Surveillance of Antimicrobial Resistance in Neisseria gonorrhoeae in Alberta from 2016–2022
Abstract
1. Introduction
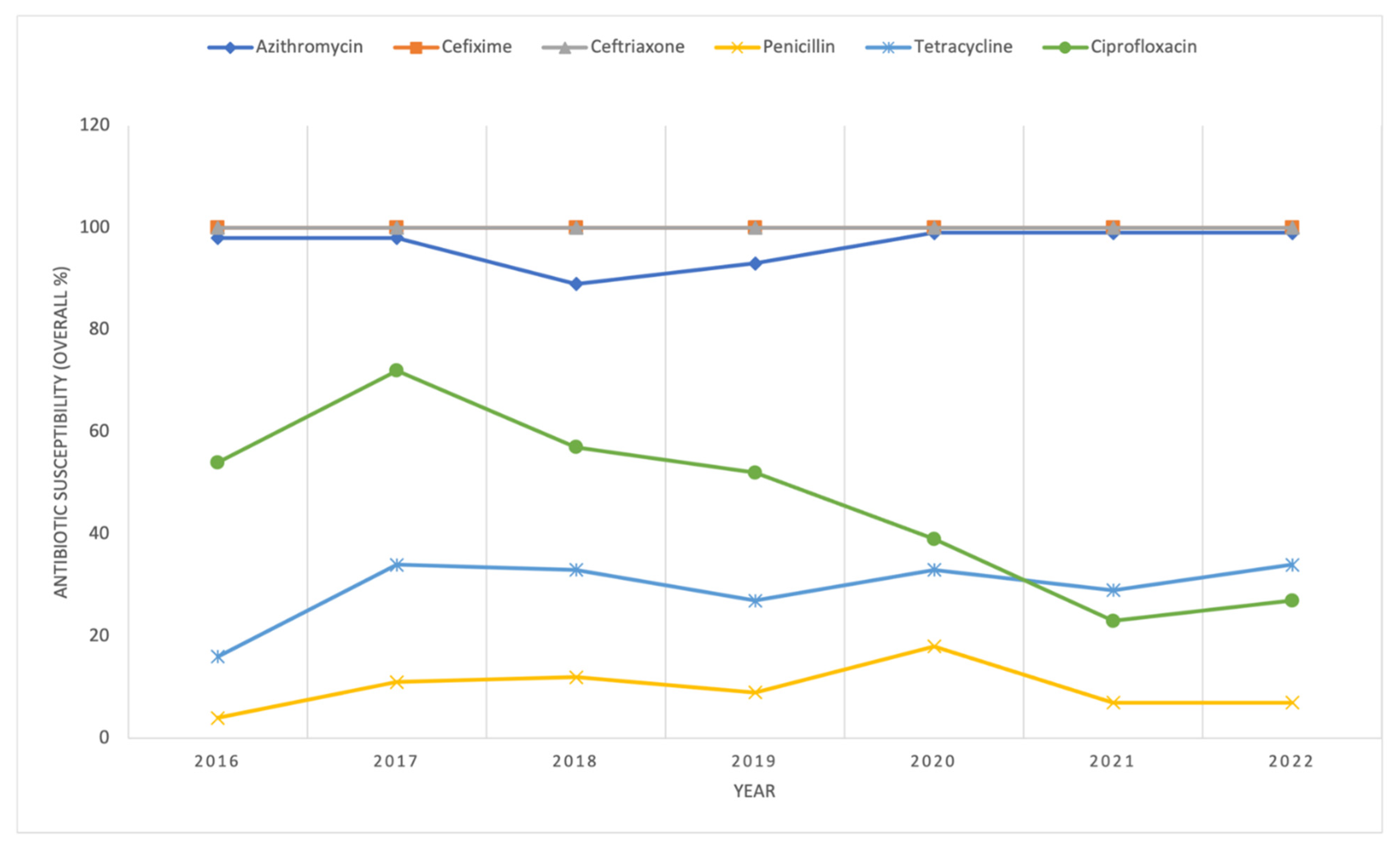
2. Results and Discussion
3. Materials and Methods
3.1. Database Creation and Analysis of AST Results
3.2. Database Creation and Analysis of AST Results Stratified by Demographic Variables
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Division of STD Prevention; National Center for HIV; Viral Hepatitis, STD; and TB Prevention, Centers for Disease Control and Prevention. Detailed STD Facts-Gonorrhea. 2023. Available online: https://www.cdc.gov/sti/?CDC_AAref_Val=https://www.cdc.gov/std/gonorrhea/stdfact-gonorrhea-detailed.htm (accessed on 29 July 2025).
