Abstract
Background: Urinary tract infections (UTIs) are the most common infections among kidney transplant recipients, with prevalence rates ranging from 12% to 75% in studies from North America and Australia and from 4.5% to 85% in the Middle East. These infections can significantly impact graft survival and patient quality of life, increasing the risk of hospitalization, morbidity, and mortality. Escherichia coli is the leading cause of UTIs in transplant patients, but multidrug-resistant (MDR) pathogens are a growing concern, especially in Saudi Arabia. Several factors, including advanced age, female gender, and use of urinary catheters, contribute to post-transplant UTIs. This study focuses on the Saudi population, aiming to assess the prevalence, risk factors, and treatment strategies for recurrent and multidrug-resistant UTIs in kidney transplant recipients. Methods: This retrospective cohort study reviewed the medical records of kidney transplant patients at King Faisal Specialist Hospital & Research Center, Jeddah, in addition to data from King Fahad Hospital, Madinah, Saudi Arabia, between March and May 2022. Adult patients (≥18 years) who developed recurrent UTIs within two years post-transplant were included, while those with one or no UTI episode or incomplete records were excluded. Results: Seventy-five of 491 screened patients (15.3%) experienced recurrent UTIs, contributing to a total of 219 episodes. Klebsiella pneumoniae was the most frequent pathogen, isolated in 94 episodes (42.9%). Key risk factors for recurrence included complicated UTIs (OR = 4.60, p = 0.005), multidrug-resistant organisms (MDROs) (OR = 3.14, p = 0.021), and ureteric stents (OR = 4.07, p = 0.042). Carbapenems were primarily used for complicated UTIs, while cephalosporins and penicillins were used for uncomplicated infections. A significant post-UTI rise in serum creatinine was observed (p < 0.001). Conclusions: Recurrent UTIs predominantly caused by K. pneumoniae are common in kidney transplant recipients, particularly in patients over 45, with multidrug-resistant organisms, or with ureteric stents. While a direct causal link to graft loss was not established, these infections can lead to increased creatinine levels, hospitalizations, and healthcare costs and increased carbapenem use. These findings highlight the critical need for institution-specific antimicrobial stewardship programs focused on infection prevention and optimized antibiotic use to improve outcomes in this vulnerable population.
1. Introduction
Urinary tract infections (UTIs) are the most prevalent and enduring infections observed after kidney transplantation [1,2,3]. Research indicates that the incidence of UTIs in kidney transplant recipients varies between 12% and 75%, depending on the specific population and geographic region studied [4,5,6,7]. The occurrence of a UTI in kidney transplant patients can have far-reaching consequences, affecting both the survival of the graft and the overall quality of life for the patient. In many cases, UTIs can lead to hospitalizations, prolonged recovery periods, and an increased risk of further complications, including morbidity and mortality [8,9]. Furthermore, the management of these infections is complicated by the global rise of multidrug-resistant (MDR) uropathogens, a trend particularly pronounced in the Middle East, where high rates of extended-spectrum β-lactamase (ESBL)-producing Enterobacterales and carbapenem-resistant organisms pose a significant therapeutic challenge [10,11,12,13]. The bacterial strains most commonly implicated in post-transplant urinary tract infections include Escherichia coli, which accounts for 30–80% of cases. Other notable pathogens include Klebsiella pneumoniae (12%) and Enterococcus species (5%), as well as less common strains such as Pseudomonas aeruginosa and Proteus [5,6,7]. These infections can vary from uncomplicated to severe, multidrug-resistant infections that complicate treatment outcomes. Several patient-specific and transplant-related factors contribute to an increased risk of developing UTI after transplantation. Factors include advanced age, as older patients exhibit greater susceptibility, and gender, with females at a higher risk compared to males. Diabetes mellitus, receiving a kidney from a deceased donor, continued use of urinary catheters, and acute rejection episodes are additional significant risk factors [1,4,6,14]. All these characteristics can affect the frequency and severity of UTI episodes, complicating prevention and management in this demographic. This study aims to enhance the understanding of recurrent and multidrug-resistant urinary tract infections in kidney transplant patients using a retrospective analysis of patient data collected two years post-transplant among two hospitals. The main objective is to evaluate the existing therapeutic options implemented in Saudi Arabia to manage these medical conditions. The study seeks to identify the primary risk variables that increase the likelihood of UTI recurrence, possibly influencing future prevention and treatment strategies for this patient group in Saudi Arabia.
