Bacterial Profile and Antibiotic Resistance of ESKAPEE Pathogens Isolated in Intensive Care Units from Blood Cultures: A Cross-Sectional Study from Abu Dhabi, United Arab Emirates (2018–2022)
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Study Setting
2.2.1. General Setting
2.2.2. Specific Setting
2.3. Study Population
2.4. Collection of Blood Specimens in the ICUs
2.5. Laboratory Processing, Bacteriological Culture, and Susceptibility Testing
2.6. Data Extraction and Variables
2.7. Data Management and Analysis
2.8. Ethical Approval
3. Results
3.1. Distribution of Blood Culture Positives of ESKAPEE by Sociodemographic Characteristics of Patients
3.2. Annual Distribution of Bacterial Profiles of the ESKAPEE Pathogens Isolated from Blood Specimens
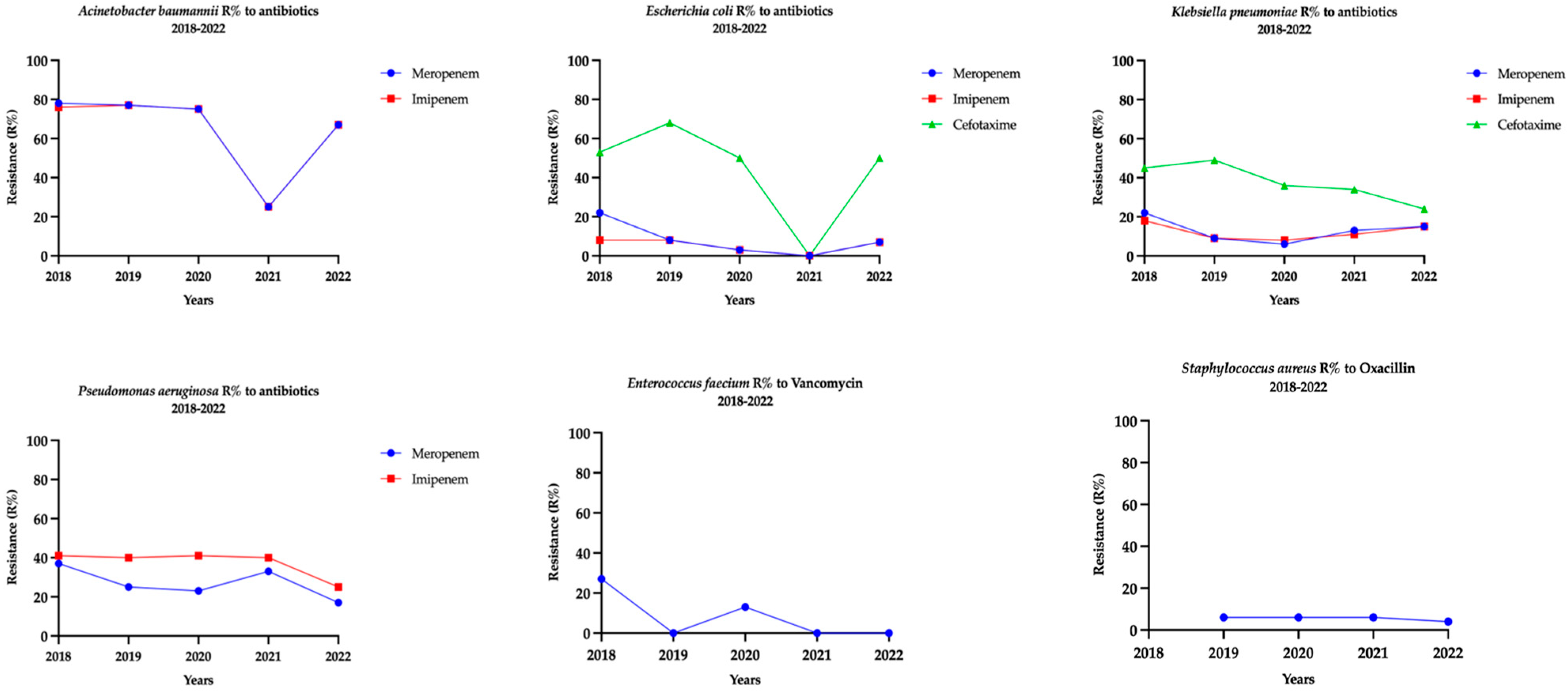
3.3. Patterns of Antibiotic Resistance Among the ESKAPEE Pathogens Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1. [Google Scholar]
- Al-Kaabi, M.R.; Tariq, W.U.; Hassanein, A.A. Rising bacterial resistance to common antibiotics in Al Ain, United Arab Emirates. East Mediterr. Health J. 2011, 17, 479–484. [Google Scholar] [CrossRef]
- World Health Organization. Comprehensive Review of the WHO Global Action Plan on Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Sommerstein, R.; Damonti, L.; Marschall, J.; Harbarth, S.; Gasser, M.; Kronenberg, A.; Buetti, N. Distribution of pathogens and antimicrobial resistance in ICU-bloodstream infections during hospitalization: A nationwide surveillance study. Sci. Rep. 2021, 11, 16876. [Google Scholar] [CrossRef]
- Costa, S.P.; Carvalho, C.M. Burden of bacterial bloodstream infections and recent advances for diagnosis. Pathog. Dis. 2022, 80, ftac027. [Google Scholar] [CrossRef]