- Unemo, M.; Seifert, H.S.; Hook, E.W., 3rd; Hawkes, S.; Ndowa, F.; Dillon, J.R. Gonorrhoea. Nat. Rev. Dis. Primers. 2019, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.R.; Joshi, K.; Brucks, E.; Ferreira, J.P. Disseminated Gonococcal Infection in an Immunosuppressed Patient. Am. J. Med. 2021, 134, e123–e124. [Google Scholar] [CrossRef] [PubMed]
- Lovett, A.; Duncan, J.A. Human Immune Responses and the Natural History of Neisseria gonorrhoeae Infection. Front. Immunol. 2019, 9, 3187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bjekić, M.; Vlajinac, H.; Sipetić, S.; Marinković, J. Risk factors for gonorrhoea: Case-control study. Genitourin. Med. 1997, 73, 518–521. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Public Health Agency of Canada. Gonorrhea Guide: Risk Factors and Clinical Manifestations. 2021. Available online: https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/gonorrhea/risk-factors-clinical-manifestation.html (accessed on 29 July 2025).
- Amies, C.R. Development of resistance of gonococci to penicillin: An eight-year study. Can. Med. Assoc. J. 1967, 96, 33–35. [Google Scholar] [PubMed] [PubMed Central]
- Lewis, D.A. The Gonococcus fights back: Is this time a knock out? Sex. Transm. Infect. 2010, 86, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Division of STD Prevention; National Center for HIV; Viral Hepatitis, STD; and TB Prevention, Centers for Disease Control and Prevention. CDC’s STI Treatment Guidelines Timeline: The Evolution of Sexual Healthcare. 2021. Available online: https://www.cdc.gov/std/treatment-guidelines/timeline.htm (accessed on 29 July 2025).
- Morse, S.A.; Johnson, S.R.; Biddle, J.W.; Roberts, M.C. High-level tetracycline resistance in Neisseria gonorrhoeae is result of acquisition of streptococcal tetM determinant. Antimicrob. Agents Chemother. 1986, 30, 664–670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gransden, W.R.; Warren, C.A.; Phillips, I.; Hodges, M.; Barlow, D. Decreased susceptibility of Neisseria gonorrhoeae to ciprofloxacin. Lancet 1990, 335, 51, Erratum in Lancet 1990, 335, 302. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Treatment of Gonorrhea in Canada. 2017. Available online: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2017-43/ccdr-volume-43-2-february-2-2017/ccdr-volume-43-2-february-2-2017-sexually-transmitted-infections.html (accessed on 29 July 2025).
- Public Health Agency of Canada. Gonorrhea Guide: Treatment and Follow-Up. 2021. Available online: https://www.canada.ca/en/public-health/services/infectious-diseases/sexual-health-sexually-transmitted-infections/canadian-guidelines/gonorrhea/treatment-follow-up.html (accessed on 29 July 2025).
- Government of Alberta. Alberta Treatment Guidelines for Sexually Transmitted Infections (STI) in Adolescents and Adults, 2018 [Updated Feb 2024]. 2024. Available online: https://open.alberta.ca/publications/treatment-guidelines-for-sti-2018 (accessed on 29 July 2025).
- Landhuis, E.W. Multidrug-Resistant “Super Gonorrhea” Rallies Multipronged Effort. JAMA 2024, 331, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Multi-Drug Resistant Gonorrhoea. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/multi-drug-resistant-gonorrhoea (accessed on 29 July 2025).