2. Result
The medical records of 491 renal transplant recipients were reviewed, and 75 patients (15.3%) met the inclusion criteria for recurrent UTIs. The mean age of the patients was 48.2 ± 15.8 years, with 43 (57.5%) being women. The average BMI was 24.9 ± 5.9. A significant majority (85.3%) of patients received living donor transplants (Table 1, Figure 1).

Table 1.
Patient Eligibility and Baseline Characteristics.
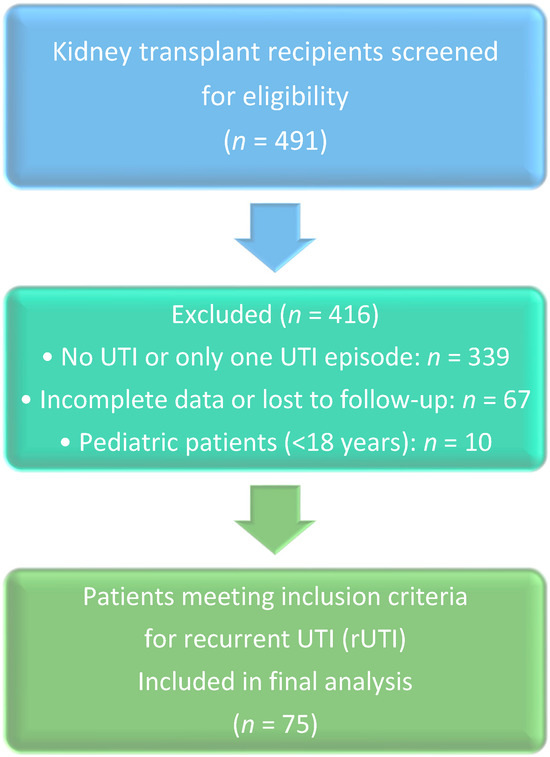
Figure 1.
PRISMA-like flow diagram of patient selection.
Key risk factors associated with recurrent UTIs included age over 45 (Mean = 48.2), DM (OR = 1.833, p = 0.039), the presence of multidrug-resistant organisms (MDRO) (OR = 3.14, 95% CI = 1.19–8.33, p = 0.02), ureteric stents (OR = 4.07, 95% CI = 1.05–15.67, p = 0.04), and complicated UTIs (OR = 4.60, 95% CI = 1.57–13.50, p = 0.005). Interestingly, patients with catheters were significantly less likely to experience recurrent UTIs compared to those without (OR = 0.19, 95% CI = 0.07–0.54, p = 0.002), and female gender was associated with lower odds of recurrence compared to male gender (OR = 0.382, 95% CI = 0.148–0.99, p = 0.048) (Table 2).

Table 2.
Univariate Logistic Regression Analysis of Risk Factors Associated with Recurrent UTI.
Logistic regression showed no significant association between baseline serum creatinine (OR = 1.006, p = 0.136) and white blood cell count (OR = 0.963, p = 0.472) with the recurrence of UTIs post-transplant. Similarly, no significant differences were found in serum creatinine levels or WBC counts between the first and second UTI episodes (p > 0.05). Additionally, the duration of prophylaxis (OR = 1.10, 95% CI = 0.97–1.22, p = 0.67) and treatment duration after the first (OR = 0.99, 95% CI = 0.86–1.14, p = 0.90) and second UTI episodes (OR = 1.06, 95% CI = 0.95–1.18, p = 0.29) did not increase the risk of recurrent UTIs. Out of 219 UTI episodes, Klebsiella pneumoniae was the most commonly identified organism (n = 94; 42.9%), followed by Escherichia coli (n = 84; 38.4%), Pseudomonas aeruginosa (n = 16; 7.3%), Proteus mirabilis (n = 7; 3.2%), and others (n = 18; 8.2%) (Figure 2).
