Phylogenomics and Antimicrobial Resistance of Clinical Bacteroides Isolates from a Tertiary Hospital in Southern Thailand
Abstract
1. Introduction
2. Results
2.1. Taxonomic Identification and Antimicrobial Susceptibility in Bacteroides spp.
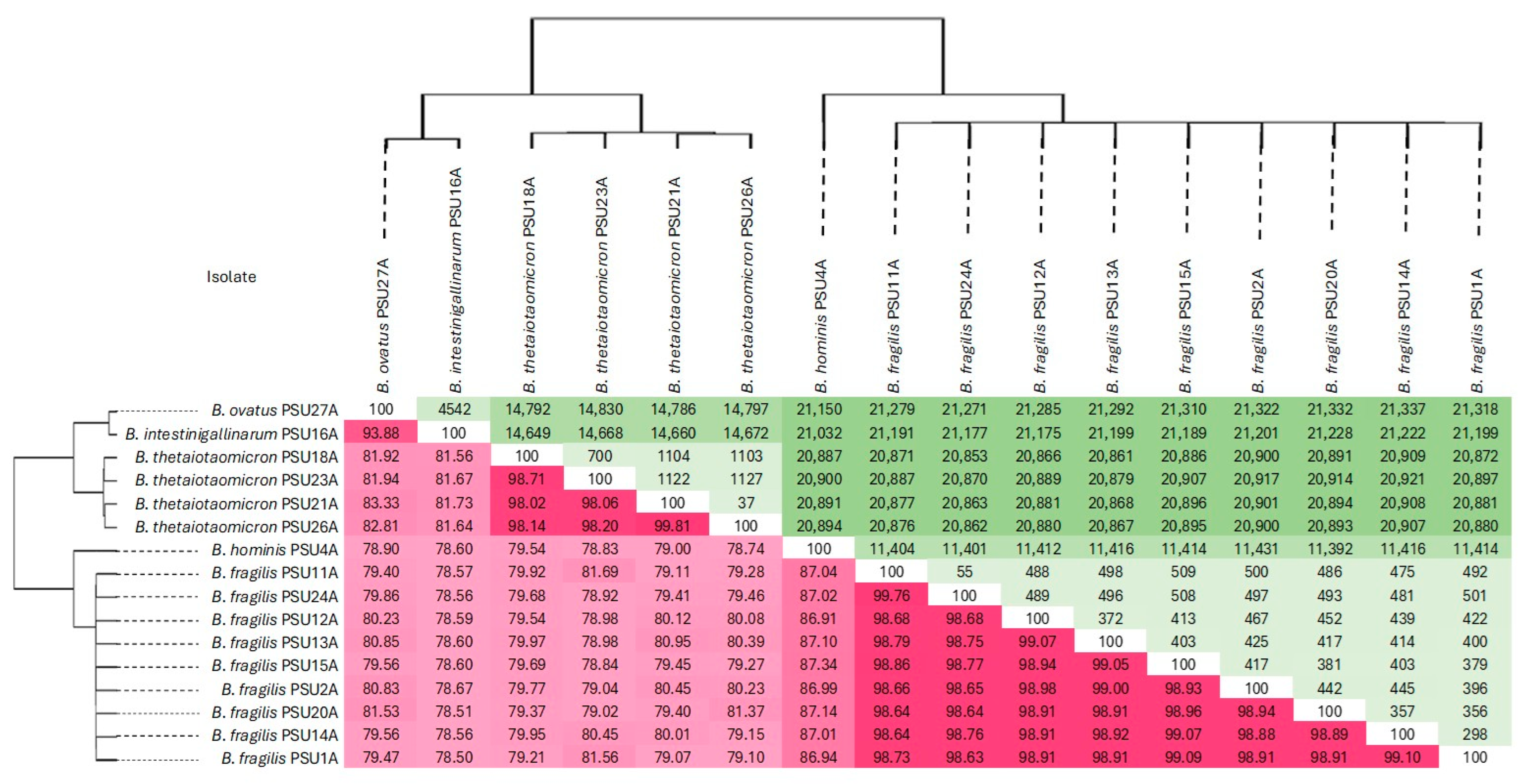
2.2. ANI and SNP-Based Genomic Relatedness of Bacteroides Isolates
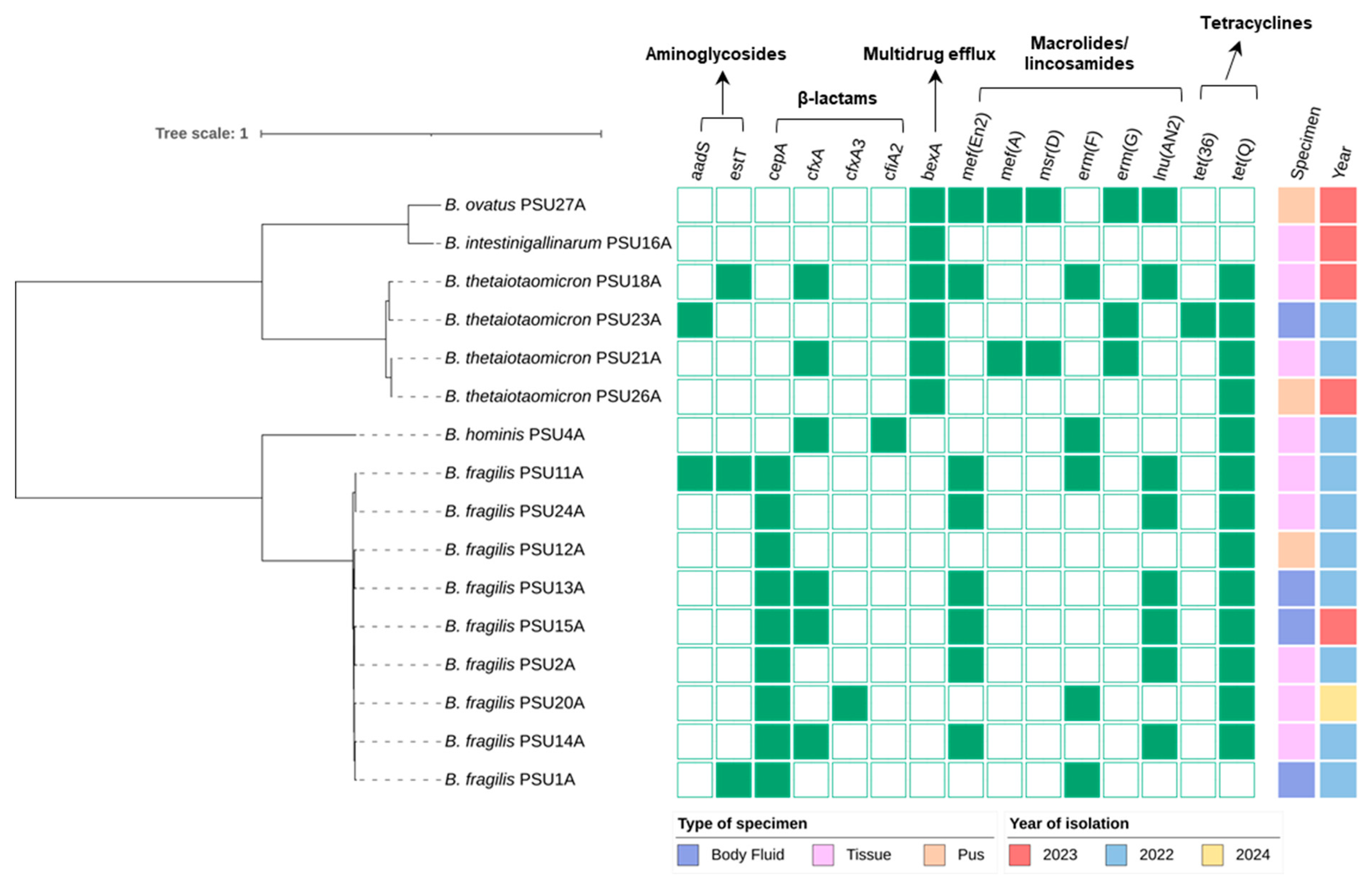
2.3. Antimicrobial Resistance Genes in Bacteroides spp.
2.4. Plasmid and Mobile Genetic Element Detection in Bacteroides spp.
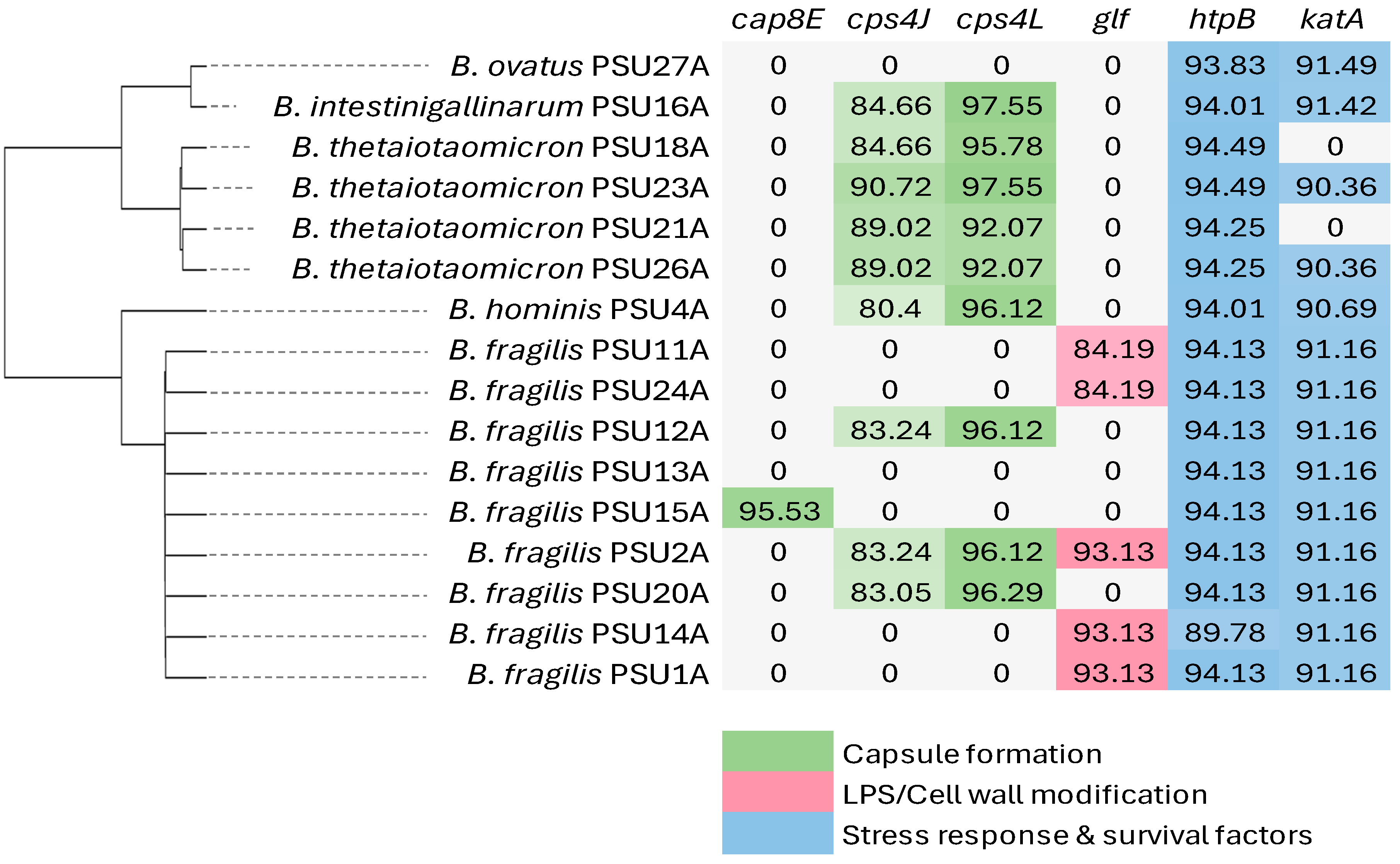
2.5. Virulence Genes in Bacteroides spp.
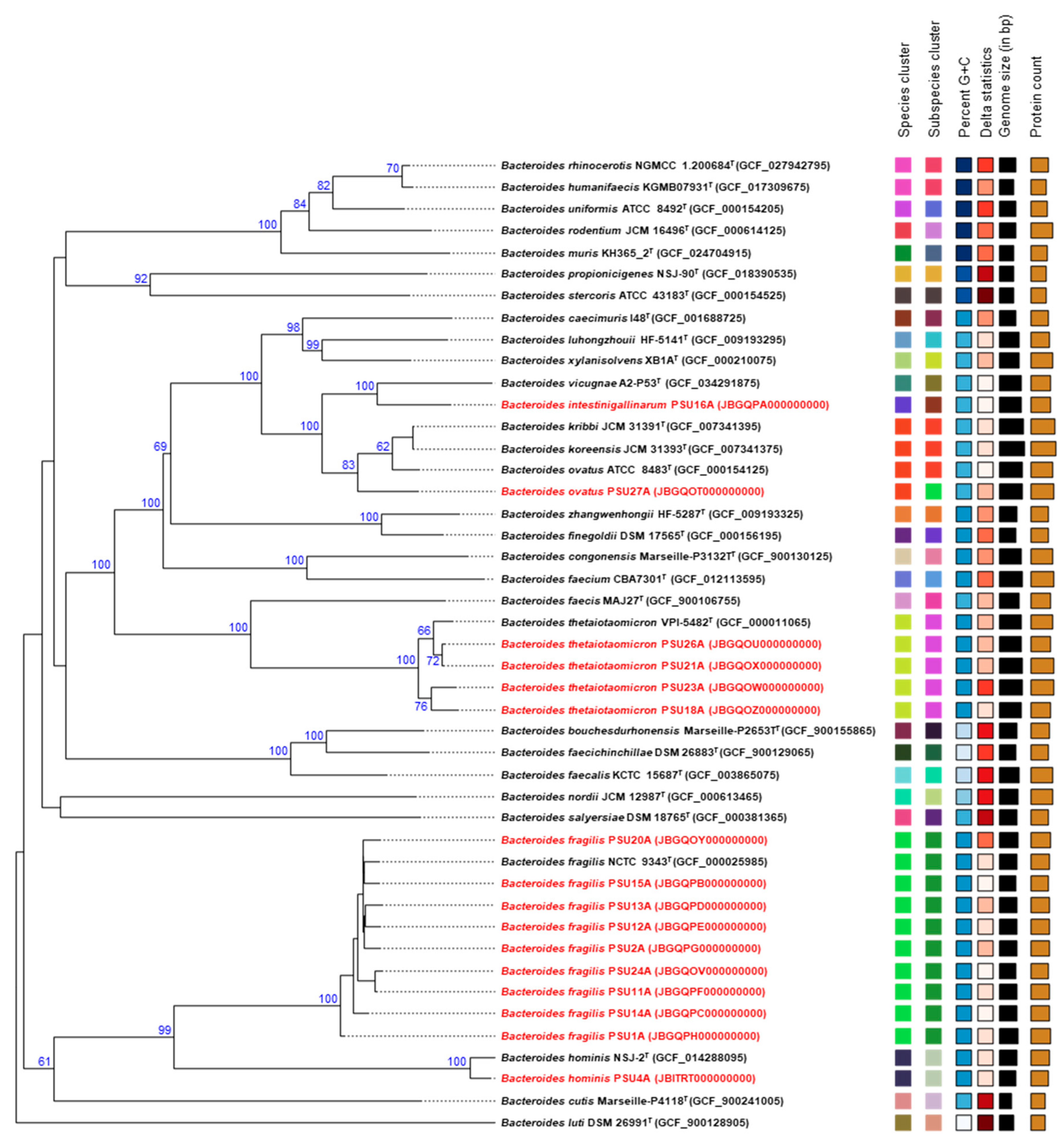
2.6. Comparative Phylogenetic Analysis of Bacteroides Isolates
3. Discussions
4. Materials and Methods
4.1. Isolates Collection, Antimicrobial Susceptibility Testing, and DNA Extraction
4.2. Bacteroides Genome Sequencing
4.3. Genomic Characterization Using Bioinformatics
4.4. Plasmid and Mobile Genetic Element Detection
4.5. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef]
- Elsaghir, H.; Reddivari, A.K.R. Bacteroides fragilis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Vu, H.; Hayashi, M.; Nguyen, T.N.; Khong, D.T.; Tran, H.T.; Yamamoto, Y.; Tanaka, K. Comparison of phenotypic and genotypic patterns of antimicrobial-resistant Bacteroides fragilis group isolated from healthy individuals in Vietnam and Japan. Infect. Drug Resist. 2021, 14, 5313–5323. [Google Scholar] [CrossRef] [PubMed]
- Shayista, H.; Prasad, M.N.N.; Raj, S.N.; Prasad, A.; Lakshmi, S.; Ranjini, H.K.; Manju, K.; Ravikumara; Chouhan, R.S.; Khohlova, O.Y.; et al. Complexity of antibiotic resistance and its impact on gut microbiota dynamics. Eng. Microbiol. 2025, 5, 100187. [Google Scholar] [CrossRef]