- Ito, M.; Yasuda, M.; Yokoi, S.; Ito, S.; Takahashi, Y.; Ishihara, S.; Maeda, S.; Deguchi, T. Remarkable increase in central Japan in 2001-2002 of Neisseria gonorrhoeae isolates with decreased susceptibility to penicillin, tetracycline, oral cephalosporins, and fluoroquinolones. Antimicrob Agents Chemother. 2004, 48, 3185–3187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fifer, H.; Natarajan, U.; Jones, L.; Alexander, S.; Hughes, G.; Golparian, D.; Unemo, M. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. N. Engl. J. Med. 2016, 374, 2504–2506. [Google Scholar] [CrossRef] [PubMed]
- Berenger, B.M.; Demczuk, W.; Gratrix, J.; Pabbaraju, K.; Smyczek, P.; Martin, I. Genetic Characterization and Enhanced Surveillance of Ceftriaxone-Resistant Neisseria gonorrhoeae Strain, Alberta, Canada, 2018. Emerg. Infect. Dis. 2019, 25, 1660–1667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakayama, S.; Shimuta, K.; Furubayashi, K.; Kawahata, T.; Unemo, M.; Ohnishi, M. New Ceftriaxone- and Multidrug-Resistant Neisseria gonorrhoeae Strain with a Novel Mosaic penA Gene Isolated in Japan. Antimicrob. Agents Chemother. 2016, 60, 4339–4341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization. Gonococcal Antimicrobial Surveillance Programme (GASP). Available online: https://www.who.int/initiatives/gonococcal-antimicrobial-surveillance-programme (accessed on 29 July 2025).
- Sawatzky, P.; Lefebvre, B.; Diggle, M.; Hoang, L.; Wong, J.; Patel, S.; Van Caessele, P.; Minion, J.; Garceau, R.; Jeffrey, S.; et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae in Canada, 2021. Can. Commun. Dis. Rep. 2023, 49, 388–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gratrix, J.; Kamruzzaman, A.; Martin, I.; Smyczek, P.; Read, R.; Bertholet, L.; Naidu, P.; Singh, A.E. Surveillance for Antimicrobial Resistance in Gonorrhea: The Alberta Model, 2012−2016. Antibiotics 2018, 7, 63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lahra, M.M.; Martin, I.; Demczuk, W.; Jennison, A.V.; Lee, K.I.; Nakayama, S.I.; Lefebvre, B.; Longtin, J.; Ward, A.; Mulvey, M.R.; et al. Cooperative Recognition of Internationally Disseminated Ceftriaxone-Resistant Neisseria gonorrhoeae Strain. Emerg. Infect. Dis. 2018, 24, 735–740. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whiley, D.M.; Mhango, L.; Jennison, A.V.; Nimmo, G.; Lahra, M.M. Direct Detection of penA Gene Associated with Ceftriaxone-Resistant Neisseria gonorrhoeae FC428 Strain by Using PCR. Emerg. Infect. Dis. 2018, 24, 1573–1575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Public Health Agency of Canada. Chlamydia, Gonorrhea and Infectious Syphilis in Canada: 2021 Surveillance Data Update. 2023. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/chlamydia-gonorrhea-infectious-syphilis-2021-surveillance-data.html (accessed on 29 July 2025).
- Government of Alberta. Alberta Public Health Disease Management Guidelines: Gonorrhea. 2022. Available online: https://open.alberta.ca/publications/gonorrhea#summary (accessed on 29 July 2025).
- Alberta Health, Government of Alberta. Alberta Sexually Transmitted Infections and HIV 2022. Annu Rep. 2023. Available online: https://open.alberta.ca/publications/9781460145449 (accessed on 29 July 2025).
