Point Prevalence Survey of Antibiotic Use in Latin American Hospitals: 2022–2023
Abstract
1. Introduction
2. Methodology
2.1. Selection of Hospitals
2.2. Selection of Patients
2.3. Training and Procedures for Data Collection
2.4. Variables and Antibiotics Collected
2.5. Data Collection, Safety, and Review
2.6. Data Analysis
3. Results
3.1. Demographic and Clinical Information of Patients Enrolled
3.2. Antibiotic Use
3.3. Diagnosis Supporting the Indication for Antibiotic Prescriptions
3.4. Compliance with Guidelines for Treatment and Prophylaxis Prescriptions
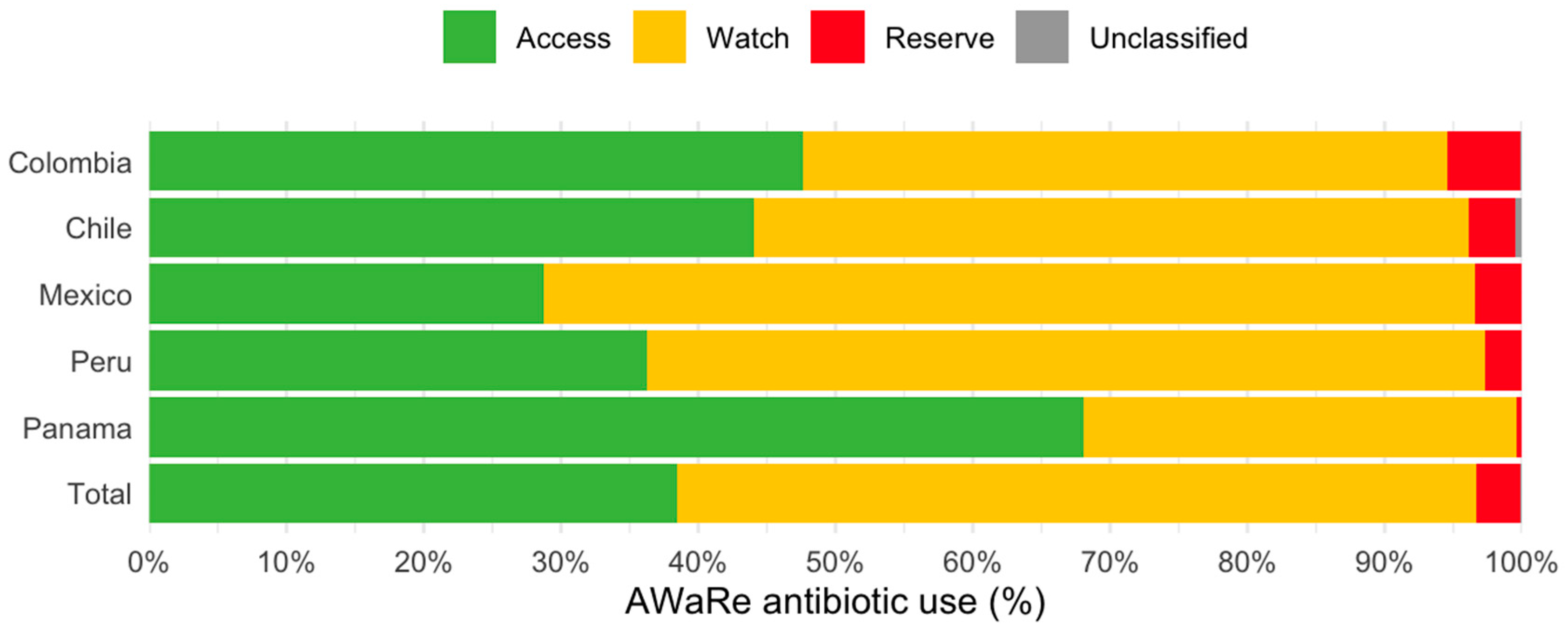
3.5. AWaRe Classification of Antibiotics Prescribed
3.6. Factors Associated with Antibiotic Use
4. Discussion
4.1. Antimicrobial Use Patterns
4.2. Adherence to Guidelines and Influencing Factors
4.3. Antimicrobial Stewardship (ASP) and National Policies
4.4. Study Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. The Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 12 November 2018).
- WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals. Version 1.1. February 2019. Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2018.01 (accessed on 12 December 2023).
- 2009 EU-U.S. Summit Declaration. 3 November 2009. Available online: https://www.europarl.europa.eu/cmsdata/122880/Joint%20Statement%202009%20EU-US%20Summit.pdf (accessed on 1 March 2025).
