Current Clinical Practice on the Management of Invasive Streptococcus Pyogenes Infections in Children: A Survey-Based Study
Abstract
1. Introduction
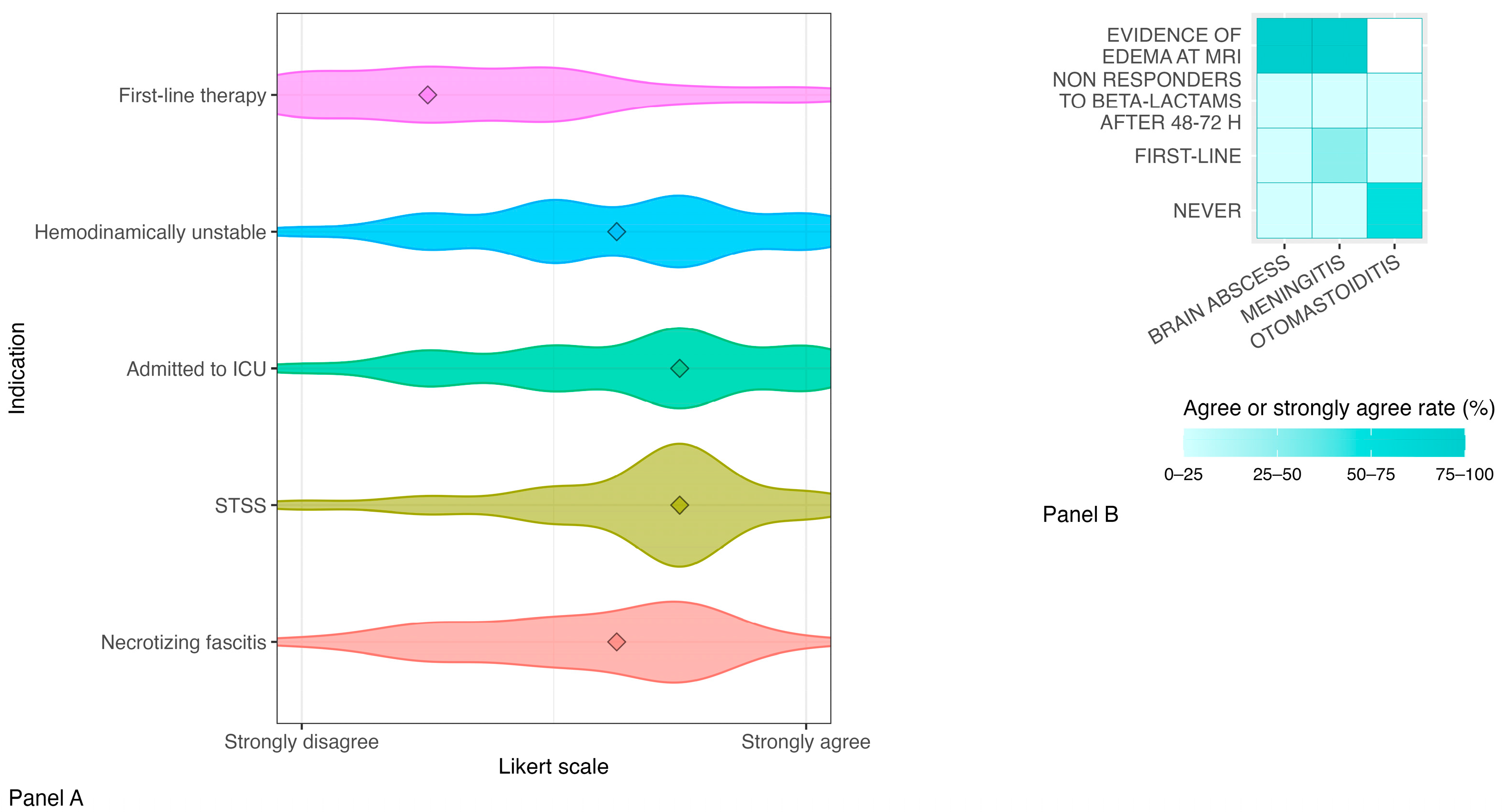
2. Results
- Sepsis
- Streptococcal toxic shock syndrome
- Necrotizing fasciitis
- Pneumonia
- Otomastoiditis
- Meningitis and brain abscess
- Septic arthritis and osteomyelitis
- Use of intravenous immunoglobulins
- Use of steroids
- Prophylaxis of close contacts
3. Discussion
4. Materials and Methods
4.1. Study Design, Data Collection and Analysis
4.2. Definitions
- ▯
- Invasive group A Streptococcus infection: detection of S. pyogenes by culture or accredited molecular methods (such as PCR), from a normally sterile body site, such as blood, cerebrospinal fluid, joint aspirate, pericardial-peritoneal-pleural fluids, bone, endometrium, deep tissue or deep abscess at operation.