- Sawatzky, P.; Thorington, R.; Barairo, N.; Lefebvre, B.; Diggle, M.; Hoang, L.; Patel, S.; Van Caessele, P.; Minion, J.; Desnoyers, G.; et al. Antimicrobial susceptibilities of Neisseria gonorrhoeae in Canada, 2022. Can. Commun. Dis. Rep. 2025, 51, 129–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, X.; Xi, Y.; Gong, X.; Chen, S. Ceftriaxone-Resistant Gonorrhea-China, 2022. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 255–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Commonwealth of Massachusetts: Bureau of Infectious Disease and Laboratory Sciences. Department of Public Health Announces First Cases of Concerning Gonorrhea Strain|Mass.gov. Available online: https://www.mass.gov/news/department-of-public-health-announces-first-cases-of-concerning-gonorrhea-strain (accessed on 29 July 2025).
- Centers for Disease Control and Prevention. GISP Profiles National Data, CDC Archive. Sexually Transmitted Disease Surveillance 2022: Gonococcal Isolate Surveillance Project Profile Division of STD Prevention April 2024. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/sti-statistics/media/pdfs/2024/07/2022-GISP-Profiles_National_CLEARED_CLEAN.pdf (accessed on 17 October 2025).
- Ouk, V.; Heng, L.S.; Virak, M.; Deng, S.; Lahra, M.M.; Frankson, R.; Kreisel, K.; McDonald, R.; Escher, M.; et al.; EGASP Cambodia Working Group High prevalence of ceftriaxone-resistant and XDR Neisseria gonorrhoeae in several cities of Cambodia, 2022–2023: WHO Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP). JAC-Antimicrob. Resist. 2024, 6, dlae053. [Google Scholar] [CrossRef]
- Lan, P.T.; Nguyen, H.T.; Golparian, D.; Thuy Van, N.T.; Maatouk, I.; Unemo, M.; EGASP-Vietnam WGS Study Group. The WHO Enhanced Gonococcal Antimicrobial Surveillance Programme (EGASP) identifies high levels of ceftriaxone resistance across Vietnam, 2023. Lancet Reg. Health West Pac. 2024, 48, 101125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unemo, M.; Ross, J.; Serwin, A.B.; Gomberg, M.; Cusini, M.; Jensen, J.S. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int. J. STD AIDS 2020, ahead of print, 956462420949126. [Google Scholar] [CrossRef] [PubMed]
- Fifer, H.; Saunders, J.; Soni, S.; Sadiq, S.T.; FitzGerald, M. 2018 UK national guideline for the management of infection with Neisseria gonorrhoeae. Int. J. STD AIDS 2020, 31, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.N.; Marrazzo, J.; Batteiger, B.E.; Hook, E.W., 3rd; Seña, A.C.; Long, J.; Wierzbicki, M.R.; Kwak, H.; Johnson, S.M.; Lawrence, K.; Mueller, J. Single-Dose Zoliflodacin (ETX0914) for Treatment of Urogenital Gonorrhea. N. Engl. J. Med. 2018, 379, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.R.; Scangarella-Oman, N.E.; Millns, H.; Flight, W.; Gatsi, S.; Jakielaszek, C.; Janmohamed, S.; Lewis, D.A. Efficacy and Safety of Gepotidacin as Treatment of Uncomplicated Urogenital Gonorrhea (EAGLE-1): Design of a Randomized, Comparator-Controlled, Phase 3 Study. Infect. Dis. Ther. 2023, 12, 2307–2320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- GSK. GSK Announces Positive Headline Results from EAGLE-1 Phase III Trial for Gepotidacin in Uncomplicated Urogenital Gonorrhoea (GC)|GSK. 2024. Available online: https://www.gsk.com/en-gb/media/press-releases/gsk-announces-positive-headline-results-from-eagle-1-phase-iii-trial-for-gepotidacin-in-uncomplicated-urogenital-gonorrhoea-gc/ (accessed on 29 July 2025).
