The Domination of Penicillin G Degradation in Natural Surface Water: Effect of Calcium Ion and Biological Dissolved Organic Matter
Abstract
1. Introduction
2. Results and Discussion
2.1. PG Hydrolysis in Different Surface Waters
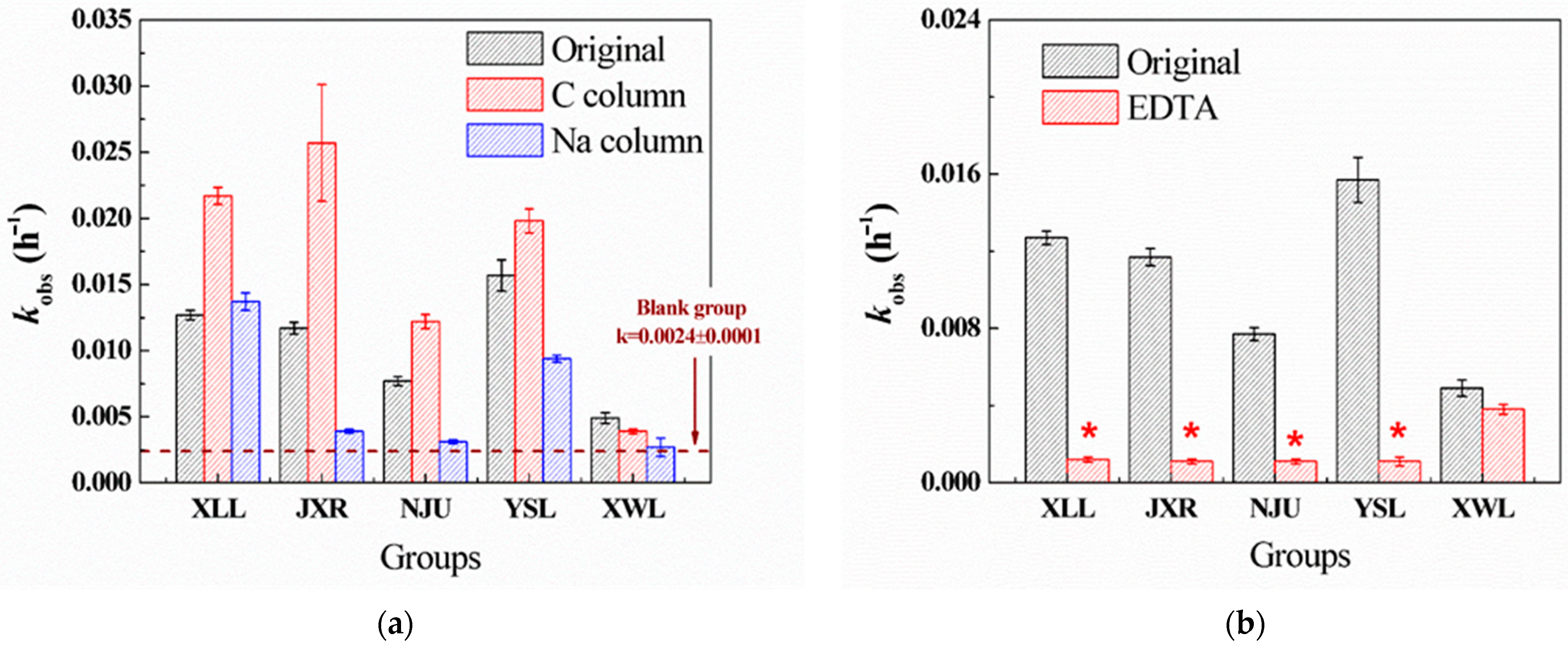
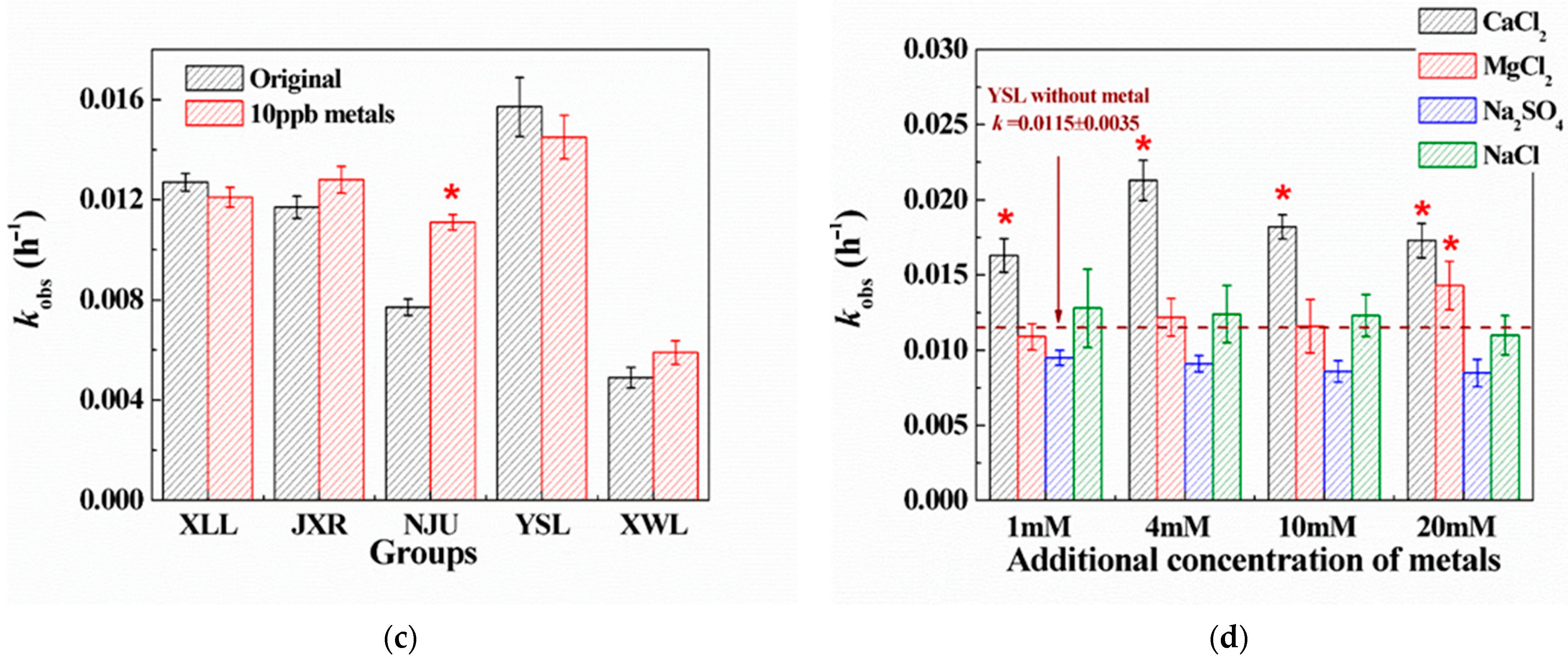
2.2. Effects of Aquatic Parameters on PG Hydrolysis
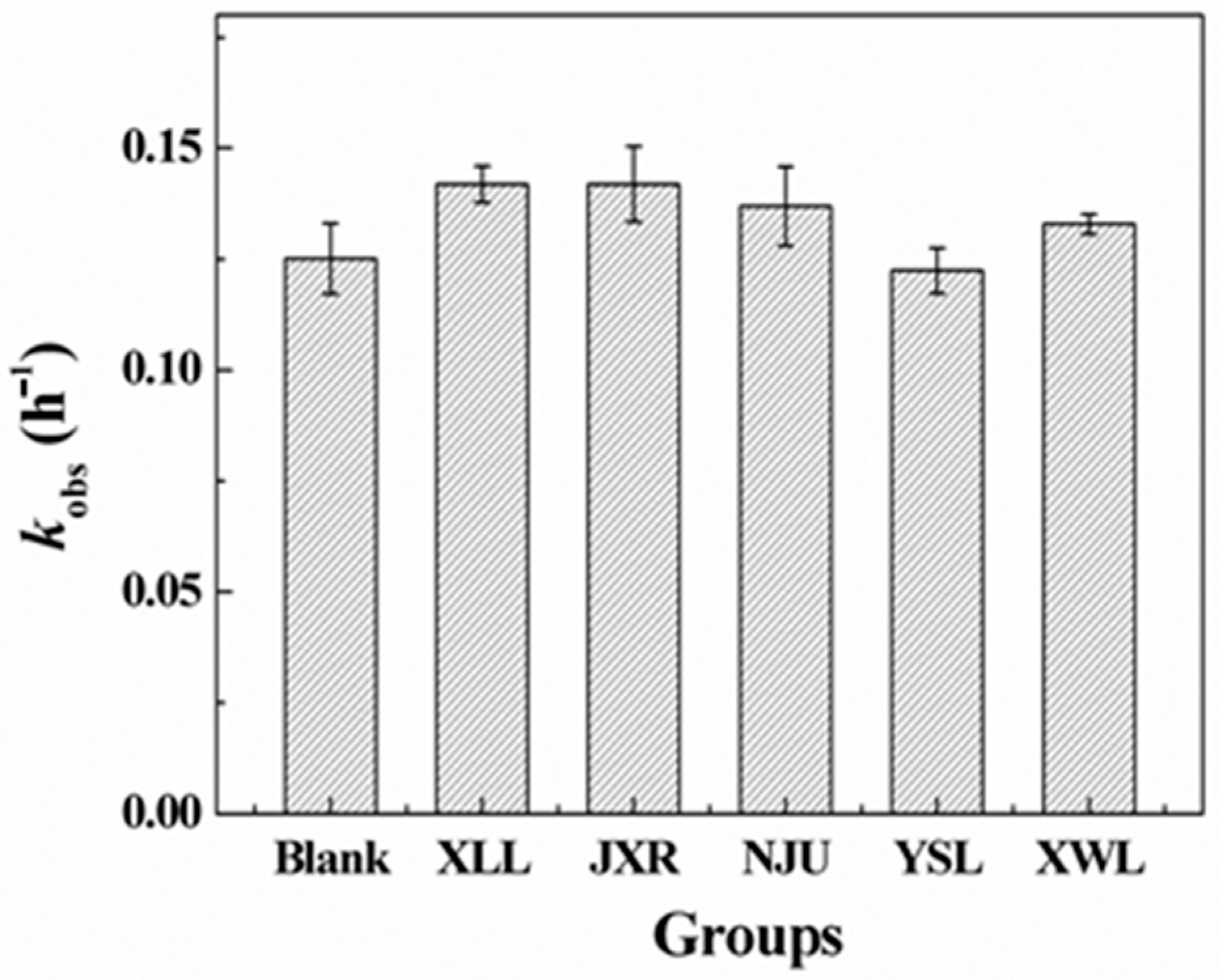
2.3. PG Photolysis in Different Surface Waters
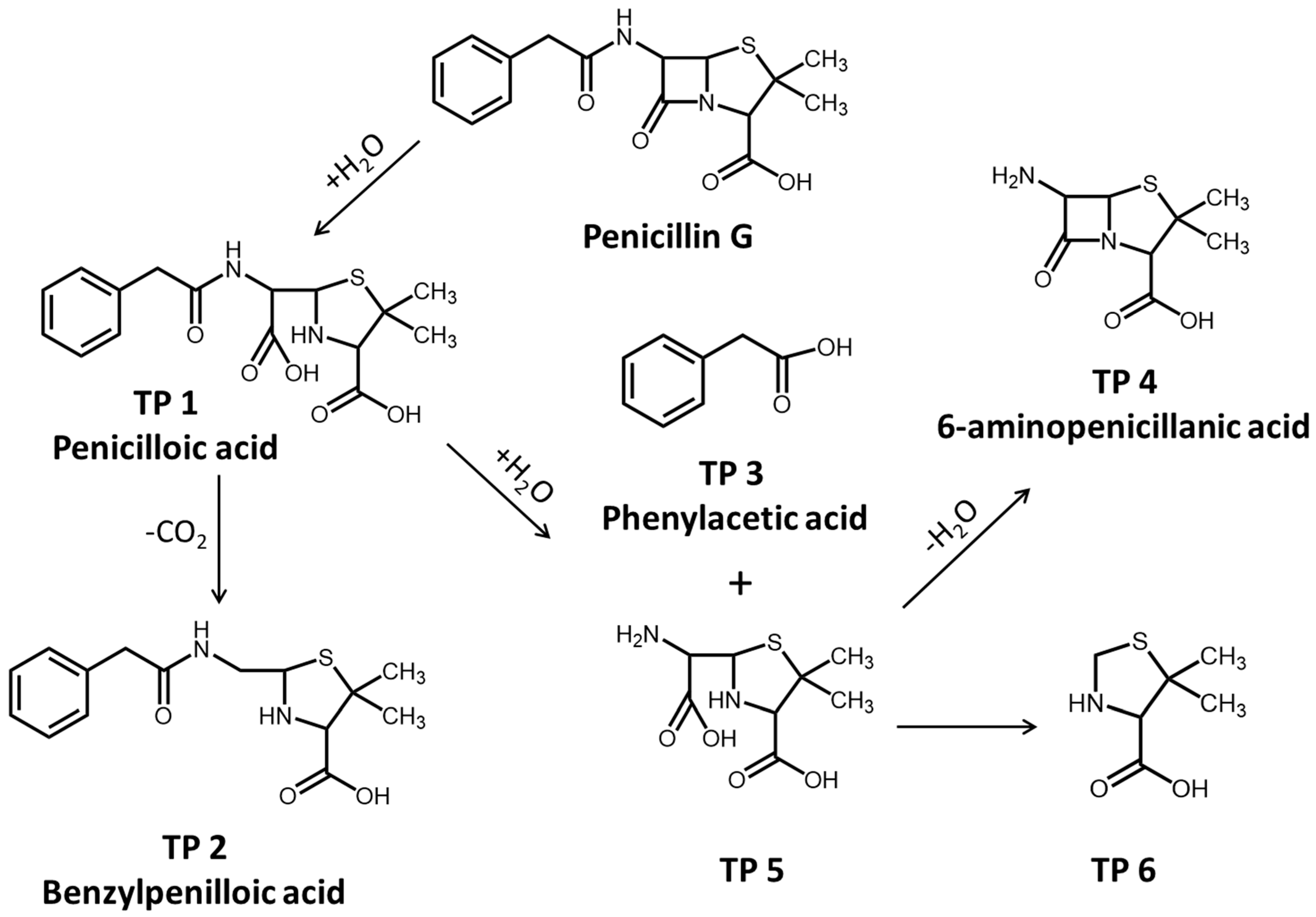
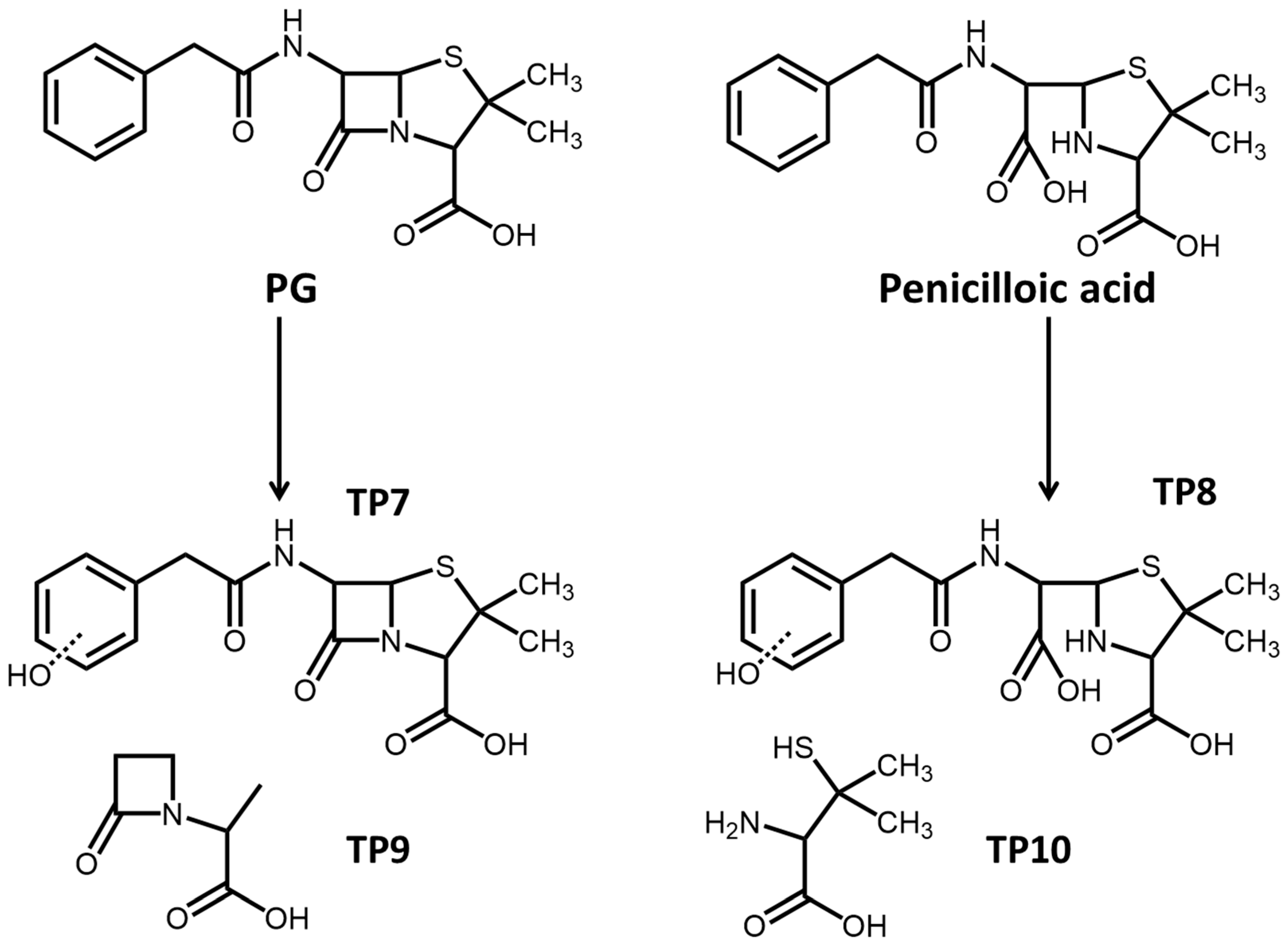
2.4. Product Analysis
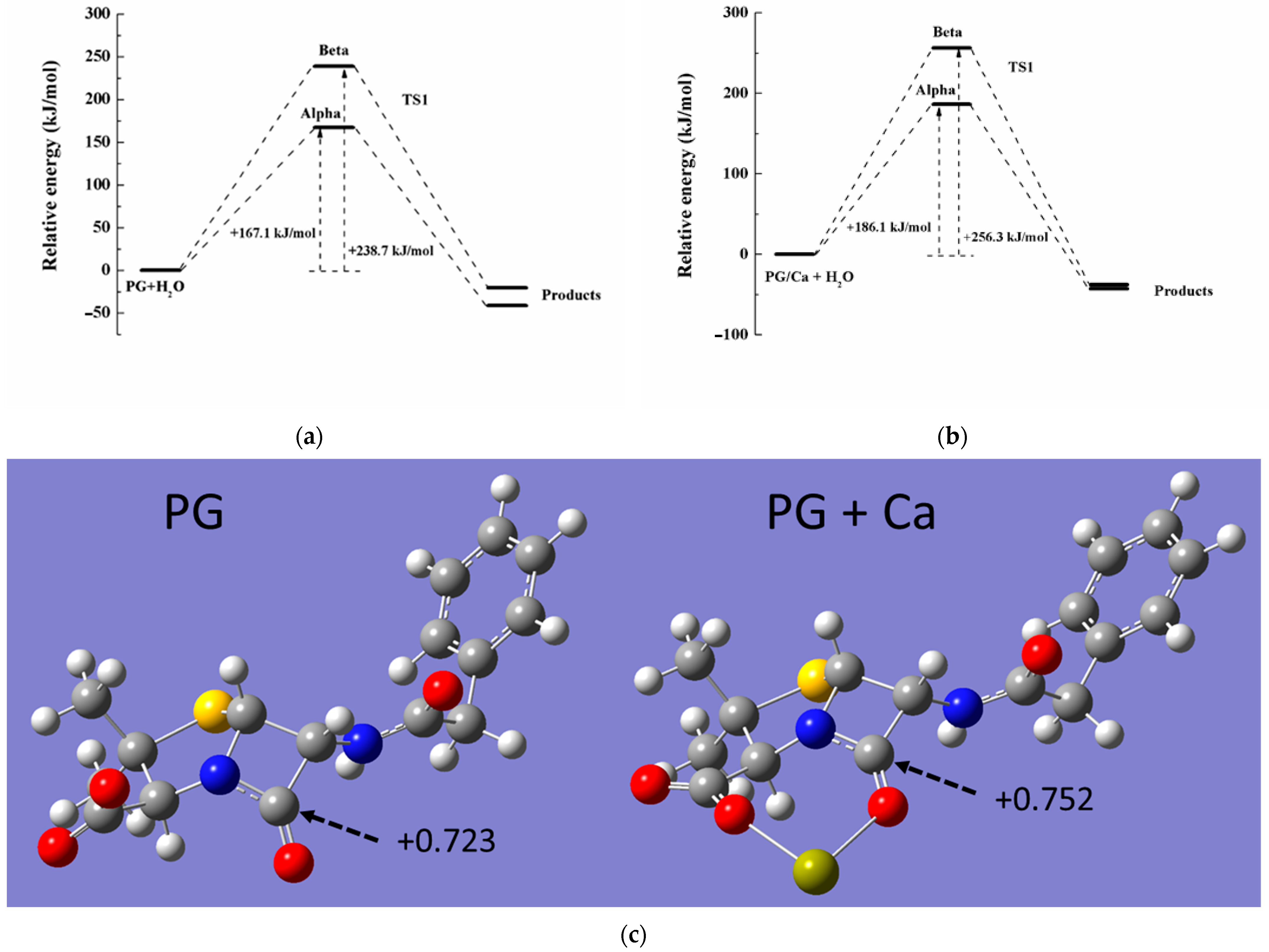
2.5. DFT Calculation
3. Materials and Methods
3.1. Chemicals
3.2. Sampling of Natural Water
3.3. Analysis Method
3.4. Hydrolysis and Photolysis of PG in Surface Water
3.5. Density Functional Theory (DFT) Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Yang, M.; Hu, J.Y.; Zhang, Y.; Chang, H.; Jin, F. Determination of penicillin G and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Res. 2008, 42, 307–317. [Google Scholar] [CrossRef]
- Raynor, B.D. Penicillin and ampicillin. Prim. Care Update OB/GYNS 1997, 4, 147–152. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef]
