Antimicrobial Resistance Phenotypes and Genotypes of Escherichia coli Isolates from Artisanal Minas Frescal Cheeses from the Federal District, Brazil
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of E. coli from Cheese Samples and Determination of the Phenotypic Profile of Antimicrobial Resistance
2.2. Determination of the Genotypic Profile of Antimicrobial Resistance of E. coli Isolates
3. Material and Methods
3.1. Sample Collection
3.2. Enumeration of Thermotolerant Coliforms and Isolation of E. coli
3.3. Bacterial DNA Extraction
3.4. Identification of E. coli
3.5. Antimicrobial Susceptibility Profile of E. coli
3.6. Detection of Antimicrobial Resistance Genes in E. coli
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carneiro Aguiar, R.A.; Ferreira, F.A.; Dias, R.S.; Nero, L.A.; Miotto, M.; Verruck, S.; De Marco, I.; De Dea Lindner, J. Graduate student literature review: Enterotoxigenic potential and antimicrobial resistance of Staphylococci from Brazilian artisanal raw milk cheeses. J. Dairy Sci. 2022, 105, 5685–5699. [Google Scholar] [CrossRef] [PubMed]
- Castro, C. Brazil: Dairy and Products Annual USDA. 2023. Available online: https://fas.usda.gov/data/brazil-dairy-and-products-annual-10 (accessed on 14 April 2025).
- Siqueira, K.B. Na Era do Consumidor: Uma Visão do Mercado Lácteo Brasileiro; Edição do Autor: Juiz de Fora, MG, Brazil, 2021; 220p, Available online: http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1134890 (accessed on 14 April 2025).
- Rocha, R.S.; Silva, R.; Guimarães, J.T.; Balthazar, C.F.; Pimentel, T.C.; Neto, R.P.C.; Tavares, M.I.B.; Esmerino, E.A.; Freitas, M.Q.; Cappato, L.P.; et al. Possibilities for using ohmic heating in Minas Frescal cheese production. Food Res. Int. 2020, 131, 109027. [Google Scholar] [CrossRef]
- Mercado Comum do Sul. Instrução Normativa n° 4, de 1 de Março de 2004. Technical Regulation on Minas Frescal Cheese—Standards of Identity and Quality. 2004. Available online: https://www.gov.br/agricultura/pt-br/assuntos/defesa-agropecuaria/suasa/regulamentos-tecnicos-de-identidade-e-qualidade-de-produtos-de-origem-animal-1/rtiq-leite-e-seus-derivados (accessed on 14 April 2025).
- Teider, P.I.; Ribeiro, J.C.; Ossugui, E.H.; Tamanini, R.; Ribeiro, J.; Santos, G.A.; Alfieri, A.A.; Beloti, V. Pseudomonas spp. and other psychrotrophic microorganisms in inspected and non-inspected Brazilian Minas Frescal Cheese: Proteolytic, lipolytic and AprX production potential. Pesqui. Vet. Bras. 2019, 39, 807–815. [Google Scholar] [CrossRef]
- de Campos, A.C.L.P.; Puño-Sarmiento, J.J.; Medeiros, L.P.; Gazal, L.E.S.; Maluta, R.P.; Navarro, A.; Kobayashi, R.K.T.; Fagan, E.P.; Nakazato, G. Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathog. Dis. 2018, 15, 94–100. [Google Scholar] [CrossRef]
- Camargo, A.C.; de Araújo, J.P.A.; Fusieger, A.; de Carvalho, A.F.; Nero, L.A. Microbiological quality and safety of Brazilian artisanal cheeses. Braz. J. Microbiol. 2021, 52, 393–409. [Google Scholar] [CrossRef]
- Cardozo, M.V.; Nespolo, N.; Delfino, T.C.; Almeida, C.C.; Pizauro, L.J.L.; Valmorbida, M.K.; Pereira, N.; Ávila, F.A. Raw milk cheese as a potential infection source of pathogenic and toxigenic foodborne pathogens. Food Sci. Technol. 2021, 41, 355–358. [Google Scholar] [CrossRef]
- Pena, R.H.R.; Freitas, F.; Castro, B.G. Hygienic-sanitary quality and antimicrobial sensitivity profile of Escherichia coli in milk and cheese sold illegally in municipalities of northern Mato Grosso, Brazil. Arq. Inst. Biol. 2021, 88, e0702019. [Google Scholar] [CrossRef]
- Oliveira, M.D.; Santos, I.G.; Dias, B.P.; Nascimento, C.A.; Rodrigues, É.M.; Ribeiro Júnior, J.C.; Alfieri, A.A.; Alexandrino, B. Hygienic-health quality and microbiological hazard of clandestine Minas Frescal cheese commercialized in north Tocantins, Brazil. Semin. Cienc. Agrar. 2021, 42, 679–694. [Google Scholar] [CrossRef]
- Pineda, A.P.A.; Campos, G.Z.; Pimentel-Filho, N.J.; Franco, B.D.G.M.; Pinto, U.M. Brazilian Artisanal Cheeses: Diversity, Microbiological Safety, and Challenges for the Sector. Front. Microbiol. 2021, 12, 666922. [Google Scholar] [CrossRef] [PubMed]
- Pakbin, B.; Brück, W.M.; Rossen, J.W.A. Virulence factors of enteric pathogenic Escherichia coli: A review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.Y.; Lupo, A.; Schink, A.K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef]
- Fang, J.; Shen, Y.; Qu, D.; Han, J. Antimicrobial resistance profiles and characteristics of integrons in Escherichia coli strains isolated from a large-scale centralized swine slaughterhouse and its downstream markets in Zhejiang, China. Food Control 2019, 95, 215–222. [Google Scholar] [CrossRef]