- Russotto, V.; Cortegiani, A.; Graziano, G.; Saporito, L.; Raineri, S.M.; Mammina, C.; Giarratano, A. Bloodstream infections in intensive care unit patients: Distribution and antibiotic resistance of bacteria. Infect. Drug Resist. 2015, 8, 287–296. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990-2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Willemsen, A.; Reid, S.; Assefa, Y. A review of national action plans on antimicrobial resistance: Strengths and weaknesses. Antimicrob. Resist. Infect. Control. 2022, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Kalantar, E.; Bahmani, N.; Fatemi, A.; Naseri, N.; Ghotbi, N.; Naseri, M.H. Neonatal bacteriemia isolates and their antibiotic resistance pattern in neonatal insensitive care unit (NICU) at Beasat Hospital, Sanandaj, Iran. Acta Med. Iran. 2014, 52, 337–340. [Google Scholar] [PubMed]
- Al-Dhaheri, A.S.; Al-Niyadi, M.S.; Al-Dhaheri, A.D.; Bastaki, S.M. Resistance patterns of bacterial isolates to antimicrobials from 3 hospitals in the United Arab Emirates. Saudi Med. J. 2009, 30, 618–623. [Google Scholar]
- Sonnevend, Á.; Ghazawi, A.; Al Munthari, N.; Pitout, M.; Hamadeh, M.B.; Hashmey, R.; Girgis, S.K.; Sheikh, F.A.; Al Haj, M.; Nagelkerke, N.; et al. Characteristics of epidemic and sporadic strains of Acinetobacter baumannii isolated in Abu Dhabi hospitals. J. Med. Microbiol. 2013, 62 Pt 4, 582–590. [Google Scholar] [CrossRef][Green Version]
- Carey Roberta, B.; Bhattacharyya, S.; Kehl Sue, C.; Matukas Larissa, M.; Pentella Michael, A.; Salfinger, M.; Schuetz, A.N. Practical Guidance for Clinical Microbiology Laboratories: Implementing a Quality Management System in the Medical Microbiology Laboratory. Clin. Microbiol. Rev. 2018, 31, e00062-17. [Google Scholar] [CrossRef]
- Quiros-Roldan, E.; Sottini, A.; Natali, P.G.; Imberti, L. The Impact of Immune System Aging on Infectious Diseases. Microorganisms 2024, 12, 775. [Google Scholar] [CrossRef]
- Fintech Career Accelerator Scheme (FCAS). UAE Statistics UAE2024. Available online: https://uaestat.fcsc.gov.ae/en (accessed on 5 January 2025).