- Chen, J.; Sun, P.; Zhou, X.; Zhang, Y.; Huang, C.H. Cu(II)–catalyzed transformation of benzylpenicillin revisited: The overlooked oxidation. Environ. Sci. Technol. 2015, 49, 4218–4225. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhong, Y.; Lim, T.T. Comparison of amoxicillin photodegradation in the UV/H2O2 and UV/persulfate systems: Reaction kinetics, degradation pathways, and antibacterial activity. Chem. Eng. J. 2019, 372, 420–428. [Google Scholar] [CrossRef]
- Kümmerer, K. Antibiotics in the aquatic environment—A review—Part II. Chemosphere 2009, 75, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, Y.; Qian, Y.; Huang, T. Fe(III)-promoted transformation of β-lactam antibiotics: Hydrolysis vs oxidation. J. Hazard. Mater. 2017, 335, 117–124. [Google Scholar] [CrossRef]
- Walsh, C. Molecular mechanisms that confer antibacterial drug resistance. Nature 2000, 406, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Sui, Q.; Wang, B.; Zhao, W.T.; Huang, J.; Yu, G.; Deng, S.B.; Qiu, Z.F.; Lu, S.G. Identification of priority pharmaceuticals in the water environment of China. Chemosphere 2012, 89, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.W.; Minh, T.B.; Murphy, M.B.; Lam James, C.W.; So, M.K.; Martin, M.; Lam Paul, K.S.; Richardson, B.J. Distribution, fate and risk assessment of antibiotics in sewage treatment plants in Hong Kong, South China. Environ. Int. 2012, 42, 1–9. [Google Scholar] [CrossRef]