- Bhat, B.A.; Mir, R.A.; Qadri, H.; Dhiman, R.; Almilaibary, A.; Alkhanani, M.; Mir, M.A. Integrons in the development of antimicrobial resistance: Critical review and perspectives. Front. Microbiol. 2023, 14, 1231938. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Valdezate, S.; Cobo, F.; Monzón, S.; Medina-Pascual, M.J.; Zaballos, Á.; Cuesta, I.; Pino-Rosa, S.; Villalón, P. Genomic background and phylogeny of cfiA-positive Bacteroides fragilis strains resistant to meropenem-EDTA. Antibiotics 2021, 10, 304. [Google Scholar] [CrossRef]
- Pricop, G.R.; Gheorghe, I.; Pircalabioru, G.G.; Cristea, V.; Popa, M.; Marutescu, L.; Chifiriuc, M.C.; Mihaescu, G.; Bezirtzoglou, E. Resistance and virulence features of Bacteroides spp. isolated from abdominal infections in Romanian patients. Pathogens 2020, 9, 940. [Google Scholar] [CrossRef]
- Kierzkowska, M.; Majewska, A.; Karłowicz, K.; Pituch, H. Phenotypic and genotypic identification of carbapenem resistance in Bacteroides fragilis clinical strains. Med. Microbiol. Immunol. 2023, 212, 231–240. [Google Scholar] [CrossRef]
- Jasemi, S.; Emaneini, M.; Ahmadinejad, Z.; Fazeli, M.S.; Sechi, L.A.; Sadeghpour Heravi, F.; Feizabadi, M.M. Antibiotic resistance pattern of Bacteroides fragilis isolated from clinical and colorectal specimens. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.-F.; Xie, X.-L.; Xiao, M.; Yang, Y.; Zhang, G.; Zhang, J.-j.; Duan, S.-m.; Zhang, Q.; Zhang, P.; et al. Evaluation of VITEK MS, Clin-ToF-II MS, Autof MS 1000 and VITEK 2 ANC card for identification of Bacteroides fragilis group isolates and antimicrobial susceptibilities of these isolates in a Chinese university hospital. J. Microbiol. Immunol. Infect. 2019, 52, 456–464. [Google Scholar] [CrossRef]