- ▯
- Severe group A Streptococcus infection: isolation of S. pyogenes from a normally non-sterile site such as the throat, sputum, vagina or a wound in combination with severe clinical presentation, such as STSS, NF, pneumonia, septic arthritis, meningitis, peritonitis, osteomyelitis, myositis and puerperal sepsis.
4.3. Ethical Statement
5. Conclusions
Future Directions
- Enhanced surveillance of local GAS strains to monitor antibiotic susceptibility trends;
- Clinical trials to assess outcomes in patients treated with combination therapy (including anti-toxin antibiotics) versus monotherapy;
- Comparative studies on the timing of anti-toxin antibiotic initiation (first-line versus delayed use at 48–72 h) across various infectious sites;
- Evaluation of the efficacy, safety and cost-effectiveness of clindamycin versus linezolid as anti-toxin agents;
- Research into the role of IVIG in different clinical scenarios (i.e., hemodynamic instability, ICU admission, NF or STSS), including optimal dosing regimens;
- Investigation of corticosteroid use in iGAS meningitis, analogous to pneumococcal meningitis treatment strategies;
- Development of comprehensive clinical guidelines to support the management of iGAS infections and strengthen outbreak preparedness among ID specialists.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | US Centers for Disease Control and Prevention |
| CROSS | Checklist for Reporting of Survey Studies |
| GAS | Group A Streptococcus |
| iGAS | Invasive GAS |
| IVIG | Intravenous Immunoglobulins |
| NF | Necrotizing Fasciitis |
| PID | Pediatric Infectious Diseases |
| SITIP | Italian Society of Pediatric Infectious Diseases |
| STSS | Streptococcal Toxic Shock Syndrome |
References
- Carapetis, J.R.; Steer, A.C.; Mulholland, E.K.; Weber, M. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 2005, 5, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Cobo-Vázquez, E.; Aguilera-Alonso, D.; Grandioso-Vas, D.; Gamell, A.; Rello-Saltor, V.; Oltra-Benavent, M.; Cervantes, E.; Sanz-Santaeufemia, F.; Carrasco-Colom, J.; Manzanares-Casteleiro, Á.; et al. Sharp increase in the incidence and severity of invasive Streptococcus pyogenes infections in children after the COVID-19 pandemic (2019–2023): A nationwide multicenter study. Int. J. Infect Dis. 2025, 16, 107982. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, U.; Hartling, U.B.; Munkstrup, C.; Nielsen, A.B.; Dungu, K.H.S.; Schmidt, L.S.; Glenthøj, J.; Matthesen, A.T.; Rytter, M.J.H.; Holm, M. Invasive group A streptococcal infections in children and adolescents in Denmark during 2022–2023 compared with 2016–2017 to 2021–2022: A nationwide, multicentre, population-based cohort study. Lancet Child Adolesc. Health 2024, 8, 112–121. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. Group A Streptococcal Infections: Report on Seasonal Activity in England (2023). Available online: https://www.gov.uk/government/publications/gp-in-hours-weekly-bulletins-for-2022 (accessed on 15 February 2025).