- European Centre for Disease Prevention and Control. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/point-prevalence-survey-healthcare-associated-infections-and-antimicrobial-use-4 (accessed on 1 March 2025).
- Magill, S.S.; O’Leary, E.; Ray, S.M.; Kainer, M.A.; Evans, C.; Bamberg, W.M.; Johnston, H.; Janelle, S.J.; Oyewumi, T.; Lynfield, R. Emerging Infections Program Hospital Prevalence Survey Team. Antimicrobial Use in US Hospitals: Comparison of Results from Emerging Infections Program Prevalence Surveys, 2015 and 2011. Clin. Infect. Dis. 2021, 72, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Global PPS Development Group. Point Prevalence Survey of Antimicrobial Consumption and Resistance (2017 GLOBAL-PPS). 2016. Available online: https://www.global-pps.com/ (accessed on 12 December 2023).
- Massele, A.; Burger, J.; Kalemeera, F.; Jande, M.; Didimalang, T.; Kalungia, A.C.; Matshotyana, K.; Law, M.; Malone, B.; Ogunleye, O.; et al. Outcome of the second Medicines Utilisation Research in Africa Group meeting to promote sustainable and appropriate medicine use in Africa. Expert. Rev. Pharmacoecon Outcomes Res. 2017, 17, 149–152. [Google Scholar] [CrossRef][Green Version]
- Huerta-Gutiérrez, R.; Braga, L.; Camacho-Ortiz, A.; Díaz-Ponce, H.; García-Mollinedo, L.; Guzmán-Blanco, M.; Valderrama-Beltráng, S.; Landaeta-Nezerh, E.; Moreno-Espinosa, S. One-day point prevalence of healthcConare-associated infections and antimicrobial use in four countries in Latin America. Int. J. Infect. Dis. 2019, 86, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Levy Hara, G.; Rojas-Cortés, R.; Molina León, H.F.; Mansilla, A.D.; Orta, I.A.; Rizo-Amezquita, J.N.; Herrera, R.G.S.; de Ayala, S.M.; Villalobos, M.A.; Latin American Point Prevalent Survey Study Group. Point prevalence survey of antibiotic use in hospitals in Latin American countries. J. Antimicrob. Chemother. 2022, 77, 807–815, Erratum in: J. Antimicrob. Chemother. 2022, 77, 2051. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization. Tratamiento de las Enfermedades Infecciosas 2020-2022, 8th ed; PAHO: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- World Health Organization. AWaRe (Access, Watch, Reserve) Classification of Antibiotics for Evaluation and Monitoring of Use. 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 7 April 2025).
- Chen, R.; Li, J.; Wang, C.; Zhou, P.; Song, Q.; Wu, J.; Li, Q.; Li, H.; Gong, Y.; Zeng, T.; et al. Global antibiotic prescription practices in hospitals and associated factors: A systematic review and meta-analysis. J. Glob. Health 2025, 15, 04023. [Google Scholar] [CrossRef] [PubMed]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Global-PPS network. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health 2018, 6, e619–e629, Erratum in: Lancet Glob. Health 2018, 6, e968. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, C.; Versporten, A.; Severino, N.; Cifuentes, M.; Silva, F.; Rojas, A.; Goossens, H.; Labarca, J. The Global Point Prevalence Survey of Antimicrobial Consumption and Resistance (Global-PPS): First Results of Antimicrobial Prescribing in 12 Chilean Hospitals. 2016. Available online: https://www.global-pps.com/wp-content/uploads/2016/04/ECCMID-2016_CHILE.pdf (accessed on 1 March 2025).
- Menkem, E.Z.; Labo Nanfah, A.; Takang, T.; Ryan Awah, L.; Awah Achua, K.; Ekane Akume, S.; Fekam Boyom, F. Attitudes and Practices of the Use of Third-Generation Cephalosporins among Medical Doctors Practicing in Cameroon. Int. J. Clin. Pract. 2023, 2023, 8074413. [Google Scholar] [CrossRef] [PubMed]
- Tafere, C.; Endeshaw, D.; Demsie, D.G.; Yismaw, M.B.; Tefera, B.B.; Yehualaw, A.; Feyisa, K.; Siraj, E.A.; Yayehrad, A.T.; Addisu, Z.D.; et al. Inappropriate ceftriaxone utilization and predictor factors in Ethiopia: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 25035. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.V.; Kattan, J.N.; Quinteros, M.G.; Casellas, J.M. Prevalence of extended-spectrum beta-lactamases in South America. Clin. Microbiol. Infect. 2008, 14 (Suppl. S1), 154–158, Erratum in: Clin. Microbiol. Infect. 2008, 14 (Suppl. S5), 21–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fabre, V.; Cosgrove, S.E.; Secaira, C.; Tapia Torrez, J.C.; Lessa, F.C.; Patel, T.S.; Quiros, R. Antimicrobial stewardship in Latin America: Past, present, and future. Antimicrob. Steward. Heal. Epidemiol. 2022, 2, e68. [Google Scholar] [CrossRef] [PubMed]
- Pan American Health Organization website. Latin American Network for Antimicrobial Resistance Surveillance—ReLAVRA data Visualization Dashboards. Available online: https://www.paho.org/en/topics/antimicrobial-resistance/latin-american-and-caribbean-network-antimicrobial-resistance (accessed on 10 September 2024).