- Narimani, T.; Douraghi, M.; Owlia, P.; Rastegar, A.; Esghaei, M.; Nasr, B.; Talebi, M. Heterogeneity in resistant fecal Bacteroides fragilis group collected from healthy people. Microb. Pathog. 2016, 95, 1–6. [Google Scholar] [CrossRef]
- Cao, H.; Liu, M.C.-J.; Tong, M.-K.; Jiang, S.; Chow, K.-H.; To, K.K.-W.; Tse, C.W.-S.; Ho, P.-L. Comprehensive investigation of antibiotic resistance gene content in cfiA-harboring Bacteroides fragilis isolates of human and animal origins by whole genome sequencing. Int. J. Med. Microbiol. 2022, 312, 151559. [Google Scholar] [CrossRef]
- Reissier, S.; Penven, M.; Guérin, F.; Cattoir, V. Recent trends in antimicrobial resistance among anaerobic clinical isolates. Microorganisms 2023, 11, 1474. [Google Scholar] [CrossRef]
- Roberts, M.C. Environmental macrolide-lincosamide-streptogramin and tetracycline resistant bacteria. Front. Microbiol. 2011, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.T.; Hryckowian, A.J.; Merrill, B.D.; Fuentes, J.J.; Gardner, J.O.; Glowacki, R.W.P.; Singh, S.; Crawford, R.D.; Snitkin, E.S.; Sonnenburg, J.L.; et al. Phase-variable capsular polysaccharides and lipoproteins modify bacteriophage susceptibility in Bacteroides thetaiotaomicron. Nat. Microbiol. 2020, 5, 1170–1181. [Google Scholar] [CrossRef]