- Hu, Z.; Periyannan, G.; Bennett, B.; Crowder, M.W. Role of the Zn1 and Zn2 sites in Metallo-β-lactamase L1. J. Am. Chem. Soc. 2008, 130, 14207–14216. [Google Scholar] [CrossRef] [PubMed]
- Kaminskaia, N.V.; Spingler, B.; Lippard, S.J. Hydrolysis of β-lactam antibiotics catalyzed by dinuclear zinc(ii) complexes: Functional mimics of metallo-β-lactamases. J. Am. Chem. Soc. 2000, 122, 6411–6422. [Google Scholar] [CrossRef]
- Bahr, G.; González, L.J.; Vila, A.J. Metallo-β-lactamases in the age of multidrug resistance: From structure and mechanism to evolution, dissemination, and inhibitor design. Chem. Rev. 2021, 121, 7957–8094. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J. pH and temperature effects on the hydrolysis of three β-lactam antibiotics: Ampicillin, cefalotin and cefoxitin. Sci. Total Environ. 2014, 466–467, 547–555. [Google Scholar] [CrossRef]
- Mabey, W.; Mill, T. Critical review of hydrolysis of organic compounds in water under environmental conditions. J. Phys. Chem. Ref. Data 1978, 7, 383–415. [Google Scholar] [CrossRef]