- Stasiak, M.; Mackiw, E.; Kowalska, J.; Kucharek, K.; Postupolski, J. Silent genes: Antimicrobial resistance and antibiotic production. Pol. J. Microbiol. 2021, 70, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Lipszyc, A.; Szuplewska, M.; Bartosik, D. How do transposable elements activate expression of transcriptionally silent antibiotic resistance genes? Int. J. Mol. Sci. 2022, 23, 8063. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, W.; Hu, X.; Xie, C.; Yang, X.; Li, C.; Li, G.; Lu, Y.; You, X. Silent or low expression of blaTEM and blaSHV suggests potential for targeted proteomics in clinical detection of β-lactamase-related antimicrobial resistance. J. Pharm. Anal. 2025, 15, 101220. [Google Scholar] [CrossRef]
- Deekshit, V.K.; Srikumar, S. ‘To be, or not to be’—The dilemma of ‘silent’ antimicrobial resistance genes in bacteria. J. Appl. Microbiol. 2022, 133, 2902–2914. [Google Scholar] [CrossRef]
- Vk, D.; Srikumar, S.; Shetty, S.; Van Nguyen, S.; Karunasagar, I.; Fanning, S. Silent antibiotic resistance genes: A threat to antimicrobial therapy. Int. J. Infect. Dis. 2019, 79, 20. [Google Scholar] [CrossRef]
- Ombarak, R.A.; Hinenoya, A.; Elbagory, A.M.; Yamasaki, S. Prevalence and molecular characterization of antimicrobial resistance in Escherichia coli isolated from raw milk and raw milk cheese in Egypt. J. Food Prot. 2018, 81, 226–232. [Google Scholar] [CrossRef]
- Shoaib, M.; He, Z.; Geng, X.; Tang, M.; Hao, R.; Wang, S.; Shang, R.; Wang, X.; Zhang, H.; Pu, W. The emergence of multi-drug resistant and virulence gene carrying Escherichia coli strains in the dairy environment: A rising threat to the environment, animal, and public health. Front. Microbiol. 2023, 14, 1197579. [Google Scholar] [CrossRef]
- Belaynehe, K.M.; Shin, S.W.; Yoo, H.S. Interrelationship between tetracycline resistance determinants, phylogenetic group affiliation and carriage of class 1 integrons in commensal Escherichia coli isolates from cattle farms. BMC Vet. Res. 2018, 14, 340. [Google Scholar] [CrossRef]
- Brasil. Agência Nacional de Vigilância Sanitária. Instrução Normativa N° 161, 1 de Julho de 2022. Establishes the Microbiological Standards for Food. 2022. Available online: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-in-n-161-de-1-de-julho-de-2022-413366880 (accessed on 14 April 2025).
- Mohamed, M.-Y.I.; Habib, I. Pathogenic E. coli in the food chain across the Arab countries: A descriptive review. Foods 2023, 12, 3726. [Google Scholar] [CrossRef] [PubMed]
- Barbau-Piednoir, E.; Denayer, S.; Botteldoorn, N.; Dierick, K.; De Keersmaecker, S.C.J.; Roosens, N.H. Detection and discrimination of five E. coli pathotypes using a combinatory SYBR® Green qPCR screening system. Appl. Microbiol. Biotechnol. 2018, 102, 3267–3285. [Google Scholar] [CrossRef]
- Molina, F.; López-Acedo, E.; Tabla, R.; Roa, I.; Gómez, A.; Rebollo, J.E. Improved detection of Escherichia coli and coliform bacteria by multiplex PCR. BMC Biotechnol. 2015, 15, 48. [Google Scholar] [CrossRef]
- Zarei Ahmady, A.; Aliyan Aliabadi, R.; Amin, M.; Ameri, A.; Abbasi Montazeri, E. Occurrence of diarrheagenic Escherichia coli pathotypes from raw milk and unpasteurized buttermilk by culture and multiplex polymerase chain reaction in southwest Iran. Mol. Biol. Rep. 2023, 50, 3661–3667. [Google Scholar] [CrossRef]
- Alsanjary, L.H.; Sheet, O.H. Molecular detection of uidA gene in Escherichia coli isolated from the dairy farms in Nineveh governorate, Iraq. Iraqi J. Vet. Sci. 2022, 36, 599–603. [Google Scholar] [CrossRef]
- Ribeiro, L.F.; Rossi, G.A.M.; Sato, R.A.; de Souza Pollo, A.; Cardozo, M.V.; Amaral, L.A.; Fairbrother, J.M. Epidemiology, virulence and antimicrobial resistance of Escherichia coli isolated from small Brazilian farms producers of raw milk fresh cheese. Microorganisms 2024, 12, 1739. [Google Scholar] [CrossRef]
- Messele, Y.E.; Abdi, R.D.; Tegegne, D.T.; Bora, S.K.; Babura, M.D.; Emeru, B.A.; Weird, G.M. Analysis of milk-derived isolates of E. coli indicating drug resistance in central Ethiopia. Trop. Anim. Health Prod. 2019, 51, 661–667. [Google Scholar] [CrossRef]
- Hassanien, A.A.; Shaker, E.M. Investigation of the effect of chitosan and silver nanoparticles on the antibiotic resistance of Escherichia coli O157:H7 isolated from some milk products and diarrheal patients in Sohag city, Egypt. Vet. World 2020, 13, 1647–1653. [Google Scholar] [CrossRef] [PubMed]
- Joubrane, K.; Jammoul, A.; Daher, R.; Ayoub, S.; El Jed, M.; Hneino, M.; El Hawari, K.; Al Iskandarani, M.; Daher, Z. Microbiological contamination, antimicrobial residues, and antimicrobial resistance in raw bovine milk in Lebanon. Int. Dairy J. 2022, 134, 105455. [Google Scholar] [CrossRef]
- Food and Drug Administration. Antimicrobials Sold or Distributed for Use in Food-Producing Animals. 2021. Available online: https://www.fda.gov/animal-veterinary/cvm-updates/fda-releases-annual-summary-report-antimicrobials-sold-or-distributed-2021-use-food-producing (accessed on 14 April 2025).