- Yang, H.; Gan, Y.; Jiang, S.; Zhu, X.; Xia, Y.; Gong, D.; Xie, X.; Gong, Y.; Zhang, Y.; Lei, Q.; et al. Genomic alterations in Bacteroides fragilis favor adaptation in colorectal cancer microenvironment. BMC Genom. 2025, 26, 269. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M.J.; Tzianabos, A.O.; Mallory, B.C.; Carey, V.J.; Kasper, D.L.; Comstock, L.E. Polysaccharide biosynthesis locus required for virulence of Bacteroides fragilis. Infect. Immun. 2001, 69, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.S.; O’Connor, S.P.; Holloway, B.P.; Plikaytis, B.B.; Carlone, G.M.; Mayer, L.W. Nucleotide sequence of htpB, the Legionella pneumophila gene encoding the 58-kilodalton (kDa) common antigen, formerly designated the 60-kDa common antigen. Infect. Immun. 1990, 58, 3154–3157. [Google Scholar] [CrossRef]
- Sevilla, E.; Bes, M.T.; Peleato, M.L.; Fillat, M.F. Fur-like proteins: Beyond the ferric uptake regulator (Fur) paralog. Arch. Biochem. Biophys. 2021, 701, 108770. [Google Scholar] [CrossRef]
- Busch, A.; Homeier-Bachmann, T.; Abdel-Glil, M.Y.; Hackbart, A.; Hotzel, H.; Tomaso, H. Using affinity propagation clustering for identifying bacterial clades and subclades with whole-genome sequences of Francisella tularensis. PLoS Neglected Trop. Dis. 2020, 14, e0008018. [Google Scholar] [CrossRef]
- Muto, Y.; Tanaka, K. Comparative evolutionary genomics reveals genetic diversity and differentiation in Bacteroides fragilis. Genes 2024, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.; Nakayama-Imaohji, H.; Hashimoto, M.; Tada, A.; Yamasaki, H.; Nagao, T.; Kuwahara, T. The human gut microbe Bacteroides thetaiotaomicron suppresses toxin release from Clostridium difficile by inhibiting autolysis. Antibiotics 2021, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Henz, S.R.; Huson, D.H.; Auch, A.F.; Nieselt-Struwe, K.; Schuster, S.C. Whole-genome prokaryotic phylogeny. Bioinformatics 2005, 21, 2329–2335. [Google Scholar] [CrossRef]