- Guo, Y.; Tsang, D.C.W.; Zhang, X.; Yang, X. Cu(II)-catalyzed degradation of ampicillin: Effect of pH and dissolved oxygen. Environ. Sci. Pollut. Res. 2018, 25, 4279–4288. [Google Scholar] [CrossRef]
- Gensmantel, N.P.; Proctor, P.; Page, M.I. Metal-ion catalysed hydrolysis of some β-lactam antibiotics. J. Chem. Soc. Perkin Trans. 1980, 2, 1725–1732. [Google Scholar] [CrossRef]
- Sheng, F.; Ling, J.Y.; Wang, C.; Jin, X.; Gu, X.Y.; Li, H.; Zhao, J.T.; Wang, Y.J.; Gu, C. Rapid Hydrolysis of penicillin antibiotics mediated by adsorbed zinc on goethite surfaces. Environ. Sci. Technol. 2019, 53, 10705–10713. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, D.D.; Ling, J.Y.; Wang, C.; Liu, C.; Gu, C. Hydrolysis of chloramphenicol catalyzed by clay minerals under nonaqueous conditions. Environ. Sci. Technol. 2019, 53, 10645–10653. [Google Scholar] [CrossRef]
- Hirte, K.; Seiwert, B.; Schüürmann, G.; Reemtsma, T. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water Res. 2016, 88, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, P.; Zhang, Y.; Huang, C.H. Multiple roles of Cu(II) in catalyzing hydrolysis and oxidation of β-lactam antibiotics. Environ. Sci. Technol. 2016, 50, 12156–12165. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Fang, C.; Qian, Y.; Gu, H.; Chen, J. Insight into Mn(II)-mediated transformation of β-lactam antibiotics: The overlooked hydrolysis. Chem. Eng. J. 2017, 321, 662–668. [Google Scholar] [CrossRef]
- Diaz, N.; Sordo, T.L.; Suarez, D.; Mendez, R.; Martin-Villacorta, J. Zn2+ catalysed hydrolysis of β-lactams: Experimental and theoretical studies on the influence of the β-lactam structure. New J. Chem. 2004, 28, 15–25. [Google Scholar] [CrossRef]
- Morris, J.J.; Page, M.I. Intra- and inter-molecular catalysis in the aminolysis of benzylpenicillin. J. Chem. Soc. Perkin Trans. 1980, 2, 212–219. [Google Scholar] [CrossRef]
- Llinás, A.; Donoso, J.; Vilanova, B.; Frau, J.; Muñoz, F.; Page, M.I. Thiol-catalysed hydrolysis of benzylpenicillin. J. Chem. Soc. Perkin Trans. 2000, 2, 1521–1525. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, L.; Ji, R. Biotic and abiotic degradation of four cephalosporin antibiotics in a lake surface water and sediment. Chemosphere 2010, 80, 1399–1405. [Google Scholar] [CrossRef]
- Xu, H.; Cooper, W.J.; Jung, J.; Song, W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011, 45, 632–638. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Ciniglia, C. Antibiotics in the environment: Occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef]