- Waltmann, A.; Chen, J.S.; Duncan, J.A. Promising developments in gonococcal vaccines. Curr. Opin. Infect. Dis. 2024, 37, 63–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gulati, S.; Mattsson, A.H.; Schussek, S.; Zheng, B.; DeOliveira, R.B.; Shaughnessy, J.; Lewis, L.A.; Rice, P.A.; Comstedt, P.; Ram, S. Preclinical efficacy of a cell division protein candidate gonococcal vaccine identified by artificial intelligence. mBio 2023, 14, e0250023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Islam, E.A.; Fegan, J.E.; Zeppa, J.J.; Ahn, S.K.; Ng, D.; Currie, E.G.; Lam, J.; Moraes, T.F.; Gray-Owen, S.D. Adjuvant-dependent impacts on vaccine-induced humoral responses and protection in preclinical models of nasal and genital colonization by pathogenic Neisseria. Vaccine 2025, 48, 126709. [Google Scholar] [CrossRef] [PubMed]
- Perrin, A.; Nassif, X.; Tinsley, C. Identification of regions of the chromosome of Neisseria meningitidis and Neisseria gonorrhoeae which are specific to the pathogenic Neisseria species. Infect. Immun. 1999, 67, 6119–6129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petousis-Harris, H.; Paynter, J.; Morgan, J.; Saxton, P.; McArdle, B.; Goodyear-Smith, F.; Black, S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet 2017, 390, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Robison, S.G.; Leman, R.F. Association of Group B Meningococcal Vaccine Receipt with Reduced Gonorrhea Incidence Among University Students. JAMA Netw. Open 2023, 6, e2331742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abara, W.E.; Bernstein, K.T.; Lewis, F.M.T.; Schillinger, J.A.; Feemster, K.; Pathela, P.; Hariri, S.; Islam, A.; Eberhart, M.; Cheng, I.; et al. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: A retrospective observational study. Lancet Infect. Dis. 2022, 22, 1021–1029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Looker, K.J.; Booton, R.; Begum, N.; Beck, E.; Shen, J.; Turner, K.M.E.; Christensen, H. The potential public health impact of adolescent 4CMenB vaccination on Neisseria gonorrhoeae infection in England: A modelling study. BMC Public Health 2023, 23, 1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ladhani, S.N.; Mandal, S.; Mohammed, H.; Saunders, J.; Andrews, N.; Ramsay, M.E.; Fifer, H. The United Kingdom meningococcal vaccine (4CMenB) programme against gonorrhoea: A review of the evidence and knowledge gaps. J. Infect. 2025, 91, 106582. [Google Scholar] [CrossRef] [PubMed]
- Government of Alberta. Gonorrhea Test 2022. Available online: https://myhealth.alberta.ca:443/Health/tests-treatments/pages/conditions.aspx?Hwid=hw4905 (accessed on 29 July 2025).
- Clinical and Laboratory Standards Institute. CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 35th ed.; CLSI supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2025. [Google Scholar]
- Alberta Health and Alberta Health Services. Official Standard Geographic Areas. 2018. Available online: https://open.alberta.ca/dataset/a14b50c9-94b2-4024-8ee5-c13fb70abb4a/resource/70fd0f2c-5a7c-45a3-bdaa-e1b4f4c5d9a4/download/official-standard-geographic-area-document.pdf (accessed on 17 October 2025).