- Tian, L.; Zhang, Z.; Sun, Z. Antimicrobial resistance trends in bloodstream infections at a large teaching hospital in China: A 20-year surveillance study (1998–2017). Antimicrob. Resist. Infect. Control. 2019, 8, 86. [Google Scholar] [CrossRef]
- Bereanu, A.S.; Bereanu, R.; Mohor, C.; Vintilă, B.I.; Codru, I.R.; Olteanu, C.; Sava, M. Prevalence of Infections and Antimicrobial Resistance of ESKAPE Group Bacteria Isolated from Patients Admitted to the Intensive Care Unit of a County Emergency Hospital in Romania. Antibiotics 2024, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, J.; Abdulrazzaq, N.M.; AlRand, H.; The UAE AMR Surveillance Consortium. Surveillance of antimicrobial resistance in the United Arab Emirates: The early implementation phase. Front. Public Health 2023, 11, 1247627. [Google Scholar] [CrossRef] [PubMed]
- Banawas, S.S.; Alobaidi, A.S.; Dawoud, T.M.; AlDehaimi, A.; Alsubaie, F.M.; Abdel-Hadi, A.; Manikandan, P. Prevalence of Multidrug-Resistant Bacteria in Healthcare-Associated Bloodstream Infections at Hospitals in Riyadh, Saudi Arabia. Pathogens 2023, 12, 1075. [Google Scholar] [CrossRef]
- Müller, C.; Reuter, S.; Wille, J.; Xanthopoulou, K.; Stefanik, D.; Grundmann, H.; Higgins, P.G.; Seifert, H. A global view on carbapenem-resistant Acinetobacter baumannii. mBio 2023, 14, e02260-23. [Google Scholar] [CrossRef]
- Haque, S.; Ahmed, A.; Islam, N.; Haque, F.K.M. High Prevalence of Multidrug-Resistant Bacteria in the Trachea of Intensive Care Units Admitted Patients: Evidence from a Bangladeshi Hospital. Antibiotics 2024, 13, 62. [Google Scholar] [CrossRef]
- Zowawi, H.M.; Sartor, A.L.; Sidjabat, H.E.; Balkhy, H.H.; Walsh, T.R.; Al Johani, S.M.; AlJindan, R.Y.; Alfaresi, M.; Ibrahim, E.; Al-Jardani, A.; et al. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii Isolates in the Gulf Cooperation Council States: Dominance of OXA-23-Type Producers. J. Clin. Microbiol. 2015, 53, 896–903. [Google Scholar] [CrossRef]
- Ayoub Moubareck, C.; Hammoudi Halat, D.; Nabi, A.; AlSharhan, M.A.; AlDeesi, Z.O.; Han, A.; Celiloglu, H.; Karam Sarkis, D. Detection of OXA-23, GES-11 and NDM-1 among carbapenem-resistant Acinetobacter baumannii in Dubai: A preliminary study. J. Glob. Antimicrob. Resist. 2021, 24, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Ture, Z.; Güner, R.; Alp, E. Antimicrobial stewardship in the intensive care unit. J. Intensive Med. 2023, 3, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Konwar, D.; Chaudhary, N.K.; Yadav, P. Carbapenem-Resistant Gram-Negative Bacterial Infections at a Tertiary Health Care Center in Nepal: An Observational Study. JNMA J. Nepal. Med. Assoc. 2025, 63, 47–51. [Google Scholar]
- Piscitelli, P.; Costigliola, V.; Azamfirei, L. The challenge of antimicrobial resistance in intensive care setting. J. Crit. Care Med. 2025, 11, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Sardar, S.; De, R.; Biswas, M.; Mascellino, M.T.; Miele, M.C.; Biswas, S.; Mitra, A.N. Current Trends in Antimicrobial Resistance Patterns in Bacterial Pathogens among Adult and Pediatric Patients in the Intensive Care Unit in a Tertiary Care Hospital in Kolkata, India. Antibiotics 2023, 12, 459. [Google Scholar] [CrossRef]