- Timm, A.; Borowska, E.; Majewsky, M.; Merel, S.; Zwiener, C.; Baase, S.; Horn, H. Photolysis of four β-lactam antibiotics under simulated environmental conditions: Degradation, transformation products and antibacterial activity. Sci. Total Environ. 2019, 651, 1605–1612. [Google Scholar] [CrossRef]
- Alekseev, V.G. Metal complexes of penicillins and cephalosporins (Review). Pharm. Chem. J. 2012, 45, 679–697. [Google Scholar] [CrossRef]
- Wang, H.; Yao, H.; Sun, P.; Li, D.; Huang, C.-H. Transformation of tetracycline antibiotics and Fe(II) and Fe(III) species induced by their complexation. Environ. Sci. Technol. 2016, 50, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, H.; Sun, P.Z.; Pei, J.; Li, D.S.; Huang, Q.H. Oxidation of tetracycline antibiotics induced by Fe(III) ions without light irradiation. Chemosphere 2015, 119, 1255–1261. [Google Scholar] [CrossRef]
- Navarro, P.G.; Blazquez, I.H.; Osso, B.Q.; Parras, P.J.; Puentedura, M.I.M.; Garcia, A.M. Penicillin degradation catalysed by Zn(II) ions in methanol. Int. J. Biol. Macromol. 