- Kasem, N.G.; Al-Ashmawy, M.; Elsherbini, M.; Abdelkhalek, A. Antimicrobial resistance and molecular genotyping of Escherichia coli and Staphylococcus aureus isolated from some Egyptian cheeses. J. Adv. Vet. Anim. Res. 2021, 8, 246–255. [Google Scholar]
- Hussein, N.D.; Hassan, J.W.; Osman, M.; El-Omari, K.; Kharroubi, S.A.; Toufeili, I.; Kassem, I.I. Assessment of the microbiological acceptability of white cheese (Akkawi) in Lebanon and the antimicrobial resistance profiles of associated Escherichia coli. Antibiotics 2023, 12, 610. [Google Scholar] [CrossRef]
- Adzitey, F.; Yussif, S.; Ayamga, R.; Zuberu, S.; Addy, F.; Adu-Bonsu, G.; Huda, N.; Kobun, R. Antimicrobial susceptibility and molecular characterization of Escherichia coli recovered from milk and related samples. Microorganisms 2022, 10, 1335. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, Y.; Jiao, X.; Tang, B.; Feng, T.; Li, P.; Fang, J. In vivo fitness of sul gene-dependent sulfonamide-resistant Escherichia coli in the mammalian gut. mSystems 2024, 9, e0083624. [Google Scholar] [CrossRef]
- Los Santos, E.; Laviña, M.; Poey, M.E. Strict relationship between class 1 integrons and resistance to sulfamethoxazole in Escherichia coli. Microb. Pathog. 2021, 161, 105206. [Google Scholar] [CrossRef]
- Sánchez-Osuna, M.; Cortés, P.; Barbé, J.; Erill, I. Origin of the mobile di-hydro-pteroate synthase gene determining sulfonamide resistance in clinical isolates. Front. Microbiol. 2019, 9, 3332. [Google Scholar] [CrossRef] [PubMed]
- Kuzeubayeva, A.; Ussenbayev, A.; Aydin, A.; Akanova, Z.; Rychshanova, R.; Abdullina, E.; Seitkamzina, D.; Sakharia, L.; Ruzmatov, S. Contamination of Kazakhstan cheeses originating from Escherichia coli and its resistance to antimicrobial drugs. Vet. World 2024, 17, 361–370. [Google Scholar] [CrossRef]
- Roberts, M.C.; Schwarz, S. Tetracycline and phenicol resistance genes and mechanisms: Importance for agriculture, the environment, and humans. J. Environ. Qual. 2016, 45, 576–592. [Google Scholar] [CrossRef]
- Grossman, T.H. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025387. [Google Scholar] [CrossRef]
- Perewari, D.O.; Otokunefor, K.; Agbagwa, O.E. Tetracycline-resistant genes in Escherichia coli from clinical and nonclinical sources in rivers state, Nigeria. Int. J. Microbiol. 2022, 2022, 9192424. [Google Scholar] [CrossRef] [PubMed]
- Møller, T.S.B.; Overgaard, M.; Nielsen, S.S.; Bortolaia, V.; Sommer, M.O.A.; Guardabassi, L.; Olsen, J.E. Relation between tetR and tetA expression in tetracycline resistant Escherichia coli. BMC Microbiol. 2016, 16, 39. [Google Scholar] [CrossRef] [PubMed]
- Tabaran, A.; Mihaiu, M.; Tăbăran, F.; Colobatiu, L.; Reget, O.; Borzan, M.M.; Dan, S.D. First study on characterization of virulence and antibiotic resistance genes in verotoxigenic and enterotoxigenic E. coli isolated from raw milk and unpasteurized traditional cheeses in Romania. Folia Microbiol. 2017, 62, 145–150. [Google Scholar] [CrossRef]
- Salinas, L.; Loayza, F.; Cárdenas, P.; Saraiva, C.; Johnson, T.J.; Amato, H.; Graham, J.P.; Trueba, G. Environmental spread of extended spectrum beta-lactamase (ESBL) producing Escherichia coli and ESBL genes among children and domestic animals in Ecuador. Environ. Health Perspect. 2021, 129, 27007. [Google Scholar] [CrossRef]
- Bastidas-Caldes, C.; Romero-Alvarez, D.; Valdez-Vélez, V.; Morales, R.D.; Montalvo-Hernández, A.; Gomes-Dias, C.; Calvopiña, M. Extended-spectrum beta-lactamases producing Escherichia coli in South America: A systematic review with a one health perspective. Infect. Drug Resist. 2022, 15, 5759–5779. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Eldesoukey, I.E.; Elmonir, W.; Alouffi, A.; Beleta, E.I.M.; Kelany, M.A.; Elnahriry, S.S.; Alghonaim, M.I.; alZeyadi, Z.A.; Elaadli, H. Multidrug-resistant enteropathogenic Escherichia coli isolated from diarrhoeic calves, milk, and workers in dairy farms: A potential public health risk. Antibiotics 2022, 11, 999. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa MAPA n° 09, de 27 de Junho de 2003. The Manufacture, Handling, Splitting, Marketing, Importation, and Use of the Active Ingredient’s Chloramphenicol and Nitrofurans, as Well as Any Products Containing These Substances, Are Prohibited for Veterinary Use and for Any Use in the Feeding of All Animals and Insects. Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-pecuarios/resistencia-aos-antimicrobianos/legislacao/proibicoes-de-aditivos-na-alimentacao-animal (accessed on 14 April 2025).
- Feng, P.; Weagant, S.D.; Grant, M.A.; Burkhardt, W. Chapter 4: Enumeration of Escherichia coli and the Coliform bacteria. In Bacteriological Analytical Manual (BAM); FDA: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-4-enumeration-escherichia-coli-and-coliform-bacteria (accessed on 14 April 2025).