| Penicillin (n = 3594) | Tetracycline (n = 3592) | Ciprofloxacin (n = 3593) | Azithromycin (n = 3591) | ||||||||||||
| Susceptible n (%) | Non-Susceptible n (%) | p-value * | Susceptible n (%) | Non-Susceptible n (%) | p-value * | Susceptible n (%) | Non-Susceptible n (%) | p-value * | Susceptible n (%) | Non-Susceptible n (%) | p-value * | ||||
| Age (years) (n = 3592) | 0.202 | Age (years) (n = 3590) | 0.221 | Age (years) (n = 3591) | <0.001 * | Age (years) (n = 3589) | 0.374 | ||||||||
| 10–20 a (n = 252) | 36 (10.68) | 216 (6.64) | 10–20 a (n = 251) | 76 (7.15) | 175 (6.93) | 10–20 a (n = 252) | 156 (8.35) | 96 (5.57) | 10–20 a (n = 251) | 239 (6.97) | 12 (7.59) | ||||
| 21–30 (n = 1520) | 139 (41.25 | 1381 (42.43) | 21–30 (n = 1521) | 421 (39.60) | 1100 (45.53) | 21–30 (n = 1521) | 833 (44.59) | 688 (39.93) | 21–30 (n = 1520) | 1463 (42.64) | 57 (36.08) | ||||
| 31–40 (n = 1184) | 112 (33.23) | 1072 (32.93) | 31–40 (n = 1183) | 368 (34.62) | 815 (32.25) | 31–40 (n = 1183) | 588 (31.48) | 595 (34.53) | 31–40 (n = 1183) | 1126 (32.82) | 57 (36.08) | ||||
| 41–50 (n = 399) | 33 (9.79) | 366 (11.24) | 41–50 (n = 399) | 124 (11.67) | 275 (10.88) | 41–50 (n = 399) | 186 (9.96) | 213 (12.36) | 41–50 (n = 399) | 382 (11.13) | 17 (10.76) | ||||
| 51–60 (n = 191) | 15 (4.45) | 176 (5.41) | 51–60 (n = 191) | 65 (6.11) | 126 (4.99) | 51–60 (n = 191) | 91 (4.87) | 100 (5.80) | 51–60 (n = 191) | 177 (5.16) | 14 (8.86) | ||||
| >60 (n = 46) | 2 (0.59) | 44 (1.35) | >60 (n = 45) | 9 (0.84) | 36 (1.43) | >60 (n = 45) | 14 (0.75) | 31 (1.80) | >60 (n = 45) | 44 (1.29) | 1 (0.63) | ||||
| ‡ Sex (n = 3590) | 0.006 * | ‡ Sex (n = 3588) | 0.368 | ‡ Sex (n = 3589) | <0.001 * | ‡Sex (n = 3587) | 0.001 * | ||||||||
| Male (n = 2928) | 257 (76.04) | 2,671 (82.13) | Male (n = 2928) | 877 (82.12) | 2051 (81.23) | Male (n = 2928) | 1462 (76.38) | 1502 (87.22) | Male (n = 2928) | 2783 (81.16) | 145 (91.77) | ||||
| Female (n = 662) | 81 (23.96) | 581 (17.87) | Female (n = 660) | 186 (17.88) | 474 (18.77) | Female (n = 612) | 441 (23.62) | 220 (12.78) | Female (n = 659) | 646 (18.84) | 13 (8.23) | ||||
| Health Zone (n = 3562) | <0.001 * | Health Zone (n = 3561) | 0.092 | Health Zone (n = 3562) | <0.001 * | Health Zone (n = 3560) | 0.001 * | ||||||||
| Calgary (n = 2039) | 146 (43.58) | 1893 (58.66) | Calgary (n = 2037) | 575 (54.55) | 1462 (58.32) | Calgary (n = 2037) | 885 (47.73) | 1152 (67.45) | Calgary (n = 2037) | 1968 (57.85) | 69 (43.67) | ||||
| Central (n = 59) | 8 (2.39) | 51 (1.58) | Central (n = 59) | 12 (1.14) | 47 (1.87) | Central (n = 59) | 33 (1.78) | 26 (1.52) | Central (n = 59) | 54 (1.59) | 5 (3.16) | ||||
| Edmonton (n = 1330) | 159 (47.46) | 1,171 (36.29) | Edmonton (n = 1331) | 423 (40.13) | 908 (36.22) | Edmonton (n = 1332) | 845 (45.58) | 487 (28.51) | Edmonton (n = 1331) | 1249 (36.71) | 82 (51.90) | ||||
| North (n = 113) | 20 (5.97) | 93 (2.88) | North (n = 113) | 37 (3.51) | 76 (3.03) | North (n = 113) | 81 (4.37) | 32 (1.87) | North (n = 112) | 110 (3.23) | 2 (1.27) | ||||