2003, 33, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Her, N.; Amy, G.; McKnight, D.; Sohn, J.; Yoon, Y. Characterization of DOM as a function of MW by fluorescence EEM and HPLC-SEC using UVA, DOC, and fluorescence detection. Water Res. 2003, 37, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
| Location | Fitting Equation | R2 | kobs (h−1) | T1/2 (h) |
|---|---|---|---|---|
| XXL | y = −0.0127x + 0.0444 | 0.99 | 0.0127 | 55 |
| JXR | y = −0.0117x + 0.0669 | 0.99 | 0.0117 | 59 |
| NJU | y = −0.0077x + 0.0315 | 0.99 | 0.0077 | 90 |
| YSL | y = −0.0157x + 0.0746 | 0.98 | 0.0157 | 44 |
| XWL | y = −0.0049x + 0.0344 | 0.98 | 0.0049 | 141 |
| Blank | y = −0.0024x − 0.0151 | 0.99 | 0.0024 | 289 |
| Parameter | Correlation Coefficient | Significance (p) |
|---|---|---|
| pH | −0.483 | 0.410 |
| Conductivity | 0.906 | 0.034 * |
| Cl− | −0.304 | 0.619 |
| NO3− | −0.277 | 0.651 |
| SO42− | 0.990 | 0.001 ** |
| K | 0.183 | 0.768 |
| Ca | 0.899 | 0.038 * |
| Na | −0.139 | 0.823 |
| Mg | 0.919 | 0.027 * |
| Ba | 0.540 | 0.347 |
| Total P | 0.113 | 0.857 |
| Total S | 0.958 | 0.010 * |
| Al | −0.158 | 0.800 |
| Mn | −0.937 | 0.019 * |
| Co | 0.221 | 0.721 |
| Ni | 0.735 | 0.157 |
| Cu | −0.147 | 0.814 |
| Zn | −0.228 | 0.713 |
| Fe | −0.380 | 0.528 |