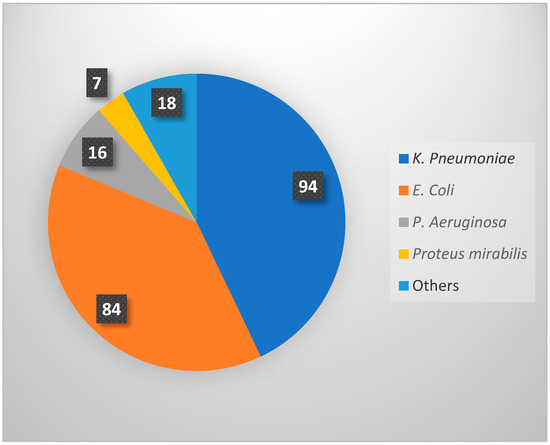
Figure 2.
Distribution of causative microorganisms identified in 219 recurrent UTI episodes.
The most frequently used antibiotics were carbapenems (n = 95; 40.8%), cephalosporins (n = 67; 28.2%), penicillins (n = 28; 12%), and fluoroquinolones (n = 27; 11.6%). The Wilcoxon signed-rank test revealed a significant rise in serum creatinine levels in post-transplant patients with recurrent UTIs compared to baseline levels (Z = −4.552, p < 0.001) (Table 3).

Table 3.
Baseline Serum Creatinine (SCr) and White Blood Cell (WBC) Counts, and Impact of First UTI Episode on SCr.
Serum creatinine increased in 58 patients, remained the same in three, and decreased in 14. The median post-transplant creatinine level was 97 µmol/L (IQR = 77), compared to a baseline median of 85 µmol/L (IQR = 48) (Z = −4.552, p < 0.001).
3. Discussion
Our results are consistent with other research regarding risk factors for recurrent urinary tract infections [1,4,6,7,15]. For instance, when we compared our findings with a meta-analysis encompassing around 72,600 individuals, both investigations recognized age exceeding 45 as a notable risk factor, with a mean age of 48 years in our investigation. Both studies also identified the use of ureteric stents as a major risk factor, with an odds ratio (OR) of 4.065 (95% CI: 1.054–15.670) in our study and OR = 1.54 (95% CI: 1.16–2.06) in the meta-analysis [4]. In cases of UTIs caused by multidrug-resistant organisms (MDROs), our findings were consistent with those of a prior study that showed this risk factor nearly tripled the chances of recurrence, with an OR of 2.75 (95% CI: 1.97–3.83). Our study produced similar results, with an OR of 3.143 (95% CI: 1.185–8.334) [16]. Patients with complicated UTIs in our study had a significantly higher risk of recurrent UTIs (OR = 4.600, 95% CI: 1.567–13.502) and DM (OR = 1.833, p = 0.039), marking them as the primary risk factors identified. Although urinary catheters are widely recognized as a risk factor for urinary tract infections (UTIs), our analysis found that their presence associated with a reduced incidence of recurrent UTIs (OR = 0.19, 95% CI = 0.07–0.54, p = 0.002), potentially attributable to the transient nature of catheter use and the growing evidence supporting the benefits of early catheter removal in reducing UTI risk [17,18,19]. Some studies suggest that early removal might reduce the incidence of urinary tract infections. Also, the unexpected association between female gender and reduced recurrence risk warrants further investigation, but it may be related to unmeasured confounding factors in our cohort. Further factors, including smoking, anatomical anomalies, and infections like CMV and BK virus, did not demonstrate significant correlations with recurrent UTIs. (Table 2). All kidney transplant recipients in our study were given induction immunosuppressants, specifically thymoglobulin (ATG) (49.3%) or basiliximab (50.7%). Maintenance therapy included steroids, tacrolimus, and mycophenolate mofetil (MMF). Only 3 out of 75 patients (4%) experienced episodes of rejection. While most studies define E. coli as the predominant cause of UTIs post-kidney transplantation, our research identified K. pneumoniae as the most prevalent pathogen, accounting for 42.9% of the cases and followed by E. coli at 38.4%, P. aeruginosa at 7.3%, P. mirabilis at 3.2%, and other organisms representing 8.2% of the total UTI episodes. Numerous organizations, including the Infectious Disease Society of America (IDSA), the American Society of Transplantation Infectious Diseases Community of Practice, and the European Association of Urology Guidelines on Urological Infections, offer recommendations regarding the classifications of urinary tract infections (UTIs) and suitable antibiotic regimens for each [5,20,21]. In our study, we found that carbapenems (40%) were the most commonly used antibiotics to treat recurrent UTIs, followed by cephalosporins (28.8%), with an average treatment duration of around 9 days (Table 4).