- Wilkins, T.D.; Thiel, T. Modified broth-disk method for testing the antibiotic susceptibility of anaerobic bacteria. Antimicrob. Agents Chemother. 1973, 3, 350–356. [Google Scholar] [CrossRef]
- Petit Robert, A.; Read Timothy, D. Bactopia: A flexible pipeline for complete analysis of bacterial genomes. mSystems 2020, 5, e00190-20. [Google Scholar] [CrossRef]
- Andrews, S. Fastqc: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Cambridge, UK, 2010. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Seemann, T. Shovill, version 1.1.0; Zenodo: Geneva, Switzerland, 2016. [Google Scholar]
- Feldgarden, M.; Brover, V.; Gonzalez-Escalona, N.; Frye, J.G.; Haendiges, J.; Haft, D.H.; Hoffmann, M.; Pettengill, J.B.; Prasad, A.B.; Tillman, G.E.; et al. AMRFinderPlus and the Reference Gene Catalog facilitate examination of the genomic links among antimicrobial resistance, stress response, and virulence. Sci. Rep. 2021, 11, 12728. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Seemann, T. Snp-Dists: Pairwise SNP Distance Matrix from a FASTA Alignment; GitHub: San Francisco, CA, USA, 2018. [Google Scholar]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. Gtdb-tk: A toolkit to classify genomes with the genome taxonomy database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Biomatters. Geneious Prime, version 2023.1.2; Biomatters Ltd.: Auckland, New Zealand, 2023. [Google