- Pauwels, I.; Versporten, A.; Drapier, N.; Vlieghe, E.; Goossens, H.; Global-PPS network. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): Results from a worldwide point prevalence survey in 69 countries. J Antimicrob. Chemother. 2021, 76, 1614–1624. [Google Scholar] [CrossRef] [PubMed]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Middle-Income Countries: A WHO Practical Toolkit; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Pan American Health Organization; Florida International University. Recommendations for Implementing Antimicrobial Stewardship Programs in Latin America and the Caribbean: Manual for Public Health Decision-Makers; PAHO, FIU: Washington, DC, USA, 2018; Available online: https://iris.paho.org/handle/10665.2/49645?locale-attribute=es (accessed on 1 March 2025).
| No. | % | Chile n (%) | Colombia n (%) | Mexico n (%) | Panama n (%) | Peru n (%) | |
|---|---|---|---|---|---|---|---|
| Number of participants | 11,094 | - | 3339 (30.1) | 1087 (9.8) | 2292 (20.7) | 298 (2.7) | 4078 (36.8) |
| Sex | |||||||
| Male | 5624 | 50.7 | 1723 (51.6) | 515 (47.4) | 1262 (55.1) | 161 (54.0) | 1963 (48.1) |
| Female | 5453 | 49.2 | 1608 (48.2) | 572 (52.6) | 1028 (44.9) | 137 (46.0) | 2108 (51.7) |
| Transgender | 11 | 0.1 | 6 (0.2) | 0 (0.0) | 2 (0.1) | 0 (0.0) | 3 (0.1) |
| Unknown | 6 | 0.1 | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (0.1) |
| Age categories | |||||||
| Less than 1 year | 1487 | 13.4 | 367 (11.0) | 125 (11.5) | 274 (12.0) | 195 (65.4) | 526 (12.9) |
| 1–4 years | 679 | 6.1 | 168 (5.0) | 17 (1.6) | 184 (8.0) | 57 (19.1) | 253 (6.2) |
| 5–17 years | 1099 | 9.9 | 205 (6.1) | 25 (2.3) | 384 (16.8) | 46 (15.4) | 439 (10.8) |
| 18–65 years | 5149 | 46.4 | 1512 (45.3) | 574 (52.8) | 1078 (47.0) | 0 (0.0) | 1985 (48.7) |
| More than 65 years | 2677 | 24.1 | 1085 (32.5) | 346 (31.8) | 372 (16.2) | 0 (0.0) | 874 (21.4) |
| Unknown | 3 | 0.0 | 2 (0.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.0) |
| Ward | |||||||
| Adult medical | 3303 | 29.8 | 1170 (35.0) | 231 (21.3) | 688 (30.0) | 0 (0.0) | 1214 (29.8) |
| Pediatric | 2360 | 21.3 | 494 (14.8) | 63 (5.8) | 672 (29.3) | 229 (76.8) | 902 (22.1) |
| Adult surgical | 1792 | 16.2 | 553 (16.6) | 63 (5.8) | 590 (25.7) | 0 (0.0) | 586 (14.4) |
| Intensive care units | 1493 | 13.5 | 607 (18.2) | 205 (18.9) | 205 (8.9) | 69 (23.2) | 407 (10.0) |
| Mixed | 1006 | 9.1 | 111 (3.3) | 406 (37.4) | 38 (1.7) | 0 (0.0) | 451 (11.1) |
| Obstetrics and gynecology | 893 | 8.0 | 318 (9.5) | 88 (8.1) | 94 (4.1) | 0 (0.0) | 393 (9.6) |