Table 4.
Most used antibiotic classes with treatment duration.
The choice of antibiotic and treatment length varies based on factors such as the patient’s condition, the type of organism, whether the infection is complicated or uncomplicated, and the administration route [22,23,24,25,26]. The dominance of Klebsiella pneumoniae and the extensive use of carbapenems in our group is not an isolated finding but reflects a serious and well-acknowledged antimicrobial resistance (AMR) crisis within the Kingdom of Saudi Arabia and the broader Gulf region [10,11,12]. National and regional surveillance reports consistently show alarmingly high rates of extended-spectrum β-lactamase (ESBL)-producing Enterobacterales and carbapenem-resistant Klebsiella pneumoniae (CRKP) [13]. This growing resistance trend, clearly demonstrated in our cohort, compromises the efficacy of first-line antibiotics and necessitates the use of last-resort agents like carbapenems. This highlights the urgent need for institution-specific, evidence-based antimicrobial stewardship programs (ASPs) tailored for the transplant population, in line with recent national efforts [27,28,29]. Such programs should focus on the following: empiric therapy guidelines based on real-time local antibiograms, rapid diagnostic testing to enable early de-escalation from broad-spectrum drugs, and strict adherence to guidelines for appropriate treatment length. Our findings add to this vital regional discussion by pointing out the unique challenges faced by highly vulnerable kidney transplant patients. Although some studies have explored antibiotic strategies for preventing recurrent infections [30,31,32], all kidney transplant recipients in our study received UTI prophylaxis with daily trimethoprim-sulfamethoxazole for at least six months post-transplant, following KDIGO guidelines for the care of KTRs [33]. In our findings, carbapenems were predominantly used for complicated infections, while cephalosporins and penicillins were more commonly selected for uncomplicated cases. This prescribing pattern reflects the serious and well-acknowledged antimicrobial resistance (AMR) crisis within the Kingdom of Saudi Arabia and the broader Gulf region. Table 5 [34,35,36]. A Chi-Square test of independence was performed to examine the relation between antibiotic class selection and UTI complexity. The relation between these variables was significant, χ2(10, N = 72) = 43.462, p < 0.001, indicating that the choice of antibiotic was not independent of whether the infection was complicated or uncomplicated.

Table 5.
Association between antibiotic class selection and UTI complexity.
Numerous studies have investigated the influence of UTIs on graft function, yielding varied outcomes. Certain studies investigating the impact of urinary tract infections [37,38] indicate no correlation between urinary tract infections (UTIs) and poor transplant function or survival, but other studies recognize UTIs as a notable risk factor for unfavorable graft outcomes. Effects of recurrent urinary tract infections on graft and patient outcomes after kidney transplantation have been described [8,9]. Our investigation revealed that blood creatinine levels were elevated subsequent to urinary tract infection episodes, especially following the initial infection. A Wilcoxon signed-rank test revealed a statistically significant rise in serum creatinine levels in KTRs following UTI incidents compared to baseline levels (Z = −4.552, p < 0.001), with serum creatinine increasing in 58 patients (77.3%). This study has several strengths, including its conduct at two major transplant hospitals in Saudi Arabia, which enabled access to an extensive dataset and supported the examination of certain risk factors over an extended duration. The findings are consistent with existing research, and the comprehensive analysis of antibiotic use, pathogen prevalence, and their impact on serum creatinine provides valuable insights for post-transplant management. However, there are some limitations. The retrospective design of the study may introduce bias resulting from differences in hospital systems, employment, and standards over time. The limited sample size and concentration on a single region may restrict the applicability of the findings to wider populations. Furthermore, a major limitation of our analysis is the use of univariate logistic regression. While this identified several significant associations, these models are unadjusted and cannot determine whether the risk factors are independent predictors or if their effects are confounded by other variables. The wide confidence intervals for some estimates also indicate statistical uncertainty, likely due to the sample size. Therefore, the odds ratios presented should be interpreted as preliminary associations. Future studies with larger cohorts are necessary to perform multivariable analysis to adjust for potential confounders and identify true independent risk factors for recurrent UTIs in this population.