- Clinical & Laboratory Standards Institute. M02 Performance Standards for Antimicrobial Susceptibility Testing, 14th ed.; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- World Organization for Animal Health. Annual Report on Antimicrobial Agents Intended for Use in Animals, 8th ed.; World Organization for Animal Health: Paris, France, 2024; pp. 1–44. [Google Scholar]
- Virto, M.; Santamarina-García, G.; Amores, G.; Hernández, I. Antibiotics in dairy production: Where is the problem? Dairy 2022, 3, 541–564. [Google Scholar] [CrossRef]
- Le, T.A.; Hiba, T.; Chaudhari, D.; Preston, A.N.; Palowsky, Z.R.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. Aminoglycoside-related nephrotoxicity and ototoxicity in clinical practice: A review of pathophysiological mechanism and treatment options. Adv. Ther. 2023, 40, 1357–1365. [Google Scholar] [CrossRef]
- Brito, D.A.P.; Costa, F.N. Antimicrobial resistance of bacterial agents of bovine mastitis from dairy properties in the metropolitan region of São Luís—MA. Rev. Bras. Saúde Prod. Anim. 2024, 25, e20230033. [Google Scholar] [CrossRef]
- European Union. Commission Delegated Regulation (EU) 2022/1255 of 19 May 2022 supplementing Regulation (EU) 2019/6 of the European Parliament and of the Council by establishing the criteria for the designation of antimicrobials to be reserved for the treatment of certain infections in humans. Off. J. Eur. Union 2022, 20, 7. [Google Scholar]
- Qiu, J.; Jiang, Z.; Ju, Z.; Zhao, X.; Yang, J.; Guo, H.; Sun, S. Molecular and phenotypic characteristics of Escherichia coli isolates from farmed minks in Zhucheng, China. BioMed Res. Int. 2019, 2019, 3917841. [Google Scholar] [CrossRef]
- Arabi, H.; Pakzad, I.; Nasrollahi, A.; Hosainzadegan, H.; Azizi Jalilian, F.; Taherikalani, M.; Samadi, N.; Monadi Sefidan, A. Sulfonamide resistance genes (sul) M in extended spectrum beta lactamase (ESBL) and non-ESBL producing Escherichia coli Isolated from Iranian hospitals. Jundishapur J. Microbiol. 2015, 8, e19961. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Clegg, P.D.; Williams, N.J.; Baptiste, K.E.; Bennett, M. Antimicrobial resistance in equine faecal Escherichia coli isolates from North West England. Ann. Clin. Microbiol. Antimicrob. 2010, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.A.; Tyler, S.; Christianson, S.; McGeer, A.; Muller, M.P.; Willey, B.M.; Bryce, E.; Gardam, M.; Nordmann, P.; Mulvey, M.R. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. Antimicrob. Agents Chemother. 2004, 48, 3758–3764. [Google Scholar] [CrossRef] [PubMed]
- Gundran, R.S.; Cardenio, P.A.; Villanueva, M.A.; Sison, F.B.; Benigno, C.C.; Kreausukon, K.; Pichpol, D.; Punyapornwithaya, V. Prevalence and distribution of blaCTX-M, blaSHV, blaTEM genes in extended- spectrum β- lactamase- producing E. coli isolates from broiler farms in the Philippines. BMC Vet. Res. 2019, 15, 227. [Google Scholar] [CrossRef] [PubMed]