| Adult high-risk units | 247 | 2.2 | 86 (2.6) | 31 (2.9) | 5 (0.2) | 0 (0.0) | 125 (3.1) |
| Presence of a peripheral catheter | 7870 | 70.9 | 2167 (64.9) | 964 (88.7) | 1536 (67.0) | 232 (77.9) | 2971 (72.9) |
| Patients intubated | 2153 | 19.4 | 626 (18.7) | 250 (23.0) | 464 (20.2) | 156 (52.3) | 657 (16.1) |
| Previous history of hospitalization (within 90 days) | 1006 | 9.1 | 244 (7.3) | 89 (8.2) | 347 (15.1) | 38 (12.8) | 288 (7.1) |
| Total | Chile | Colombia | Mexico | Peru | Panama | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admitted, n | AB Use, n (%) | Admitted, n | AB Use, n (%) | Admitted, n | AB Use, n (%) | Admitted, n | AB Use, n (%) | Admitted, n | AB Use, n (%) | Admitted, n | AB Use, n (%) | |
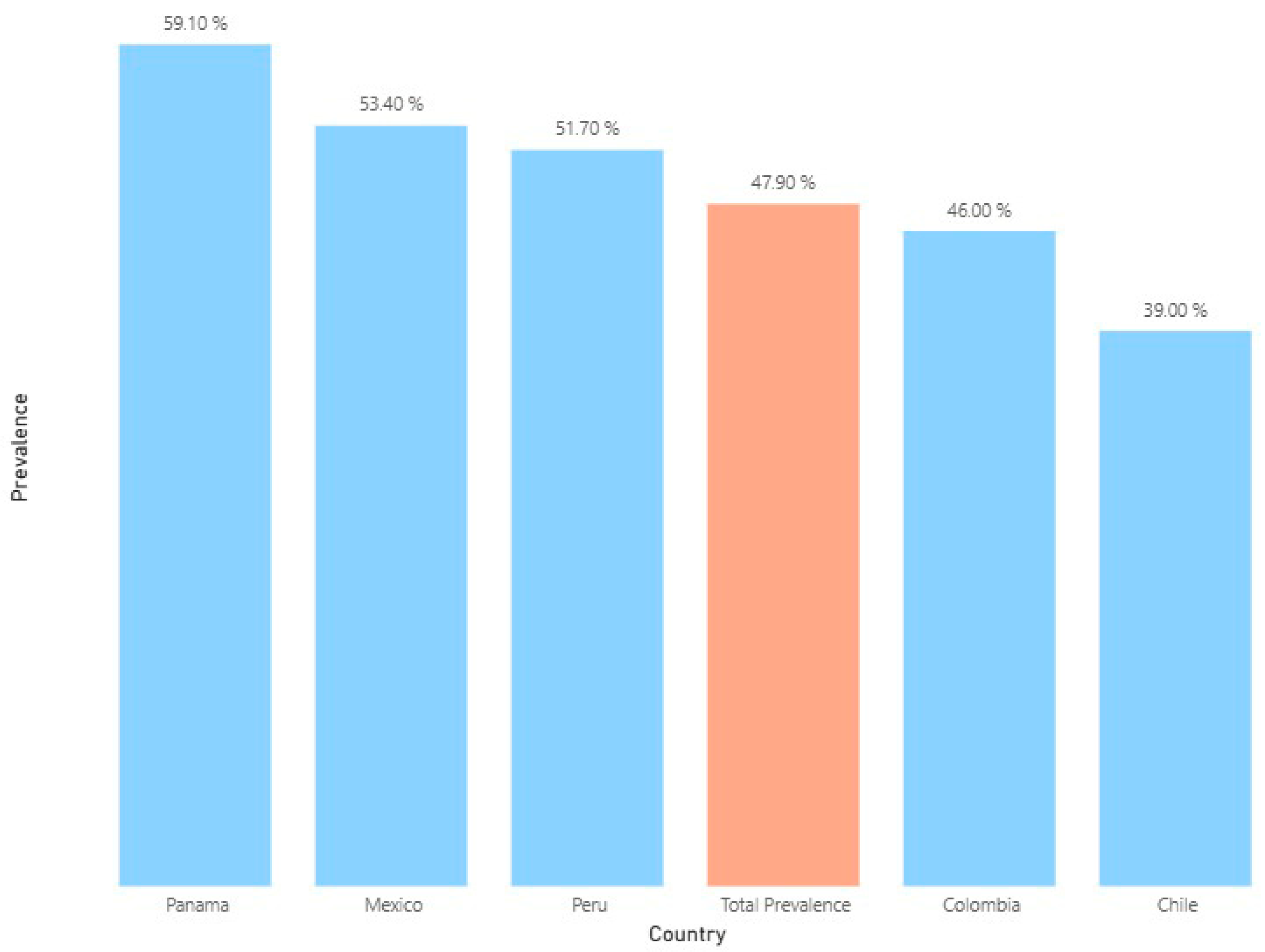
| Prevalence of antibiotics | 11,094 | 5310 (47.9) | 3339 | 1302 (39.0) | 1087 | 500 (46.0) | 2292 | 1224 (53.4) | 4078 | 2108 (51.7) | 298 | 176 (59.1) |
| Adult medical wards | 3303 | 1465 (44.4) | 1170 | 429 (36.7) | 231 | 98 (42.4) | 688 | 346 (50.3) | 1214 | 592 (48.8) | 0 | 0 (0.0) |
| Adult surgical wards | 1792 | 1012 (56.5) | 553 | 291 (52.6) | 63 | 40 (63.5) | 590 | 362 (61.4) | 586 | 319 (54.4) | 0 | 0 (0.0) |
| Intensive care units | 1493 | 836 (56.0) | 607 | 274 (45.1) | 205 | 93 (45.4) | 205 | 144 (70.2) | 407 | 267 (65.6) | 69 | 58 (84.1) |
| Pediatric wards | 2360 | 1029 (43.6) | 494 | 156 (31.6) | 63 | 15 (23.8) | 672 | 282 (42.0) | 902 | 458 (50.8) | 229 | 118 (51.5) |
| Obstetrics and gynecology | 893 | 295 (33.0) | 318 | 56 (17.6) | 88 | 24 (27.3) | 94 | 69 (73.4) | 393 | 146 (37.2) | 0 | 0 (0.0) |
| Adult high-risk units | 247 | 119 (48.2) | 86 | 45 (52.3) | 31 | 17 (54.8) | 5 | 3 (60.0) | 125 | 54 (43.2) | 0 | 0 (0.0) |
| Mixed wards | 1006 | 554 (55.1) | 111 | 51 (45.9) | 406 | 213 (52.5) | 38 | 18 (47.4) | 451 | 272 (60.3) | 0 | 0 (0.0) |
| Antibiotic Group | Total, n (%) | Chile n (%) | Colombia n (%) | Mexico n (%) | Peru n (%) | Panama n (%) |
|---|---|---|---|---|---|---|
| J01DD Third-generation cephalosporins (ceftriaxone, cefotaxime, and ceftazidime) | 1699 (22.0) | 482 (25.0) | 38 (5.9) | 482 (26.9) | 681 (21.9) | 16 (6.2) |
| J01DH Carbapenems (meropenem, imipenem, and ertapenem) | 933 (12.1) | 139 7.2) | 54 (8.4) | 263 (14.7) | 461 (14.8) | 16 (6.2) |
| J01XA Glycopeptide antibacterials (vancomycin) | 684 (8.8) | 141 (7.3) | 52 (8.1) | 152 (8.5) | 336 (10.8) | 3 (1.2) |
| A07AA09 Antibiotics (vancomycin oral) | 30 (0.4) | 20 (1) | 3 (0.5) | 6 (0.3) | 1 (0) | 0 (0) |