4. Materials and Methods
This retrospective cohort study analyzed the medical records of kidney transplant patients at King Faisal Specialist Hospital & Research Center in Jeddah, as well as data from King Fahad Hospital in Madinah, Saudi Arabia, from March to May 2022. To minimize inter-center variability and potential selection bias, a standardized set of diagnostic criteria for UTIs (detailed below) was applied uniformly to all patient records from both institutions during data collection. Furthermore, all data collectors were trained using the same protocol to ensure consistent interpretation and application of these criteria across both sites. The study was approved by the Institutional Review Boards of both hospitals (IRB 2022-22 for Jeddah and IRB 22-073 for Madinah). The inclusion criteria focused on adult patients (≥18 years) who had received a kidney transplant and developed recurrent UTIs within two years post-transplant. Exclusion criteria: Patients who experienced only one or no UTI episodes post-transplant to specifically investigate the risk factors associated with recurrent infection, a distinct clinical entity. This focus may limit the generalizability of our findings to transplant recipients with sporadic, rather than recurrent, UTIs, those with incomplete data or lost to follow-up, and patients under 18 years of age. Data collected included patient demographics, pre-transplant risk factors, transplantation-related factors, baseline lab values, immunosuppressant medications, and UTI episodes, along with their management. Definitions for classifications used in this study were based on the IDSA guidelines cited earlier in this manuscript [20]. Recurrent UTI (rUTI) was defined as two or more culture-proven UTI episodes within six months, or three or more episodes within twelve months post-transplantation, adapting the IDSA criteria for the immunocompromised transplant population. Uncomplicated UTI: Positive urine culture accompanied by urinary symptoms such as dysuria, urgency, frequency, or suprapubic pain. Complicated UTI: Systemic symptoms requiring hospital admission for intravenous antibiotic therapy. Clinical presentation: Symptoms such as fever, chills, dysuria, cloudy urine, flank pain, or tenderness in the grafted kidney. Urinalysis: Considered positive if leukocyte esterase is ≥250 and/or nitrite is positive. Risk factors recorded in the study included diabetes mellitus, smoking, structural factors (e.g., native kidney disease with urological malformations, renal cysts, calculi, benign prostate hyperplasia), neurogenic bladder, and operation-related factors (e.g., urinary catheter, ureteric stent, vesicoureteral reflux, and urinary fistula), as well as infections with CMV, BK virus, and UTI caused by E. coli.
We used statistical analyses to explore the relationship between various risk factors and recurrent UTIs in kidney transplant recipients. Descriptive statistics were applied to summarize the demographic and clinical characteristics of the participants. Categorical variables are reported as frequencies and percentages, while continuous variables are presented as means and standard deviations. To assess the association between risk factors and recurrent UTIs, we performed univariate logistic regression analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the strength of these relationships, with a p-value of less than 0.05 indicating statistical significance. For comparing paired data, we employed non-parametric tests like the Wilcoxon signed-rank test. All analyses were conducted using SPSS version 25, with a significance threshold set at p < 0.05 for all statistical tests.
5. Conclusions
In conclusion, this study identifies Klebsiella pneumoniae as the predominant pathogen causing recurrent UTIs in our cohort of Saudi kidney transplant recipients, necessitating frequent carbapenem use. This reflects a serious regional AMR challenge. The significant association of rUTIs with increased serum creatinine highlights their clinical impact beyond simple morbidity. These findings strongly support the implementation of tailored antimicrobial stewardship programs in transplant centers, emphasizing empiric therapy guided by local antibiograms, rapid diagnostics for de-escalation, and strict adherence to treatment duration guidelines. Future efforts must focus on optimizing infection prevention strategies and prospectively evaluating their impact on graft function, resistance rates, and patient outcomes in this vulnerable population.