- Van, T.T.; Chin, J.; Chapman, T.; Tran, L.T.; Coloe, P.J. Safety of raw meat and shellfish in Vietnam: An analysis of Escherichia coli isolations for antibiotic resistance and virulence genes. Int. J. Food Microbiol. 2008, 124, 217–223. [Google Scholar] [CrossRef]
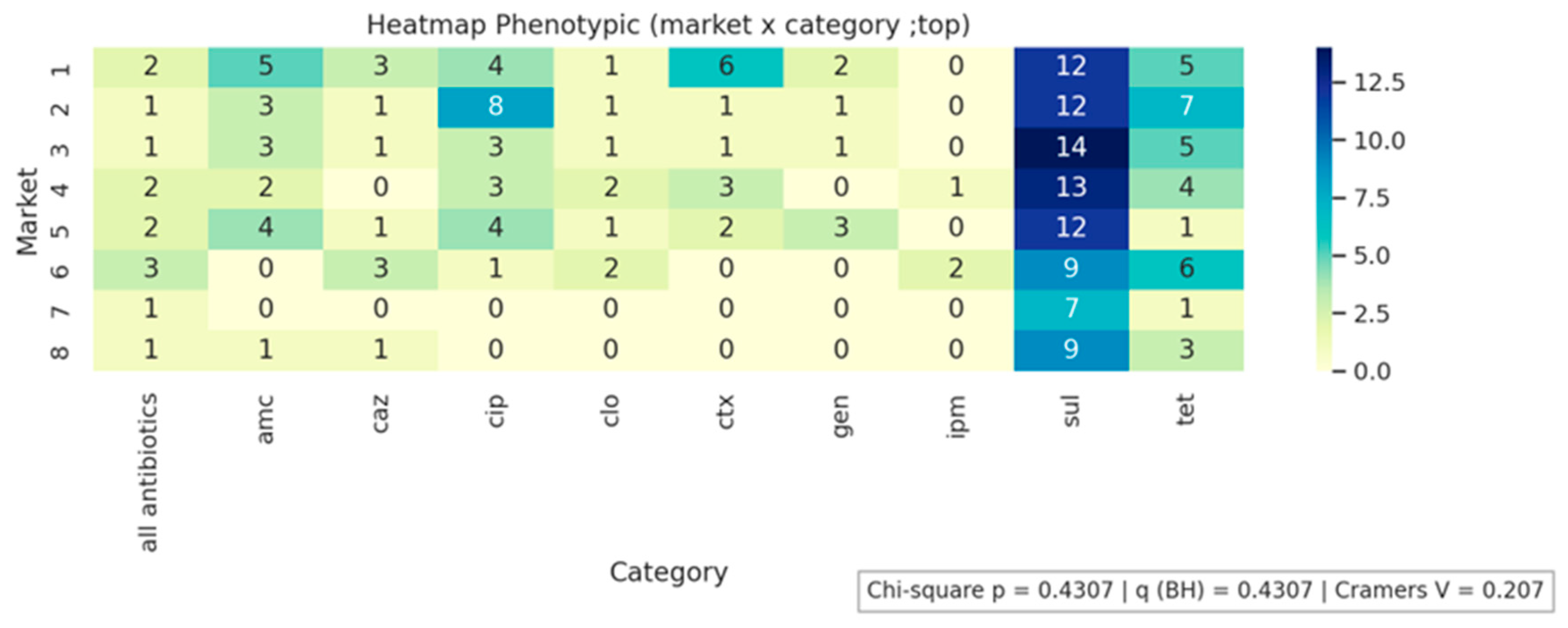
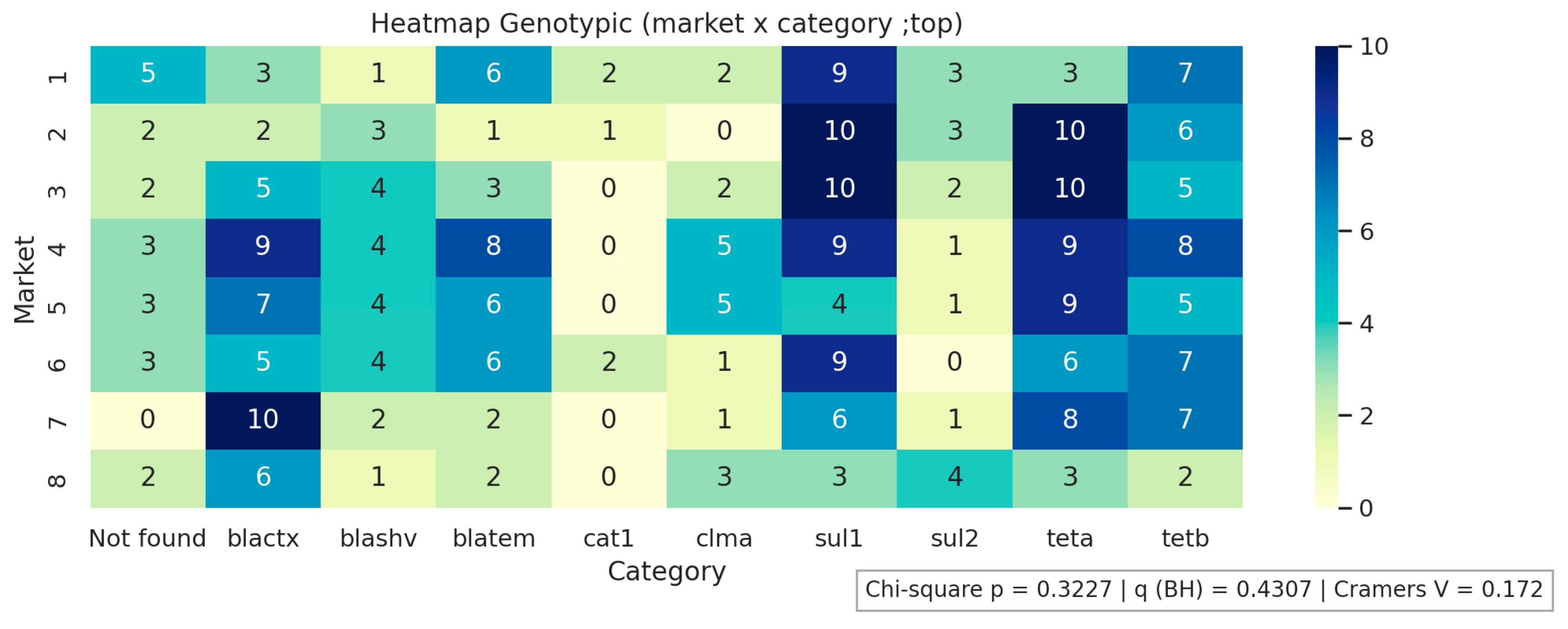
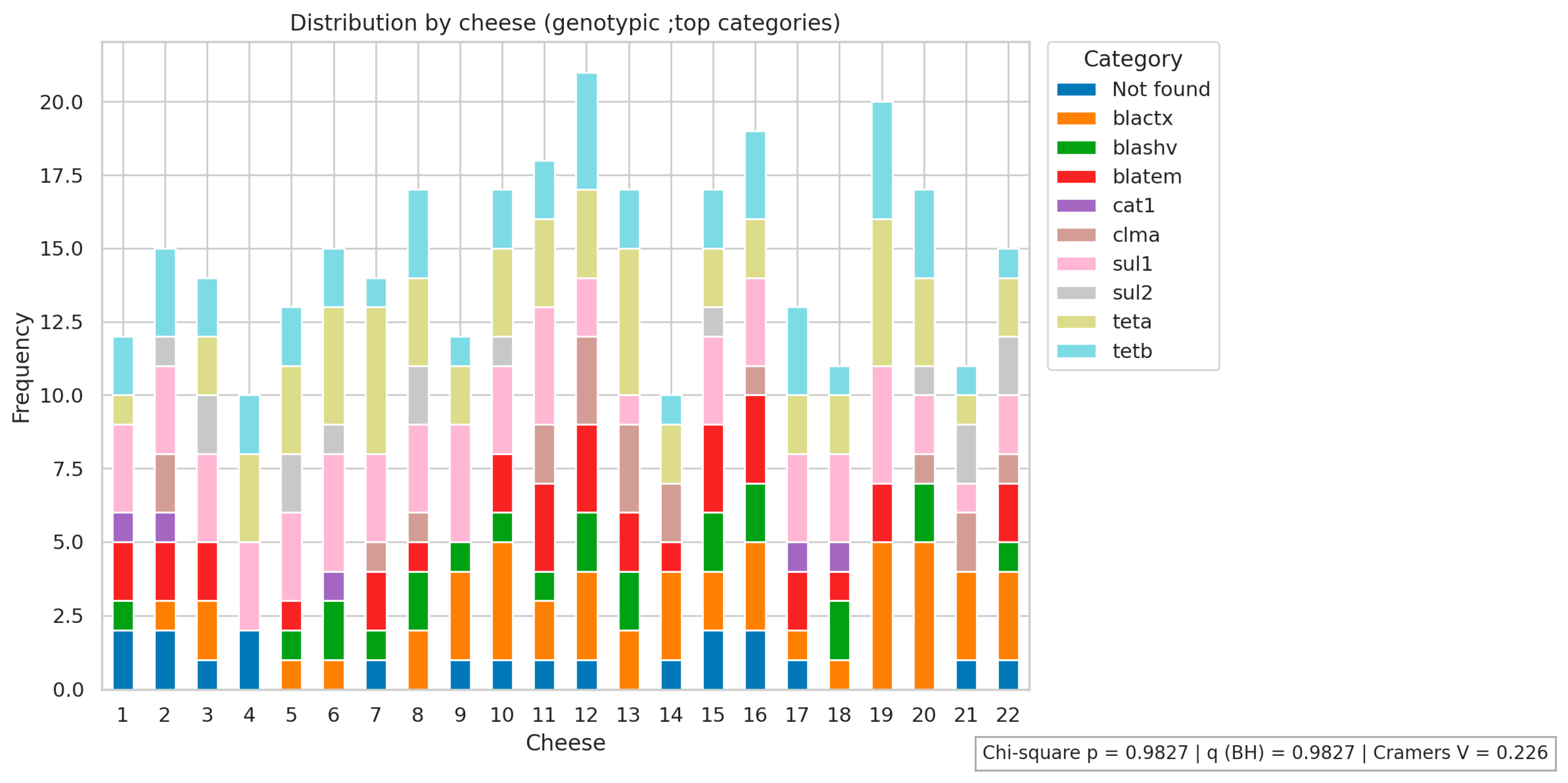
| Antimicrobials | R % (n) | I % (n) | S % (n) |
|---|---|---|---|
| Amoxicillin * (AMC) | 19.23 (20) | 3.85 (4) | 76.92 (80) |
| Cefotaxime (CTX) | 13.46 (14) | 6.73 (7) | 79.81 (83) |
| Ceftazidime (CAZ) | 10.58 (11) | 5.77 (6) | 83.65 (87) |
| Ciprofloxacin (CIP) | 23.08 (24) | 18.27 (19) | 58.65 (61) |
| Chloramphenicol (CLO) | 7.69 (8) | 2.88 (3) | 89.43 (93) |
| Gentamicin (GEN) | 8.65 (9) | 19.23 (20) | 72.12 (75) |
| Imipenem (IMP) | 2.88 (3) | 3.85 (4) | 93.27 (97) |
| Sulfonamide (SUL) | 85.58 (89) | 4.81 (5) | 9.61 (10) |
| Tetracycline (TET) | 38.46 (40) | 2.88 (3) | 58.65 (61) |
| Isolates | Antibiotics | n (%) |
|---|---|---|
| 1 | SUL | 34 (32.7) |
| 2 | TET | 2 (1.92) |
| 3 | SUL–AMC | 1 (0.96) |
| 4 | SUL–CAZ | 3 (2.88) |
| 5 | SUL–CIP | 4 (3.86) |
| 6 | SUL–CLO | 1 (0.96) |
| 7 | SUL–CTX | 1 (0.96) |
| 8 | SUL–GEN | 3 (2.88) |
| 9 | SUL–TET | 8 (7.69) |
| 10 | SUL–IMP | 1 (0.96) |
| 11 | SUL–CTX–CIP | 1 (0.96) |
| 12 | SUL–TET–CIP | 6 (5.76) |
| 13 | SUL–AMC–CIP | 2 (1.92) |
| 14 | SUL–CTX–AMC | 1 (0.96) |
| 15 | SUL–TET–AMC | 2 (1.92) |
| 16 | SUL–GEN–CIP | 1 (0.96) |
| 17 | SUL–CTX–TET–AMC | 2 (1.92) |
| 18 | SUL–CLO–AMC–CIP | 1 (0.96) |
| 19 | SUL–CTX–AMC–CAZ | 3 (2.88) |
| 20 | SUL–CLO–TET–CIP | 1 (0.96) |
| 21 | SUL–TET–AMC–CIP | 2 (1.92) |
| 22 | SUL–TET–GEN–CIP | 1 (0.96) |
| 23 | SUL–CLO–TET–AMC | 1 (0.96) |
| 24 | SUL–CTX–TET–AMC–CIP | 1 (0.96) |
| 25 | SUL–CLO–CTX–TET–CIP | 1 (0.96) |
| 26 | SUL–CLO–TET–IPM–CAZ | 2 (1.92) |
| 27 | SUL–CTX–TET–GEN–CAZ | 1 (0.96) |
| 28 | SUL–TET–GEN–AMC–CIP | 1 (0.96) |
| 29 | SUL–CTX–TET–AMC–CIP–CAZ | 1 (0.96) |
| 30 | SUL–CTX–TET–GEN–AMC–CIP | 1 (0.96) |
| 31 | SUL–CLO–CTX–TET–GEN–AMC–CAZ | 1 (0.96) |
| Total | 91 (87.5) | |
| Antimicrobial Resistance Genes | Total of E. coli Strains n (%) | Phenotypically Resistant E. coli n (%) | Phenotypically Susceptible E. coli n (%) |
|---|---|---|---|
| Sul genes | |||
| sul1 | 51 (49.04) | 48 (45.15) | 3 (2.88) |
| sul2 | 5 (4.81) | 5 (4.81) | 0 |
| sul1 + sul2 | 9 (8.65) | 9 (8.65) | 0 |
| Total sul genes | 65 (62.50) | 62 (59.61) | 3 (2.88) |
| Tet genes | |||
| tetA | 22 (21.15) | 11 (10.57) | 11 (10.57) |
| tetB | 10 (9.61) | 3 (2.88) | 7 (6.73) |
| tetA + tetB | 36 (34.61) | 23 (22.12) | 13 (12.50) |
| Total tet genes | 68 (65.38) | 37 (35.56) | 31 (29.80) |
| Bla genes | |||
| blactx-M | 22 (21.15) | 1 (0.96) | 20 (20.19) |