| J01CR Combinations of penicillins, including beta-lactamase inhibitors (amoxicillin-sulbactam, piperacillin-tazobactam, ampicillin-sulbactam, and amoxicillin-clavulanic acid) | 663 (8.6) | 167 (8.7) | 179 (27.8) | 45 (2.5) | 202 (6.5) | 70 (26.9) |
| J01XD Imidazole derivatives (metronidazole) | 587 (7.6) | 276 (14.3) | 13 (2) | 141 (7.9) | 154 (4.9) | 3 (1.2) |
| J01FF Lincosamides (clindamycin) | 480 (6.2) | 73 (3.8) | 42 (6.5) | 99 (5.5) | 255 (8.2) | 11 (4.2) |
| J01GB Other aminoglycosides (amikacin and gentamicin) | 453 (5.9) | 79 (4.1) | 30 (4.7) | 96 (5.4) | 189 (6.1) | 59 (22.7) |
| J01DB First-generation cephalosporins (cephalexin, cefadroxil, cephradine, and cefazolin) | 446 (5.8) | 112 (5.8) | 66 (10.2) | 13 (0.7) | 255 (8.2) | 0 (0) |
| J01MA Fluoroquinolones (ciprofloxacin and levofloxacin) | 392 (5.1) | 78 (4) | 10 (1.6) | 148 (8.3) | 153 (4.9) | 3 (1.2) |
| J01CA Penicillins with an extended spectrum (ampicillin and amoxicillin) | 291 (3.8) | 79 (4.1) | 24 (3.7) | 48 (2.7) | 94 (3) | 46 (17.7) |
| J01EE Combinations of sulfonamides and trimethoprim, including derivatives (trimethoprim-sulfamethoxazole) | 183 (2.4) | 59 (3.1) | 14 (2.2) | 48 (2.7) | 58 (1.9) | 4 (1.5) |
| J01FA Macrolides (azithromycin and clarithromycin) | 150 (1.9) | 54 (2.8) | 13 (2) | 28 (1.6) | 53 (1.7) | 2 (0.8) |
| J01DE Fourth-generation cephalosporins (cefepime) | 139 (1.8) | 9 (0.5) | 31 (4.8) | 74 (4.1) | 21 (0.7) | 4 (1.5) |
| J01CF Beta-lactamase-resistant penicillins (oxacillin) | 74 (1) | 0 (0) | 8 (1.2) | 0 (0) | 48 (1.5) | 18 (6.9) |
| J01XB Polymyxins (colistin) | 62 (0.8) | 3 (0.2) | 2 (0.3) | 8 (0.4) | 49 (1.6) | 0 (0) |
| J01DC Second-generation cephalosporins (cefuroxime) | 42 (0.5) | 1 (0.1) | 4 (0.6) | 8 (0.4) | 29 (0.9) | 0 (0) |
| J01AA Tetracyclines (doxycycline) | 24 (0.3) | 5 (0.3) | 4 (0.6) | 2 (0.1) | 13 (0.4) | 0 (0) |
| J01XE Nitrofuran derivatives (nitrofurantoin) | 10 (0.1) | 6 (0.3) | 0 (0) | 2 (0.1) | 2 (0.1) | 0 (0) |
| Other antibiotics | 393 (5.1) | 144 (7.5) b | 58 (9) | 127 (7.1) c | 59 (1.9) | 5 (1.9) |
| Total | 7735 | 1927 | 645 | 1790 | 3113 | 260 |
| Antibiotic Group | Pneumonia n (%) | Intra-Abdominal Sepsis n (%) | Cellulitis n (%) | Clinical Sepsis n (%) | Upper Urinary Tract Infections n (%) | Others n (%) |
|---|---|---|---|---|---|---|
| J01DD Third-generation cephalosporins (ceftriaxone, cefotaxime, and ceftazidime) | 360 (24.4) | 239 (22.9) | 144 (17.9) | 55 (8.4) | 92 (28.0) | 809 (23.6) |