- van Kempen, E.B.; Tulling, A.J.; von Asmuth, E.G.J.; van der Aa, L.B.; Bijker, E.M.; Bijlsma, M.; Borensztajn, D.M.; Brackel, C.L.H.; de Gier, B.; van Houten, M.A.; et al. Risk Factors for Severe Pediatric Invasive Group A Streptococcal Disease. JAMA Netw. Open 2025, 8, e2527717. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Executive summary: Practice guidelines for the diagnosis and management of skin and soft tissue infections. Clin. Infect. Dis. 2014, 59, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Gibbons, A.E.; Bergstrom, R.; Winn, V. The Eagle effect revisited: Efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J. Infect. Dis. 1988, 158, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs). Available online: https://www.cdc.gov/abcs/reports-findings/surv-reports.html (accessed on 19 September 2025).
- Buricchi, L.; Indolfi, G.; Renni, M.; Venturini, E.; Galli, L.; Chiappini, E. Evolving treatment strategies for invasive Streptococcus pyogenes in children in the postpandemic era. Clin. Exp. Pediatr. 2025, online ahead of print. [CrossRef]
- Watts, V.; Balasegaram, S.; Brown, C.S.; Mathew, S.; Mearkle, R.; Ready, D.; Saliba, V.; Lamagni, T. Increased risk for invasive group A Streptococcus disease for household contacts of scarlet fever cases, England, 2011–2016. Emerg. Infect. Dis. 2019, 25, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Adebanjo, T.; Apostol, M.; Alden, N.; Petit, S.; Tunali, A.; Torres, S.; Hollick, R.; Bell, A.; Muse, A.; Poissant, T.; et al. Evaluating household transmission of invasive group A Streptococcus disease in the United States. Clin. Infect. Dis. 2020, 70, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Meletis, G.; Ketikidis, A.L.; Floropoulou, N.; Tychala, A.; Kagkalou, G.; Vasilaki, O.; Mantzana, P.; Skoura, L.; Protonotariou, E. Antimicrobial resistance rates of Streptococcus pyogenes in a Greek tertiary care hospital. New Microbiol. 2023, 46, 37–42. [Google Scholar] [PubMed]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A review of the impact of streptococcal infections and antimicrobial resistance on human health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Babiker, A.; Li, X.; Lai, Y.L.; Strich, J.R.; Warner, S.; Sarzynski, S.; Dekker, J.P.; Danner, R.L.; Kadri, S.S. Effectiveness of adjunctive clindamycin in β-lactam antibiotic-treated patients with invasive β-haemolytic streptococcal infections. Lancet Infect. Dis. 2021, 21, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.A.; Cha, R.; Rybak, M.J. Influences of linezolid, penicillin, and clindamycin on streptococcal pyrogenic exotoxin A release. Antimicrob. Agents Chemother. 2003, 47, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Heil, E.L.; Kaur, H.; Atalla, A.; Basappa, S.; Mathew, M.; Seung, H.; Johnson, J.K.; Schrank, G.M. Comparison of adjuvant clindamycin vs linezolid for severe invasive group A streptococcus infections. Open Forum Infect. Dis. 2023, 10, ofad588. [Google Scholar] [CrossRef] [PubMed]
- Lapthorne, S.; McWade, R.; Scanlon, N.; Bhaoill, S.N.; Page, A.; O’DOnnell, C.; Dornikova, G.; Hannan, M.; Lynch, B.; Lynch, M.; et al. Rising clindamycin resistance in group A Streptococcus in an Irish healthcare institution. Access Microbiol. 2024, 6, 000772.v4. [Google Scholar] [CrossRef] [PubMed]