| blaTEM | 9 (8.65) | 3 (2.88) | 6 (5.77) |
| blaSHV | 10 (9.61) | 3 (2.88) | 7 (6.73) |
| blactx-M + blaTEM | 14 (13.46) | 8 (7.69) | 6 (5.77) |
| blactx-M + blaSHV | 3 (2.88) | 1 (0.96) | 2 (1.92) |
| blaSHV + blaTEM | 3 (2.88) | 1 (0.96) | 2 (1.92) |
| blactx-M + blaTEM + blaSHV | 5 (4.80) | 3 (2.88) | 2 (1.92) |
| Total bla genes | 58 (55.77) | 20 (19.23) | 45 (43.26) |
| Cat1 and clmA genes | |||
| cat1 | 4 (3.85) | 1 (0.96) | 3 (2.88) |
| clmA | 18 (17.31) | 1 (0.96) | 17 (16.35) |
| cat1 + clmA | 1 (0.96) | 0 | 1 (0.96) |
| Total cat1 and clmA | 23 (22.12) | 2 (1.92) | 21 (20.19) |
| Isolates | Genes | n | % |
|---|---|---|---|
| 1 | clmA–blaCTX-M–sul1–tetA–tetB–blaTEM–blaSHV | 1 | 0.96% |
| 2 | blaCTX-M–sul1–tetA–tetB–blaTEM–blaSHV | 2 | 1.92% |
| 3 | blaCTX-M–sul1–sul2–tetA–tetB–blaSHV | 1 | 0.96% |
| 4 | blaCTX-M–sul1–sul2–tetA–tetB–blaTEM | 2 | 1.92% |
| 5 | clmA–blaCTX-M–sul1–tetA–tetB–blaSHV | 1 | 0.96% |
| 6 | blaCTX-M–sul1–sul2–tetA–blaSHV | 1 | 0.96% |
| 7 | blaCTX-M–sul1–sul2–tetA–tetB | 1 | 0.96% |
| 8 | blaCTX-M–sul1–tetA–blaTEM–blaSHV | 1 | 0.96% |
| 9 | blaCTX-M–sul1–tetA–tetB–blaSHV | 1 | 0.96% |
| 10 | blaCTX-M–sul1–tetA–tetB–blaTEM | 5 | 4.81% |
| 11 | blaCTX-M–sul1–tetB–blaTEM–blaSHV | 1 | 0.96% |
| 12 | cat1–blaCTX-M–sul1–tetB–blaTEM | 1 | 0.96% |
| 13 | cat1–clmA–sul1–tetB–blaTEM | 1 | 0.96% |
| 14 | clmA–blaCTX-M–sul1–tetA–tetB | 1 | 0.96% |
| 15 | clmA–blaCTX-M–sul1–sul2–tetB | 1 | 0.96% |
| 16 | clmA–blaCTX-M–tetA–tetB–blaTEM | 1 | 0.96% |
| 17 | sul1–sul2–tetA–tetB–blaSHV | 1 | 0.96% |
| 18 | sul1–sul2–tetA–blaTEM–blaSHV | 1 | 0.96% |
| 19 | sul1–sul2–tetA–tetB–blaSHV | 1 | 0.96% |
| 20 | sul1–tetA–tetB–blaTEM–blaSHV | 1 | 0.96% |
| 21 | blaCTX-M–sul1–tetA–blaTEM | 1 | 0.96% |
| 22 | blaCTX-M–sul2–tetA–tetB | 1 | 0.96% |
| 23 | blaCTX-M–tetA–tetB–blaTEM | 1 | 0.96% |
| 24 | blaCTX-M–sul1–tetB–blaTEM | 1 | 0.96% |
| 25 | blaCTX-M–sul1–tetA–tetB | 2 | 1.92% |
| 26 | cat1–blaCTX-M–sul1–tetA | 1 | 0.96% |
| 27 | clmA–blaCTX-M–sul2–blaTEM | 1 | 0.96% |
| 28 | clmA–blaCTX-M–tetA–tetB | 1 | 0.96% |
| 29 | clmA–blaCTX-M–tetB–blaTEM | 1 | 0.96% |
| 30 | clmA–sul1–tetA–blaSHV | 1 | 0.96% |
| 31 | clmA–sul1–tetA–tetB | 1 | 0.96% |
| 32 | clmA–tetA–tetB–blaSHV | 1 | 0.96% |
| 33 | sul1–tetA–blaTEM–blaSHV | 1 | 0.96% |
| 34 | sul1–tetA–tetB–blaSHV | 2 | 1.92% |
| 35 | sul1–tetA–tetB–blaTEM | 3 | 2.88% |
| 36 | blaCTX-M–sul1–sul2 | 1 | 0.96% |
| 37 | blaCTX-M–sul1–tetA | 3 | 2.88% |
| 38 | blaCTX-M–tetA–blaTEM | 1 | 0.96% |
| 39 | blaCTX-M–sul1–tetB | 1 | 0.96% |
| 40 | blaCTX-M–tetA–tetB | 2 | 1.92% |
| 41 | cat1–blaCTX-M–tetA | 1 | 0.96% |
| 42 | cat1–sul1–blaTEM | 1 | 0.96% |
| 43 | clmA–sul1–blaTEM | 1 | 0.96% |
| 44 | clmA–sul1–tetA | 1 | 0.96% |
| 45 | clmA–tetA–blaTEM | 1 | 0.96% |
| 46 | sul1–tetA–tetB | 4 | 3.85% |
| 47 | sul1–tetB–blaSHV | 1 | 0.96% |