| J01DH Carbapenems (meropenem, imipenem, and ertapenem) | 225 (15.2) | 135 (12.9) | 74 (9.2) | 108 (16.4) | 111 (33.8) | 280 (8.2) |
| J01CR Combinations of penicillins, including beta-lactamase inhibitors (amoxicillin-sulbactam, piperacillin-tazobactam, ampicillin-sulbactam, and amoxicillin-clavulanic acid) | 213 (14.4) | 129 (12.4) | 75 (9.3) | 46 (7) | 24 (7.3) | 176 (5.1) |
| J01XA Glycopeptide antibacterials (vancomycin parenteral) | 137 (9.3) | 59 (5.7) | 83 (10.3) | 120 (18.3) | 6 (1.8) | 309 (9.0) |
| J01XD/P01AB Imidazole derivatives/Nitroimidazole (metronidazol) | 26 (1.8) | 274 (26.3) | 65 (8.1) | 8 (1.2) | 7 (2.1) | 207 (6.0) |
| J01GB Other aminoglycosides (amikacin and gentamicin) | 54 (3.7) | 46 (4.4) | 11 (1.4) | 122 (18.6) | 24 (7.3) | 196 (5.7) |
| J01FF Lacosamides (clindamycin) | 74 (5.0) | 14 (1.3) | 147 (18.3) | 8 (1.2) | 1 (0.3) | 236 (6.9) |
| J01MA Fluoroquinolones (ciprofloxacin and levofloxacin) | 73 (4.9) | 63 (6) | 52 (6.5) | 7 (1.1) | 19 (5.8) | 178 (5.2) |
| J01CA Penicillins with an extended spectrum (ampicillin and amoxicillin) | 45 (3.0) | 24 (2.3) | 6 (0.7) | 120 (18.3) | 5 (1.5) | 91 (2.7) |
| J01FA Macrolides (azithromycin and clarithromycin) | 100 (6.8) | 3 (0.3) | 1 (0.1) | 1 (0.2) | 1 (0.3) | 44 (1.3) |
| J01DE Fourth-generation cephalosporins (cefepime) | 44 (3.0) | 5 (0.5) | 9 (1.1) | 18 (2.7) | 4 (1.2) | 59 (1.7) |
| J01DB First-generation cephalosporins (cephalexin, cefadroxil, cephradine, and cefazolin) | 2 (0.1) | 7 (0.7) | 39 (4.9) | 2 (0.3) | 11 (3.4) | 385 (11.2) |
| J01CF Beta-lactamase-resistant penicillins (oxacillin) | 5 (0.3) | 0 (0) | 38 (4.7) | 9 (1.4) | 0 (0) | 22 (0.6) |
| J01EE Combinations of sulfonamides and trimethoprim, including derivatives (trimethoprim-sulfamethoxazole) | 32 (2.2) | 3 (0.3) | 5 (0.6) | 8 (1.2) | 1 (0.3) | 134 (3.9) |
| J01XB Polymyxins (colistin) | 29 (2) | 4 (0.4) | 2 (0.2) | 6 (0.9) | 3 (0.9) | 18 (0.5) |
| J01DC Second-generation cephalosporins (cefuroxime) | 1 (0.1) | 2 (0.2) | 2 (0.2) | 0 (0) | 8 (2.4) | 29 (0.8) |
| J01AA Tetracyclines (doxycycline) | 7 (0.5) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 16 (0.5) |
| J01XE Nitrofuran derivatives (nitrofurantoin) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (0.3) |
| J01CE Beta-lactamase-sensitive penicillins (penamecillin) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Other antibiotics | 50 (3.4) | 36 (3.5) | 49 (6.1) | 19 (2.9) | 11 (3.4) | 228 (6.7) |
| Total | 1477 | 1043 | 803 | 657 | 328 | 3427 |