- Darenberg, J.; Ihendyane, N.; Sjölin, J.; Aufwerber, E.; Haidl, S.; Follin, P.; Andersson, J.; Norrby-Teglund, A. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: A European randomized trial. Clin. Infect. Dis. 2003, 37, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Heining, D.; Plant, A.J. Steroid use in non-pneumococcal and non-Haemophilus bacterial meningitis. Lancet 2022, 399, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, L.; De Luca, M.; Cursi, L.; Romani, L.; Pisani, M.; Musolino, A.M.; Mercadante, S.; Cortazzo, V.; Vrenna, G.; Bernaschi, P.; et al. Unraveling Pediatric Group A Streptococcus Meningitis: Lessons from Two Case Reports and a Systematic Review. Microorganisms 2025, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics; Kimberlin, D.W.; Barnett, E.D.; Lynfield, R.; Sawyer, M.H. (Eds.) Red Book: 2021–2024 Report of the Committee on Infectious Diseases, 32nd ed; American Academy of Pediatrics: Itasca, IL, USA, 2021. [Google Scholar]
- Hazelhorst, E.I.; van Ewijk, C.E.; Wielders, C.C.H.; Wierik, M.J.T.; Hahné, S.J.; de Melker, H.E.; Knol, M.J.; de Gier, B.; Wielders, L. Risk factors for invasive group A streptococcal infection in children aged 6 months to 5 years: A case-control study, the Netherlands, February–May 2023. Epidemiol. Infect. 2025, 153, e57. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Ficari, A.; Romani, L.; De Luca, M.; Tripiciano, C.; Chiurchiù, S.; Carducci, F.I.C.; Cursi, L.; Di Giuseppe, M.; Krzysztofiak, A.; et al. The Thousand Faces of Invasive Group A Streptococcal Infections: Update on Epidemiology, Symptoms, and Therapy. Children 2024, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Duc, N.T.M.; Thang, T.L.L.; Nam, N.H.; Ng, S.J.; Abbas, K.S.; Huy, N.T.; Marušić, A.; Paul, C.L.; Kwok, J.; et al. Consensus-based checklist for reporting of survey studies (CROSS). J. Gen. Intern. Med. 2021, 36, 3179–3187. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. UK Guidelines for the Management of Contacts of Invasive Group A Streptococcus (iGAS) Infection in Community Settings. 2022. Available online: https://www.gov.uk/government/publications/invasive-group-a-streptococcal-disease-managing-community-contacts (accessed on 15 February 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, M.; Tripiciano, C.; D’Amore, C.; Ciofi Degli Atti, M.L.; Romani, L.; Pagano, F.; Zama, D.; Garazzino, S.; Nicolini, G.; Bosis, S.; et al. Current Clinical Practice on the Management of Invasive Streptococcus Pyogenes Infections in Children: A Survey-Based Study. Antibiotics 2025, 14, 970. https://doi.org/10.3390/antibiotics14100970
De Luca M, Tripiciano C, D’Amore C, Ciofi Degli Atti ML, Romani L, Pagano F, Zama D, Garazzino S, Nicolini G, Bosis S, et al. Current Clinical Practice on the Management of Invasive Streptococcus Pyogenes Infections in Children: A Survey-Based Study. Antibiotics. 2025; 14(10):970. https://doi.org/10.3390/antibiotics14100970
Chicago/Turabian StyleDe Luca, Maia, Costanza Tripiciano, Carmen D’Amore, Marta Luisa Ciofi Degli Atti, Lorenza Romani, Federica Pagano, Daniele Zama, Silvia Garazzino, Giangiacomo Nicolini, Samantha Bosis, and et al. 2025. "Current Clinical Practice on the Management of Invasive Streptococcus Pyogenes Infections in Children: A Survey-Based Study" Antibiotics 14, no. 10: 970. https://doi.org/10.3390/antibiotics14100970
APA StyleDe Luca, M., Tripiciano, C., D’Amore, C., Ciofi Degli Atti, M. L., Romani, L., Pagano, F., Zama, D., Garazzino, S., Nicolini, G., Bosis, S., Chiappini, E., Colomba, C., & Lo Vecchio, A. (2025). Current Clinical Practice on the Management of Invasive Streptococcus Pyogenes Infections in Children: A Survey-Based Study. Antibiotics, 14(10), 970. https://doi.org/10.3390/antibiotics14100970