| 48 | sul1–tetB–blaTEM | 1 | 0.96% |
| 49 | blaCTX-M–blaSHV | 1 | 0.96% |
| 50 | blaCTX-M–sul2 | 1 | 0.96% |
| 51 | blaCTX-M–blaTEM | 1 | 0.96% |
| 52 | blaCTX-M–tetB | 1 | 0.96% |
| 53 | blaCTX-M–tetA | 1 | 0.96% |
| 54 | clmA–blaCTX-M | 3 | 2.88% |
| 55 | clmA–sul2 | 1 | 0.96% |
| 56 | sul1–blaSHV | 3 | 2.88% |
| 57 | sul1–blaTEM | 2 | 1.92% |
| 58 | sul1–tetA | 3 | 2.88% |
| 59 | sul2–tetA | 1 | 0.96% |
| 60 | tetA | 2 | 1.92% |
| Total | 84 | 80.77% |
| Gene | Primer Sequence (5′ → 3′) | Product Size (bp) | Reference |
|---|---|---|---|
| lacZB | F: ATGAAAGCTGGCTACAGGAAGGCC R: CACCATGCCGTGGGITICAATATT | 876 | Molina et al. [27] |
| uidA | F: TGGTAATTACCGACGAAAACGGC R: ACGCGTGGTTACAGTCTTGCG | 162 | Molina et al. [27] |
| Antimicrobials | Concentration | Class | S (mm) | I (mm) | R (mm) |
|---|---|---|---|---|---|
| Amoxicillin * (AMC) | 20 + 10 μg | β-lactam/Penicillin | ≤13 | 14–17 | ≥18 |
| Cefotaxime (CTX) | 30 μg | β-lactam/Cephalosporin | ≤22 | 23–25 | ≥26 |
| Ceftazidime (CAZ) | 30 μg | β-lactam/Cephalosporin | ≤17 | 18–20 | ≥21 |
| Ciprofloxacin (CIP) | 5 μg | Quinolone | ≤21 | 22–25 | ≥26 |
| Chloramphenicol (CLO) | 30 μg | Phenicol | ≤12 | 13–17 | ≥18 |
| Gentamicin (GEN) | 10 μg | Aminoglycoside | ≤12 | 13–14 | ≥15 |
| Imipenem (IMP) | 10 μg | β-lactam/Carbapenem | ≤19 | 20–22 | ≥23 |
| Sulfonamide (SUL) | 300 μg | Sulfonamide | ≤13 | 14–17 | ≥18 |
| Tetracycline (TET) | 30 μg | Tetracycline | ≤22 | 23–25 | ≥26 |
| Gene | Primer Sequence (5′ → 3′) F and R | bp | PCR Conditions | Reference |
|---|---|---|---|---|
| sul1 | CTTCGATGAGAGCCGGCGGC GCAAGGCGGAAACCCGCGCC | 238 | Initial denaturation at 94 °C for 5 min; 30 cycles of denaturation at 94 °C for 60 s; annealing at 56 °C for 60 s; extension at 68 °C for 60; 72 °C for 10 min for final extension | Qiu et al. [59] |
| sul2 | GCGCTCAAGGCAGATGGCATT GCGTTTGATACCGGCACCCGT | 293 | Initial denaturation at 95 °C for 10 min; 35 cycles of denaturation at 94 °C for 45 s; annealing at 55 °C for 50 s; extension at 72 °C for 50; 72 °C for 10 min for final extension | Arabi et al. [60] |
| tetA | GGCGGTCTTCTTCATCATGC CGGCAGGCAGAGCAAGTAGA | 502 | Initial denaturation at 94 °C for 60 s; 30 cycles of denaturation at 95 °C for 60 s; annealing at 55 °C for 60 s; extension at 72 °C for 60 s; 72 °C for 8 min for final extension | Belaynehe et al. [23] |
| tetB | TTGGTTAGGGGCAAGTTTTG GTAATGGGCCAATAACACCG | 659 | Initial denaturation at 94 °C for 60 s; 30 cycles of denaturation at 95 °C for 30 s; annealing at 55 °C for 30 s; extension at 72 °C for 60 s; 72 °C for 8 min for final extension | Ahmed et al. [61] |
| blactx-M | ATGTGCAGYACCAGTAARGTKATGGC TGGGTRAARTARGTSACCAGAAYCAGCGG | 592 | Initial denaturation at 94 °C for 60 s; 36 cycles of denaturation at 94° C for 30 s; annealing at 58 °C for 60 s; extension at 72 °C for 60 s; 72 °C for 10 min for final extension | Boyd et al. [62] |