| Total | Chile | Colombia | Mexico | Peru | Panama | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AB Prescribed n | Compliance n (%) | AB Prescribed n | Compliance n (%) | AB Prescribed n | Compliance n (%) | AB Prescribed n | Compliance n (%) | AB Prescribed n | Compliance n (%) | AB Prescribed n | Compliance n (%) | |
| Guidelines compliance | 7735 | 4784 (61.8) | 1927 | 1417 (73.5) | 645 | 456 (70.7) | 1790 | 1013 (56.6) | 3113 | 1741 (55.9) | 260 | 157 (60.4) |
| Adult medical wards | 2028 | 1271 (62.7) | 600 | 440 (73.3) | 123 | 97 (78.9) | 466 | 249 (53.4) | 839 | 485 (57.8) | 0 | 0 (0.0) |
| Adult surgical wards | 1487 | 729 (49.0) | 458 | 307 (67.0) | 47 | 34 (72.3) | 518 | 213 (41.1) | 464 | 175 (37.7) | 0 | 0 (0.0) |
| Intensive care units | 1345 | 952 (70.8) | 427 | 325 (76.1) | 132 | 105 (79.5) | 248 | 185 (74.6) | 447 | 290 (64.9) | 91 | 47 (51.6) |
| Pediatric wards | 1575 | 1103 (70.0) | 220 | 173 (78.6) | 18 | 16 (88.9) | 454 | 338 (74.4) | 714 | 466 (65.3) | 169 | 110 (65.1) |
| Obstetrics and gynecology | 393 | 181 (46.1) | 94 | 73 (77.7) | 32 | 13 (40.6) | 75 | 14 (18.7) | 192 | 81 (42.2) | 0 | 0 (0.0) |
| Adult high-risk units | 158 | 125 (79.1) | 57 | 47 (82.5) | 22 | 21 (95.5) | 4 | 2 (50.0) | 75 | 55 (73.3) | 0 | 0 (0.0) |
| Mixed wards | 749 | 423 (56.5) | 71 | 52 (73.2) | 271 | 170 (62.7) | 25 | 12 (48.0) | 382 | 189 (49.5) | 0 | 0 (0.0) |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| n | OR IC 95% | p value | OR IC 95% | p value | |
| Intercept | - | 0.19 (0.12–0.29) | <0.001 | ||
| Age | |||||
| Less than 1 year | 1487 | 1 | 1 | ||
| 1–4 years | 679 | 1.67 (1.39–2.01) | <0.001 | 1.69 (1.36–2.1) | <0.001 |
| 5–17 years | 1099 | 1.61 (1.37–1.89) | <0.001 | 1.60 (1.32–1.92) | <0.001 |
| 18–65 years | 5149 | 1.75 (1.54–1.98) | <0.001 | 1.99 (1.6–2.48) | <0.001 |
| More than 65 years | 2677 | 1.83 (1.60–2.10) | <0.001 | 1.84 (1.46–2.31) | <0.001 |
| Gender | |||||
| Female | 5624 | 1 | 1 | ||
| Male | 5453 | 1.17 (1.09–1.26) | <0.001 | 1.05 (0.96–1.15) | 0.296 |
| Transgender | 11 | 0.75 (0.22–2.53) | 0.642 | 0.65 (0.14–3.01) | 0.582 |
| Ward | |||||
| Adult high-risk units | 247 | 1 | 1 | ||
| Adult medical | 3303 | 0.83 (0.64–1.08) | 0.162 | 0.89 (0.67–1.18) | 0.400 |
| Adult surgical | 1792 | 1.30 (0.99–1.70) | 0.056 | 1.51 (1.12–2.02) | 0.007 |
| Intensive care units | 1493 | 1.40 (1.07–1.84) | 0.015 | 1.45 (1.05–1.99) | 0.022 |
| Mixed | 1006 | 1.29 (0.97–1.71) | 0.078 | 1.49 (1.1–2.02) | 0.01 |