| South (n = 21) | 2 (0.60) | 19 (0.59) | South (n = 21) | 7 (0.66) | 14 (0.56) | South (n = 21) | 10 (0.54) | 11 (0.64) | South (n = 21) | 21 (0.62) | 0 (0) | ||||
| Geographic Region (n = 1653) | 0.085 | Geographic Region (n = 1653) | 0.796 | Geographic Region (n = 1654) | <0.001 * | Geographic Region (n = 1652) | 0.652 | ||||||||
| Metro (n = 1478) | 144 (85.21) | 1334 (89.89) | Metro (n = 1478) | 466 (90.14) | 1012 (89.08) | Metro (n = 1479) | 705 (86.50) | 774 (92.25) | Metro (n = 1478) | 1413 (89.32) | 65 (92.86) | ||||
| Urban (n = 50) | 5 (2.96) | 45 (3.03) | Urban (n = 50) | 14 (2.71) | 36 (3.17) | Urban (n = 50) | 36 (4.42) | 14 (1.67) | Urban (n = 49) | 47 (2.97) | 2 (2.86) | ||||
| Rural (n = 125) | 20 (11.83) | 105 (7/08) | Rural (n = 125) | 37 (7.16) | 88 (7.75) | Rural (n = 125) | 74 (9.08) | 51 (6.08) | Rural (n = 125) | 122 (7.71) | 3 (4.29) | ||||
| Income Quintile (n = 1653) | 0.303 | Income Quintile (n = 1653) | 0.953 | Income Quintile (n = 1654) | 0.002 * | Income Quintile (n = 1652) | 0.133 | ||||||||
| Q1 (lowest) (n = 438) | 48 (28.40) | 390 (26.28) | Q1 (lowest) (n = 438) | 137 (26.50) | 301 (26.50) | Q1 (lowest) (n = 439) | 249 (30.55) | 190 (22.65) | Q1 (lowest) (n = 438) | 421 (26.61) | 17 (24.29) | ||||
| Q2 (n = 300) | 38 (22.49) | 262 (17.65) | Q2 (n = 300) | 98 (18.96) | 202 (17.78) | Q2 (n = 300) | 155 (19.02) | 145 (17.28) | Q2 (n = 300) | 290 (18.33) | 10 (14.29) | ||||
| Q3 (n = 300) | 25 (14.70) | 275 (18.53) | Q3 (n = 300) | 95 (18.38) | 205 (18.05) | Q3 (n = 300) | 135 (16.56) | 165 (19.67) | Q3 (n = 300) | 287 (18.14) | 13 (18.57) | ||||
| Q4 (n = 288) | 31 (18.34) | 257 (17.32) | Q4 (n = 288) | 90 (17.41) | 198 (17.43) | Q4 (n = 288) | 127 (15.58) | 161 (19.19) | Q4 (n = 288) | 268 (16.94) | 20 (28.57) | ||||
| Q5 (highest) (n = 327) | 27 (15.98) | 300 (29.22) | Q5 (highest) (n = 327) | 97 (18.76) | 230 (20.25) | Q5 (highest) (n = 327) | 149 (18.28) | 178 (21.22) | Q5 (highest) (n = 326) | 316 (19.97) | 10 (14.29) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walsh, T.M.; Plitt, S.S.; Dingle, T.C.; Charlton, C.L. Surveillance of Antimicrobial Resistance in Neisseria gonorrhoeae in Alberta from 2016–2022. Antibiotics 2025, 14, 1119. https://doi.org/10.3390/antibiotics14111119
Walsh TM, Plitt SS, Dingle TC, Charlton CL. Surveillance of Antimicrobial Resistance in Neisseria gonorrhoeae in Alberta from 2016–2022. Antibiotics. 2025; 14(11):1119. https://doi.org/10.3390/antibiotics14111119
Chicago/Turabian StyleWalsh, Taylor M., Sabrina S. Plitt, Tanis C. Dingle, and Carmen L. Charlton. 2025. "Surveillance of Antimicrobial Resistance in Neisseria gonorrhoeae in Alberta from 2016–2022" Antibiotics 14, no. 11: 1119. https://doi.org/10.3390/antibiotics14111119
APA StyleWalsh, T. M., Plitt, S. S., Dingle, T. C., & Charlton, C. L. (2025). Surveillance of Antimicrobial Resistance in Neisseria gonorrhoeae in Alberta from 2016–2022. Antibiotics, 14(11), 1119. https://doi.org/10.3390/antibiotics14111119