- Rath, S.; Padhy, R.N. Prevalence of fluoroquinolone resistance in Escherichia coli in an Indian teaching hospital and adjoining communities. J. Taibah Univ. Med. Sci. 2015, 10, 504–508. [Google Scholar] [CrossRef][Green Version]
- Munro, C.; Zilberberg, M.D.; Shorr, A.F. Bloodstream Infection in the Intensive Care Unit: Evolving Epidemiology and Microbiology. Antibiotics 2024, 13, 123. [Google Scholar] [CrossRef]
- Huang, J.; Lv, C.; Li, M.; Rahman, T.; Chang, Y.-F.; Guo, X.; Song, Z.; Zhao, Y.; Li, Q.; Ni, P.; et al. Carbapenem-resistant Escherichia coli exhibit diverse spatiotemporal epidemiological characteristics across the globe. Commun. Biol. 2024, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Alfaresi, M.; Kim Sing, G.; Senok, A. First Report of bla(CTX-M-28) in Enterobacteriaceae Isolates in the United Arab Emirates. J. Pathog. 2018, 13, 04793. [Google Scholar]
- Talaat, M.; Zayed, B.; Tolba, S.; Abdou, E.; Gomaa, M.; Itani, D.; Hutin, Y.; Hajjeh, R. Increasing Antimicrobial Resistance in World Health Organization Eastern Mediterranean Region, 2017–2019. Emerg. Infect. Dis. 2022, 28, 717–724. [Google Scholar] [CrossRef]
- Tabah, A.; Buetti, N.; Staiquly, Q.; Ruckly, S.; Akova, M.; Aslan, A.T.; Leone, M.; Conway Morris, A.; Bassetti, M.; Arvaniti, K.; et al. Epidemiology and outcomes of hospital-acquired bloodstream infections in intensive care unit patients: The EUROBACT-2 international cohort study. Intensive Care Med. 2023, 49, 178–190. [Google Scholar] [CrossRef]
- Abebe, A.A.; Birhanu, A.G. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef]
- Thomsen, J.; Abdulrazzak, N.M.; AlRand, H.; The UAE AMR Surveillance Consortium; Menezes, G.A.; Moubareck, C.A.; Everett, D.B.; Senok, A.; Podbielski, A. Epidemiology of vancomycin-resistant enterococci in the United Arab Emirates: A retrospective analysis of 12 years of national AMR surveillance data. Front. Public Health 2023, 11, 1275778. [Google Scholar] [CrossRef] [PubMed]
- Azzam, A.; Elkafas, H.; Khaled, H.; Ashraf, A.; Yousef, M.; Elkashef, A.A. Prevalence of Vancomycin-resistant enterococci (VRE) in Egypt (2010–2022): A systematic review and meta-analysis. J. Egypt. Public Health Assoc. 2023, 98, 8. [Google Scholar] [CrossRef]
- O’Toole, R.F.; Leong, K.W.C.; Cumming, V.; Van Hal, S.J. Vancomycin-resistant Enterococcus faecium and the emergence of new sequence types associated with hospital infection. Res. Microbiol. 2023, 174, 104046. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance Atlas of Infectious Diseases; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2021. [Google Scholar]
- Knight, G.M.; Glover, R.E.; McQuaid, C.F.; Olaru, I.D.; Gallandat, K.; Leclerc, Q.J.; Fuller, N.M.; Willcocks, S.J.; Hasan, R.; Van Kleef, E.; et al. Antimicrobial resistance and COVID-19: Intersections and implications. Elife 2021, 10, e64139. [Google Scholar] [CrossRef] [PubMed]