Author Contributions
Conceptualization, K.A.A., R.Y.M., A.T.K. and A.S.A. (Abdulmalik S. Alotaibi); Methodology, K.A.A. and A.S.A. (Abdulmalik S. Alotaibi); Software, A.Y.A.; Validation, K.A.A., A.Y.A., N.E.A., A.S.A. (Abdullah S. Alshammari), A.S.A. (Abdulmalik S. Alotaibi) and M.A.A.; Formal analysis, A.Y.A.; Investigation, K.A.A., R.Y.M., A.T.K., B.B.A. and A.S.A. (Abdulmalik S. Alotaibi); Resources, B.B.A., A.Y.A., N.E.A., A.S.A. (Abdullah S. Alshammari) and M.A.A.; Data curation, K.A.A., R.Y.M. and A.T.K.; Writing—original draft, K.A.A., R.Y.M., A.T.K., B.B.A., A.Y.A. and A.S.A. (Abdulmalik S. Alotaibi); Writing—review & editing, K.A.A., R.Y.M., A.T.K., B.B.A., A.S.A. (Abdulmalik S. Alotaibi) and M.A.A.; Visualization, K.A.A., A.Y.A., N.E.A., A.S.A. (Abdullah S. Alshammari), A.S.A. (Abdulmalik S. Alotaibi) and M.A.A.; Supervision, N.E.A. and A.S.A. (Abdulmalik S. Alotaibi); Project administration, K.A.A. and A.S.A. (Abdulmalik S. Alotaibi). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board. Institutional Review Board (IRB) approval was obtained from both King Faisal Specialist Hospital & Research Center, Jeddah (IRB 2022-22), and King Fahad Hospital, Madinah (IRB 22-073).
Informed Consent Statement
The need for informed consent was waived by the IRB, given that the study posed minimal risk to participants.
Data Availability Statement
Upon a justified request, the corresponding author can share the data.
Conflicts of Interest
The authors confirm that there are no conflicts of interest that could have influenced the findings of this study.
Abbreviations
The following abbreviations are used in this manuscript:
| UTI | Urinary Tract Infection |
| rUTI | Recurrent Urinary Tract Infection |
| CMV | Cytomegalovirus |
| BK | BK virus (polyomavirus) |
| MDRO | Multidrug-Resistant Organism |
| DM | Diabetes Mellitus |
| KTR | Kidney Transplant Recipient |
| AMR | Antimicrobial Resistance |
References
- Alangaden, G.J.; Thyagarajan, R.; Gruber, S.A.; Morawski, K.; Garnick, J.; El-Amm, J.M.; West, M.S.; Sillix, D.H.; Chandrasekar, P.H.; Haririan, A. Infectious complications after kidney transplantation: Current epidemiology and associated risk factors. Clin. Transplant. 2006, 20, 401–409. [Google Scholar] [CrossRef]
- Alangaden, G.J. Urinary tract infections in renal transplant recipients. Curr. Infect. Dis. Rep. 2007, 9, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Veroux, M.; Giuffrida, G.; Corona, D.; Gagliano, M.; Scriffignano, V.; Vizcarra, D.; Tallarita, T.; Zerbo, D.; Virgilio, C.; Sciacca, A.; et al. Infective complications in renal allograft recipients: Epidemiology and outcome. Transplant. Proc. 2008, 40, 1873–1876. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Pezeshgi, A.; Mahdiabadi, M.Z.; Sabzghabaei, F.; Hajishah, H.; Mahdavynia, S. Prevalence and risk factors of urinary tract infection in kidney recipients: A meta-analysis study. BMC Nephrol. 2023, 24, 284. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Julian, K. Urinary tract infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13507. [Google Scholar] [CrossRef]
- Wu, X.; Dong, Y.; Liu, Y.; Li, Y.; Sun, Y.; Wang, J.; Wang, S. The prevalence and predictive factors of urinary tract infection in patients undergoing renal transplantation: A meta-analysis. Am. J. Infect. Control 2016, 44, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Behzad, D.; Hakimeh, A.; Hossein, R.; Khaledi, A. A middle east systematic review and meta-analysis of bacterial urinary tract infection among renal transplant recipients; Causative microorganisms. Microb. Pathog. 2020, 148, 104458. [Google Scholar] [CrossRef]
- Nascimento, E.H.G.; Nascimento, E.; Lasmar, M.F.; Fabreti-Oliveira, R.A. Effects of Bacterial Urinary Tract Infection on Clinical Outcome and Survival of Kidney Transplant Patients. Transplant. Proc. 2022, 54, 1262–1269. [Google Scholar] [CrossRef]