Scholar]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, D.; Liu, B.; Yang, J.; Jin, Q. VFDB 2016: Hierarchical and refined dataset for big data analysis—10 years on. Nucleic Acids Res. 2016, 44, D694–D697. [Google Scholar] [CrossRef]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. Fasttree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
 | |||||||
|---|---|---|---|---|---|---|---|
| Isolates | AMP | CH | PEN | CHL | ERY | TET | Resistance Category |
| B. fragilis PSU1A | R | R | R | S | R | R | MDR |
| B. fragilis PSU2A | R | R | R | R | R | R | MDR |
| B. hominis PSU4A | R | R | R | S | R | R | MDR |
| B. fragilis PSU11A | R | R | R | R | R | R | MDR |
| B. fragilis PSU12A | R | R | R | S | S | R | Non-MDR |
| B. fragilis PSU13A | R | R | R | S | R | R | MDR |
| B. fragilis PSU14A | R | R | R | S | R | R | MDR |
| B. fragilis PSU15A | R | R | R | R | R | R | MDR |
| B. intestinigallinarum PSU16A | R | R | R | R | R | S | MDR |
| B. thetaiotaomicron PSU18A | R | R | R | R | R | R | MDR |
| B. fragilis PSU20A | R | R | R | R | R | R | MDR |
| B. thetaiotaomicron PSU21A | R | R | R | S | R | R | MDR |
| B. thetaiotaomicron PSU23A | R | R | R | S | R | R | MDR |
| B. fragilis PSU24A | R | R | R | S | R | R | MDR |
| B. thetaiotaomicron PSU26A | R | R | R | S | S | R | Non-MDR |
| B. ovatus PSU27A | R | R | R | S | R | R | MDR |
| Resistance (%) | 100 | 100 | 100 | 37.5 | 87.5 | 93.75 | |
| Isolate | Name | Type | ARGs | Length (bp) |
|---|---|---|---|---|
| B. fragilis PSU11A | repUS2 | Plasmid | - | 2751 |
| B. ovatus PSU27A | repUS2 | Plasmid | - | 2752 |
| ISBaov1 | Insertion sequence | - | 1594 | |
| ISOdsp1 | Insertion sequence | - | 1098 | |