- Bolikas, E.; Astrinaki, E.; Panagiotaki, E.; Vitsaxaki, E.; Saplamidou, S.; Drositis, I.; Stafylaki, D.; Chamilos, G.; Gikas, A.; Kofteridis, D.P.; et al. Impact of SARS-CoV-2 Preventive Measures against Healthcare-Associated Infections from Antibiotic-Resistant ESKAPEE Pathogens: A Two-Center, Natural Quasi-Experimental Study in Greece. Antibiotics 2023, 12, 1088. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Nasef, R.; El Lababidi, R.; Alatoom, A.; Krishnaprasad, S.; Bonilla, F. The Impact of Integrating Rapid PCR-Based Blood Culture Identification Panel to an Established Antimicrobial Stewardship Program in the United Arab Emirates. Int. J. Infect. Dis. 2020, 91, 124–1288. [Google Scholar] [CrossRef]
| Characteristics | n (%) |
|---|---|
| Total ¥ | 838 |
| Age Category (yrs) | |
| 18–35 | 96 (12) |
| 36–49 | 103 (12) |
| 50–65 | 213 (25) |
| ≥65 | 426 (51) |
| Gender | |
| Female | 315 (38) |
| Male | 523 (62) |
| Nationality | |
| Emirati | 307 (37) |
| Non-Emirati | 501 (60) |
| Unknown | 30 (3) |
| Region | |
| Abu Dhabi | 574 (68) |
| Al Ain | 225 (27) |
| Al Dhafra | 39 (5) |
| Isolates | All Years n (%) | 2018 n (%) | 2019 n (%) | 2020 n (%) | 2021 n (%) | 2022 n (%) | p-Value * |
|---|---|---|---|---|---|---|---|
| Total | 965 (100) | 275 (29) | 216 (22) | 187 (19) | 143 (15) | 144 (15) | |
| Acinetobacter baumannii | 46 (5) | 18 (7) | 13 (6) | 8 (4) | 4 (3) | 3 (2) | 0.01 |
| Enterobacter spp. | 43 (4) | 14 (5) | 10 (5) | 11 (6) | 5 (3) | 3 (2) | 0.17 |
| Enterococcus faecium | 43 (4) | 11 (4) | 8 (4) | 8 (4) | 8 (6) | 8 (6) | 0.35 |
| Escherichia coli | 217 (22) | 70 (25) | 41 (19) | 38 (20) | 38 (27) | 30 (21) | 0.66 |
| Klebsiella pneumoniae | 297 (31) | 84 (30) | 59 (27) | 66 (35) | 47 (33) | 41 (28) | 0.82 |
| Pseudomonas aeruginosa | 132 (14) | 35 (13) | 36 (17) | 22 (12) | 15 (10) | 24 (17) | 0.88 |
| Staphylococcus aureus | 187 (20) | 43(16) | 49 (22) | 34(19) | 26 (18) | 35 (24) | 0.14 |
| Acinetobacter baumannii n = 46 | Enterobacter spp. n = 43 | Escherichia coli n = 217 | Klebsiella pneumoniae n = 297 | Pseudomonas aeruginosa n = 132 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Antibiotics | Tested N | R * (%) | Tested N | R (%) | Tested N | R (%) | Tested N | R (%) | Tested N | R (%) |
| Aminoglycosides | Amikacin | 27 | (81) | 34 | (0) | 165 | (1) | 222 | (7) | 128 | (4) |
| Gentamicin | 46 | (65) | 43 | (5) | 216 | (21) | 297 | (16) | 132 | (5) | |
| Tobramycin | 45 | (58) | 18 | (0) | 76 | (28) | 103 | (28) | 131 | (5) | |
| Pencillins | Ampicillin | - | - | - | - | 217 | (76) | - | - | - | - |
| Pencillins + β-lactamase inhibitor combination | Amoxicillin/Clavulanic acid | - | - | - | - | 208 | (38) | 285 | (36) | - | - |
| Piperacillin/Tazobactam | 46 | (72) | 43 | (33) | 217 | (18) | 297 | (27) | 128 | (13) | |
| Carbapenems | Ertapenem | - | - | 31 | (10) | 164 | (5) | 214 | (11) | - | - |
| Meropenem | 46 | (72) | 43 | (5) | 213 | (5) | 296 | (13) | 132 | (27) | |
| Imipenem | 36 | (78) | 30 | (13) | 149 | (42) | 206 | (37) | 127 | (13) | |
| Cephalosporins | Cefepime | 36 | (78) | 30 | (13) | 149 | (42) | 206 | (37) | 127 | (13) |