- Britt, N.S.; Hagopian, J.C.; Brennan, D.C.; Pottebaum, A.A.; Santos, C.A.; Gharabagi, A.; Horwedel, T.A. Effects of recurrent urinary tract infections on graft and patient outcomes after kidney transplantation. Nephrol. Dial. Transplant. 2017, 32, 1758–1766. [Google Scholar] [CrossRef]
- Alqasim, A.; Abu Jaffal, A.; Alyousef, A.A. Prevalence of Multidrug Resistance and Extended-Spectrum β-Lactamase Carriage of Clinical Uropathogenic Escherichia coli Isolates in Riyadh, Saudi Arabia. Int. J. Microbiol. 2018, 2018, 3026851. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M. Antimicrobial resistance in Saudi Arabia. Saudi Med. J. 2016, 37, 935–940. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Sartor, A.L.; Balkhy, H.H.; Walsh, T.R.; Al Johani, S.M.; Aljindan, R.Y.; Alfaresi, M.; Ibrahim, E.; Al-Jardani, A.; Al-Abri, S.; et al. Molecular Characterization of Carbapenemase-Producing Escherichia coli and Klebsiella pneumoniae in the Countries of the Gulf Cooperation Council: Dominance of OXA-48 and NDM Producers. Antimicrob. Agents Chemother. 2014, 58, 3085–3090. [Google Scholar] [CrossRef]
- Aljeldah, M.M. Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef]
- Fiorentino, M.; Pesce, F.; Schena, A.; Simone, S.; Castellano, G.; Gesualdo, L. Updates on urinary tract infections in kidney transplantation. J. Nephrol. 2019, 32, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Velioglu, A.; Guneri, G.; Arikan, H.; Asicioglu, E.; Tigen, E.T.; Tanidir, Y.; Tinay, I.; Yegen, C.; Tuglular, S. Incidence and risk factors for urinary tract infections in the first year after renal transplantation. PLoS ONE 2021, 16, e0251036. [Google Scholar] [CrossRef]
- Alevizakos, M.; Nasioudis, D.; Mylonakis, E. Urinary tract infections caused by ESBL-producing Enterobacteriaceae in renal transplant recipients: A systematic review and meta-analysis. Transpl. Infect. Dis. 2017, 19, e12759. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, D.G.; Stifelman, M.D.; Birkhoff, J.; Richardson, K.A.; Cohen, D.; Nowygrod, R.; Benvenisty, A.I.; Hardy, M.A. Early catheter removal decreases incidence of urinary tract infections in renal transplant recipients. Transplant. Proc. 1998, 30, 4314–4316. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Cimen, S.; Hurton, S.; Molinari, M. Risks and Benefits of Early Catheter Removal After Renal Transplantation. Transplant. Proc. 2015, 47, 2855–2859. [Google Scholar] [CrossRef]
- Werneburg, G.T. Catheter-Associated Urinary Tract Infections: Current Challenges and Future Prospects. Res. Rep. Urol. 2022, 14, 109–133. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International Clinical Practice Guidelines for the Treatment of Acute Uncomplicated Cystitis and Pyelonephritis in Women: A 2010 Update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- Kranz, J.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.; Köves, B.; Schubert, S.; Pilatz, A.; Veeratterapillay, R.; E Wagenlehner, F.M.; et al. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur. Urol. 2024, 86, 27–41. [Google Scholar] [CrossRef]
- Barber, A.E.; Norton, J.P.; Spivak, A.M.; Mulvey, M.A. Urinary Tract Infections: Current and Emerging Management Strategies. Clin. Infect. Dis. 2013, 57, 719–724. [Google Scholar] [CrossRef]
- Pitout, J.D.D. Infections with Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. Drugs 2010, 70, 313–333. [Google Scholar] [CrossRef]
- El-Gamal, M.I.; Brahim, I.; Hisham, N.; Aladdin, R.; Mohammed, H.; Bahaaeldin, A. Recent updates of carbapenem antibiotics. Eur. J. Med. Chem. 2017, 131, 185–195. [Google Scholar] [CrossRef]
- Tan, X.; Pan, Q.; Mo, C.; Li, X.; Liang, X.; Li, Y.; Lan, Y.; Chen, L. Carbapenems vs alternative antibiotics for the treatment of complicated urinary tract infection. Medicine 2020, 99, e18769. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Therapy of Complicated Urinary. 2012. Available online: https://swab.nl/exec/file/download/84 (accessed on 1 October 2025).