| blaTEM | TTGGGTGCACGAGTGGGTTA TAATTGTTGCCGGGAAGCTA | 506 | Initial denaturation at 95 °C for 3 min; 35 cycles of denaturation at 94 °C for 60 s; annealing at 55 °C for 60 s; extension at 72 °C for 1 s; 72 °C for 7 min for final extension | Gundran et al. [58] |
| blaSHV | TCGGGCCGCGTAGGCATGAT AGCAGGGCGACAATCCCGCG | 628 | Initial denaturation at 95 °C for 3 min; 35 cycles of denaturation at 94 °C for 60 s; annealing at 55 °C for 60 s; extension at 72 °C for 1 s; 72 °C for 7 min for final extension | Gundran et al. [63] |
| cat1 | AGTTGCTCAATGTACCTATAACC TTGTAATTCATTAAGCATTCTGCC | 547 | Initial denaturation at 94 °C for 5 min; 30 cycles of denaturation at 94 °C for 30 s; annealing at 50 °C for 30 s; extension at 72 °C for 1 s; 72 °C for 10 min for final extension | Van et al. [64] |
| clmA | CCGCCACGGTGTTGTTGTTATC CACCTTGCCTGCCCATCATTAG | 698 | Initial denaturation at 94 °C for 5 min; 30 cycles of denaturation at 94 °C for 30 s; annealing at 50 °C for 30 s; extension at 72 °C for 1 s; 72 °C for 10 min for final extension | Van et al. [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.F.S.; Melo, R.A.d.; Borges, N.M.; Aragão, A.C.S.; Araújo, M.O.d.; dos Santos, R.D.; Bilac, C.A.; Gomes, K.O.; do Prado, B.A.; Sá Barreto, L.C.L.d.; et al. Antimicrobial Resistance Phenotypes and Genotypes of Escherichia coli Isolates from Artisanal Minas Frescal Cheeses from the Federal District, Brazil. Antibiotics 2025, 14, 1101. https://doi.org/10.3390/antibiotics14111101
Rodrigues LFS, Melo RAd, Borges NM, Aragão ACS, Araújo MOd, dos Santos RD, Bilac CA, Gomes KO, do Prado BA, Sá Barreto LCLd, et al. Antimicrobial Resistance Phenotypes and Genotypes of Escherichia coli Isolates from Artisanal Minas Frescal Cheeses from the Federal District, Brazil. Antibiotics. 2025; 14(11):1101. https://doi.org/10.3390/antibiotics14111101
Chicago/Turabian StyleRodrigues, Letícia Fernandes Silva, Rodrigo Araújo de Melo, Nathalia Mateus Borges, Anna Cléa Silva Aragão, Marta Oliveira de Araújo, Rebeca Dias dos Santos, Carla Azevedo Bilac, Karolina Oliveira Gomes, Bruno Alcântara do Prado, Lívia Cristina Lira de Sá Barreto, and et al. 2025. "Antimicrobial Resistance Phenotypes and Genotypes of Escherichia coli Isolates from Artisanal Minas Frescal Cheeses from the Federal District, Brazil" Antibiotics 14, no. 11: 1101. https://doi.org/10.3390/antibiotics14111101
APA StyleRodrigues, L. F. S., Melo, R. A. d., Borges, N. M., Aragão, A. C. S., Araújo, M. O. d., dos Santos, R. D., Bilac, C. A., Gomes, K. O., do Prado, B. A., Sá Barreto, L. C. L. d., Silva, I. C. R. d., & Orsi, D. C. (2025). Antimicrobial Resistance Phenotypes and Genotypes of Escherichia coli Isolates from Artisanal Minas Frescal Cheeses from the Federal District, Brazil. Antibiotics, 14(11), 1101. https://doi.org/10.3390/antibiotics14111101