| Cefotaxime | - | - | 39 | (31) | 210 | (54) | 286 | (39) | - | - | |
| Ceftazidime | 46 | (72) | - | - | - | - | - | - | 132 | (20) | |
| Fluoroquinolones | Ciprofloxacin | 46 | (70) | 43 | (7) | 217 | (60) | 296 | (34) | 132 | (15) |
| Monobactams | Aztreonam | - | - | 1 | (0) | 6 | (100) | 2 | (100) | 55 | (18) |
| Folate pathway inhibitor | Trimethoprim–sulfamethoxazole | 46 | (50) | 43 | (10) | 217 | (55) | 296 | (30) | - | - |
| Tetracyclines | Tetracycline | 12 | (42) | 14 | (14) | 69 | (55) | 92 | (37) | - | - |
| Enterococcus faecium n = 43 | Staphylococcus aureus n = 187 | ||||
|---|---|---|---|---|---|
| Class | Antibiotic | Tested N | R * (%) | Tested N | R (%) |
| Aminoglycosides | Gentamicin | - | - | 122 | (2) |
| High-level Gentamicin | 40 | (65) | - | - | |
| Pencillins + β-lactamase inhibitor combination | Amoxicillin/Clavulanic acid | - | - | 6 | (0) |
| Penicillin G | - | - | 122 | (80) | |
| Oxacillin | - | - | 122 | (4) | |
| Lincosamides | Clindamycin | - | - | 122 | (16) |
| Fluoroquinolones | Ciprofloxacin | 10 | (60) | 11 | (45) |
| Levofloxacin | 7 | (71) | 39 | (36) | |
| Moxifloxacin | - | - | 121 | (35) | |
| Macrolides | Erythromycin | 43 | (84) | 122 | (32) |
| Glycopeptides | Teicoplanin | 36 | (11) | - | - |
| Vancomycin | 43 | (9) | - | - | |
| Tetracyclines | Tetracycline | - | - | 122 | (10) |
| Folate pathway inhibitor | Trimethoprim–sulfamethoxazole | - | - | 122 | (15) |
| Oxazolidinones | Linezolid | 43 | (2) | 122 | (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Marzooqi, A.A.; Bashir, M.M.; Khogali, M.A.; Suliman, A.; Timire, C.; Al Hosani, F.I.; Al Ahbabi, F.M. Bacterial Profile and Antibiotic Resistance of ESKAPEE Pathogens Isolated in Intensive Care Units from Blood Cultures: A Cross-Sectional Study from Abu Dhabi, United Arab Emirates (2018–2022). Antibiotics 2025, 14, 1142. https://doi.org/10.3390/antibiotics14111142
Al Marzooqi AA, Bashir MM, Khogali MA, Suliman A, Timire C, Al Hosani FI, Al Ahbabi FM. Bacterial Profile and Antibiotic Resistance of ESKAPEE Pathogens Isolated in Intensive Care Units from Blood Cultures: A Cross-Sectional Study from Abu Dhabi, United Arab Emirates (2018–2022). Antibiotics. 2025; 14(11):1142. https://doi.org/10.3390/antibiotics14111142
Chicago/Turabian StyleAl Marzooqi, Ayesha Abdulla, Maryam Mohammed Bashir, Mohammed Ahmed Khogali, Abubaker Suliman, Collins Timire, Farida Ismail Al Hosani, and Faisal Musleh Al Ahbabi. 2025. "Bacterial Profile and Antibiotic Resistance of ESKAPEE Pathogens Isolated in Intensive Care Units from Blood Cultures: A Cross-Sectional Study from Abu Dhabi, United Arab Emirates (2018–2022)" Antibiotics 14, no. 11: 1142. https://doi.org/10.3390/antibiotics14111142
APA StyleAl Marzooqi, A. A., Bashir, M. M., Khogali, M. A., Suliman, A., Timire, C., Al Hosani, F. I., & Al Ahbabi, F. M. (2025). Bacterial Profile and Antibiotic Resistance of ESKAPEE Pathogens Isolated in Intensive Care Units from Blood Cultures: A Cross-Sectional Study from Abu Dhabi, United Arab Emirates (2018–2022). Antibiotics, 14(11), 1142. https://doi.org/10.3390/antibiotics14111142