| cn_11070_ISBaov1 | Composite transposon | msr(D), erm(G), mef(A) | 11,070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yingkajorn, M.; Yaikhan, T.; Duangsi-Ngoen, W.; Klaysubun, C.; Dechathai, T.; Chusri, S.; Singkhamanan, K.; Pomwised, R.; Wonglapsuwan, M.; Surachat, K. Phylogenomics and Antimicrobial Resistance of Clinical Bacteroides Isolates from a Tertiary Hospital in Southern Thailand. Antibiotics 2025, 14, 1143. https://doi.org/10.3390/antibiotics14111143
Yingkajorn M, Yaikhan T, Duangsi-Ngoen W, Klaysubun C, Dechathai T, Chusri S, Singkhamanan K, Pomwised R, Wonglapsuwan M, Surachat K. Phylogenomics and Antimicrobial Resistance of Clinical Bacteroides Isolates from a Tertiary Hospital in Southern Thailand. Antibiotics. 2025; 14(11):1143. https://doi.org/10.3390/antibiotics14111143
Chicago/Turabian StyleYingkajorn, Mingkwan, Thunchanok Yaikhan, Worawut Duangsi-Ngoen, Chollachai Klaysubun, Thitaporn Dechathai, Sarunyou Chusri, Kamonnut Singkhamanan, Rattanaruji Pomwised, Monwadee Wonglapsuwan, and Komwit Surachat. 2025. "Phylogenomics and Antimicrobial Resistance of Clinical Bacteroides Isolates from a Tertiary Hospital in Southern Thailand" Antibiotics 14, no. 11: 1143. https://doi.org/10.3390/antibiotics14111143
APA StyleYingkajorn, M., Yaikhan, T., Duangsi-Ngoen, W., Klaysubun, C., Dechathai, T., Chusri, S., Singkhamanan, K., Pomwised, R., Wonglapsuwan, M., & Surachat, K. (2025). Phylogenomics and Antimicrobial Resistance of Clinical Bacteroides Isolates from a Tertiary Hospital in Southern Thailand. Antibiotics, 14(11), 1143. https://doi.org/10.3390/antibiotics14111143