- Alshehri, A.A.; Aldali, J.A.; Abdelhamid, M.A.; Alanazi, A.A.; Alhuraiz, R.B.; Alanazi, L.Z.; Alshmrani, M.A.; Alqahtani, A.M.; Alrshoud, M.I.; Alharbi, R.F. Implementation of Antimicrobial Stewardship Programs in Saudi Arabia: A Systematic Review. Microorganisms 2025, 13, 440. [Google Scholar] [CrossRef]
- Ababneh, M.A.; Nasser, S.A.; Rababa’h, A.M. A systematic review of Antimicrobial Stewardship Program implementation in Middle Eastern countries. Int. J. Infect. Dis. 2021, 105, 746–752. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Chen, H.-P.; Lin, C.-W.; Tang, J.-J.; Hsu, T.-Y.; Weng, Y.-C.; Lee, Y.-M.; Wang, W.-S.; Lo, S.-S. Implementation and outcomes of an antimicrobial stewardship program: Effectiveness of education. J. Chin. Med. Assoc. 2017, 80, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Vahlensieck, W.; Bauer, H.W.; Weidner, W.; Piechota, H.J.; Naber, K.G. Prevention of recurrent urinary tract infections. Minerva Urol. Nefrol. 2013, 65, 9–20. [Google Scholar] [PubMed]
- Dason, S.; Dason, J.T.; Kapoor, A. Guidelines for the diagnosis and management of recurrent urinary tract infection in women. Can. Urol. Assoc. J. 2011, 5, 316–322. [Google Scholar] [CrossRef]
- Al-Badr, A.; Al-Shaikh, G. Recurrent Urinary Tract Infections Management in Women: A Review. Sultan Qaboos Univ. Med. J. 2013, 13, 359–367. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9 (Suppl. 3), S1–S155. [Google Scholar] [CrossRef]
- Mehnert-Kay, S.A. Diagnosis and management of uncomplicated urinary tract infections. Am. Fam. Physician 2005, 72, 451–456. [Google Scholar] [PubMed]
- Bono, M.J.; Leslie, S.W.; Reygaert, W.C. Uncomplicated Urinary Tract Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hooton, T.M. Uncomplicated Urinary Tract Infection. N. Engl. J. Med. 2012, 366, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Halskov, A.C.L.; Dagnæs-Hansen, J.; Stroomberg, H.V.; Sørensen, S.S.; Røder, A. Incidence of and Risk Factors for Recurrent Urinary Tract Infections in Renal Transplant Recipients. Eur. Urol. Open Sci. 2023, 52, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Al Tamimi, A.R.; Alotaibi, W.S.; Aljohani, R.M.; Aldharman, S.S.; Alharbi, N.M.; Khair, H.S. The Impact of Urinary Tract Infections in Kidney Transplant Recipients: A Six-Year Single-Center Experience. Cureus 2023, 15, e44458. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).