| TOC | 0.553 | 0.334 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, F.; Ling, J.; Mi, N.; Wan, J.; Yang, L.; Li, M.; Wang, C.; Shi, J. The Domination of Penicillin G Degradation in Natural Surface Water: Effect of Calcium Ion and Biological Dissolved Organic Matter. Antibiotics 2025, 14, 1144. https://doi.org/10.3390/antibiotics14111144
Sheng F, Ling J, Mi N, Wan J, Yang L, Li M, Wang C, Shi J. The Domination of Penicillin G Degradation in Natural Surface Water: Effect of Calcium Ion and Biological Dissolved Organic Matter. Antibiotics. 2025; 14(11):1144. https://doi.org/10.3390/antibiotics14111144
Chicago/Turabian StyleSheng, Feng, Jingyi Ling, Na Mi, Jixing Wan, Lu Yang, Ming Li, Chao Wang, and Jiaqi Shi. 2025. "The Domination of Penicillin G Degradation in Natural Surface Water: Effect of Calcium Ion and Biological Dissolved Organic Matter" Antibiotics 14, no. 11: 1144. https://doi.org/10.3390/antibiotics14111144
APA StyleSheng, F., Ling, J., Mi, N., Wan, J., Yang, L., Li, M., Wang, C., & Shi, J. (2025). The Domination of Penicillin G Degradation in Natural Surface Water: Effect of Calcium Ion and Biological Dissolved Organic Matter. Antibiotics, 14(11), 1144. https://doi.org/10.3390/antibiotics14111144






