to Beat the CRAB out of Resistance: Towards an Orally Active Ga-Based Antiplantonic and Antibiofilm Agent" Antibiotics 14, no. 12: 1175. https://doi.org/10.3390/antibiotics14121175
APA StyleChen, G., Whitley, L., Tong, X., Bunge, S. D., Kim, M.-H., Shin, W. S., & Huang, S. D. (2025). Repurposing the Anticancer Drug KP46 to Beat the CRAB out of Resistance: Towards an Orally Active Ga-Based Antiplantonic and Antibiofilm Agent. Antibiotics, 14(12), 1175. https://doi.org/10.3390/antibiotics14121175