| Obstetrics and gynecology | 893 | 0.52 (0.39–0.69) | <0.001 | 0.69 (0.5–0.95) | 0.023 |
| Pediatric | 2360 | 0.70 (0.54–0.92) | 0.010 | 1.08 (0.76–1.52) | 0.675 |
| Previous history of hospitalization (within 90 days) | |||||
| No | 7464 | 1 | 1 | ||
| Yes | 1006 | 1.54 (1.35–1.76) | <0.001 | 1.47 (1.27–1.69) | <0.001 |
| Patients with a catheter | |||||
| No | 1951 | 1 | 1 | ||
| Yes | 9127 | 3.17 (2.83–3.54) | <0.001 | 2.71 (2.37–3.09) | <0.001 |
| Patients intubated | |||||
| No | 8899 | 1 | 1 | ||
| Yes | 2153 | 1.92 (1.74–2.12) | <0.001 | 1.53 (1.35–1.74) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© Pan American Health Organization 2025. Licensee MDPI, Basel, Switzerland. This is an open access article distributed under the terms of the Creative Commons Attribution IGO License (http://creativecommons.org/licenses/by/3.0/igo/legalcode), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In any reproduction of this article there should not be any suggestion that PAHO or this article endorse any specific organisation or products. The use of the PAHO logo is not permitted.
Share and Cite
Lichtenberger, P.; Levy-Hara, G.; Rojas-Cortés, R.; Orjuela, T.; Diaz-Madriz, J.P.; Ramon-Pardo, P.; Bustos, J.L.; Dreser, A.; Herrera, T.; Rojas-Diaz, M.P.; et al. Point Prevalence Survey of Antibiotic Use in Latin American Hospitals: 2022–2023. Antibiotics 2025, 14, 1078. https://doi.org/10.3390/antibiotics14111078
Lichtenberger P, Levy-Hara G, Rojas-Cortés R, Orjuela T, Diaz-Madriz JP, Ramon-Pardo P, Bustos JL, Dreser A, Herrera T, Rojas-Diaz MP, et al. Point Prevalence Survey of Antibiotic Use in Latin American Hospitals: 2022–2023. Antibiotics. 2025; 14(11):1078. https://doi.org/10.3390/antibiotics14111078
Chicago/Turabian StyleLichtenberger, Paola, Gabriel Levy-Hara, Robin Rojas-Cortés, Tatiana Orjuela, Jose Pablo Diaz-Madriz, Pilar Ramon-Pardo, Jose Luis Bustos, Anahí Dreser, Tania Herrera, Marcela Pilar Rojas-Diaz, and et al. 2025. "Point Prevalence Survey of Antibiotic Use in Latin American Hospitals: 2022–2023" Antibiotics 14, no. 11: 1078. https://doi.org/10.3390/antibiotics14111078
APA StyleLichtenberger, P., Levy-Hara, G., Rojas-Cortés, R., Orjuela, T., Diaz-Madriz, J. P., Ramon-Pardo, P., Bustos, J. L., Dreser, A., Herrera, T., Rojas-Diaz, M. P., Huaquipaco, G., Sagastume, D., Castro, J. L., & on behalf of the Latin American PPS Group. (2025). Point Prevalence Survey of Antibiotic Use in Latin American Hospitals: 2022–2023. Antibiotics, 14(11), 1078. https://doi.org/10.3390/antibiotics14111078






