Evaluating Outer Membrane Vesicle Isolation Techniques for Borrelia burgdorferi and Their Impact on Vesicle Composition, Gene Expression Profile and Uptake
Abstract
1. Introduction
2. Results
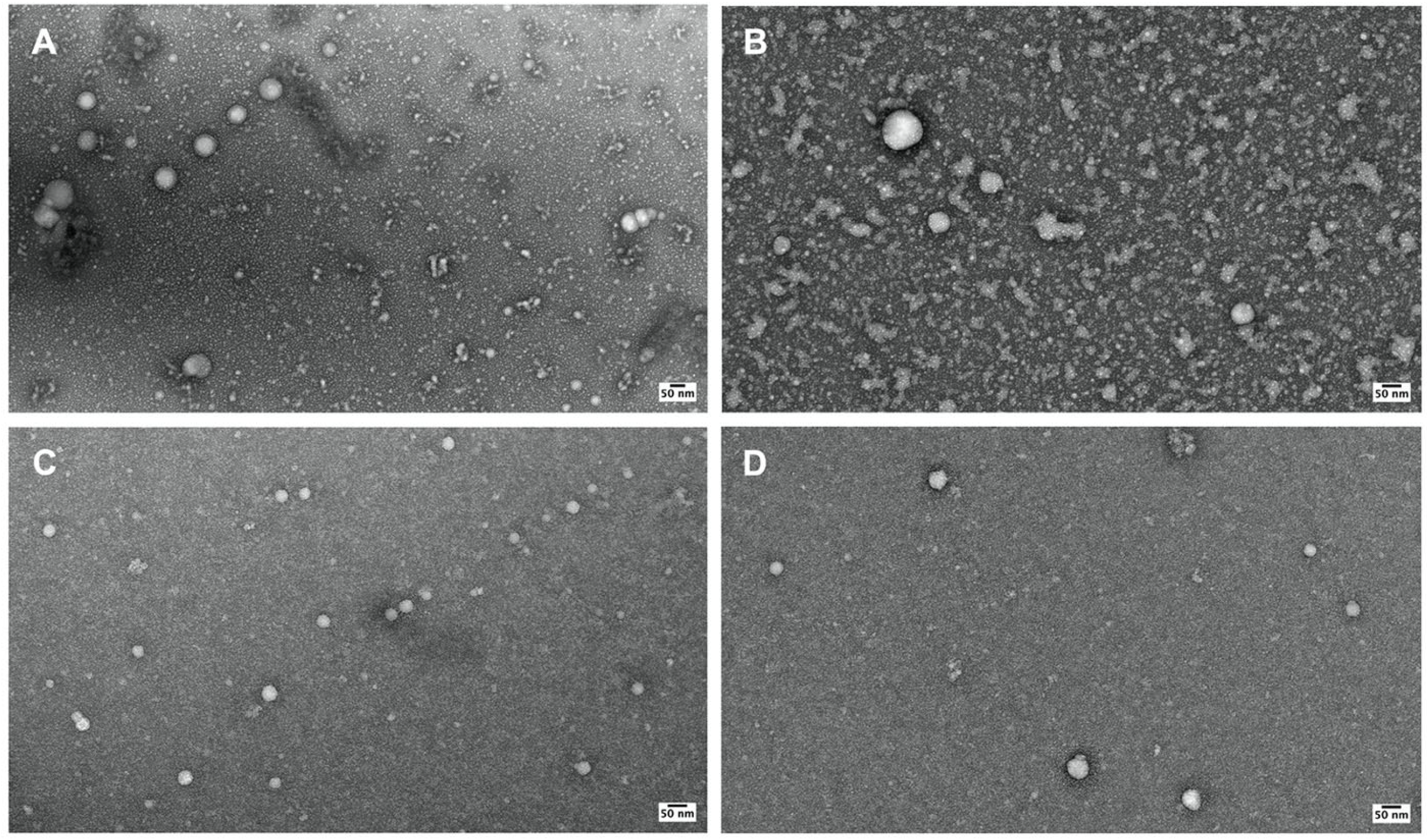
2.1. Identification of Bb-OMVs Derived from B. burgdorferi
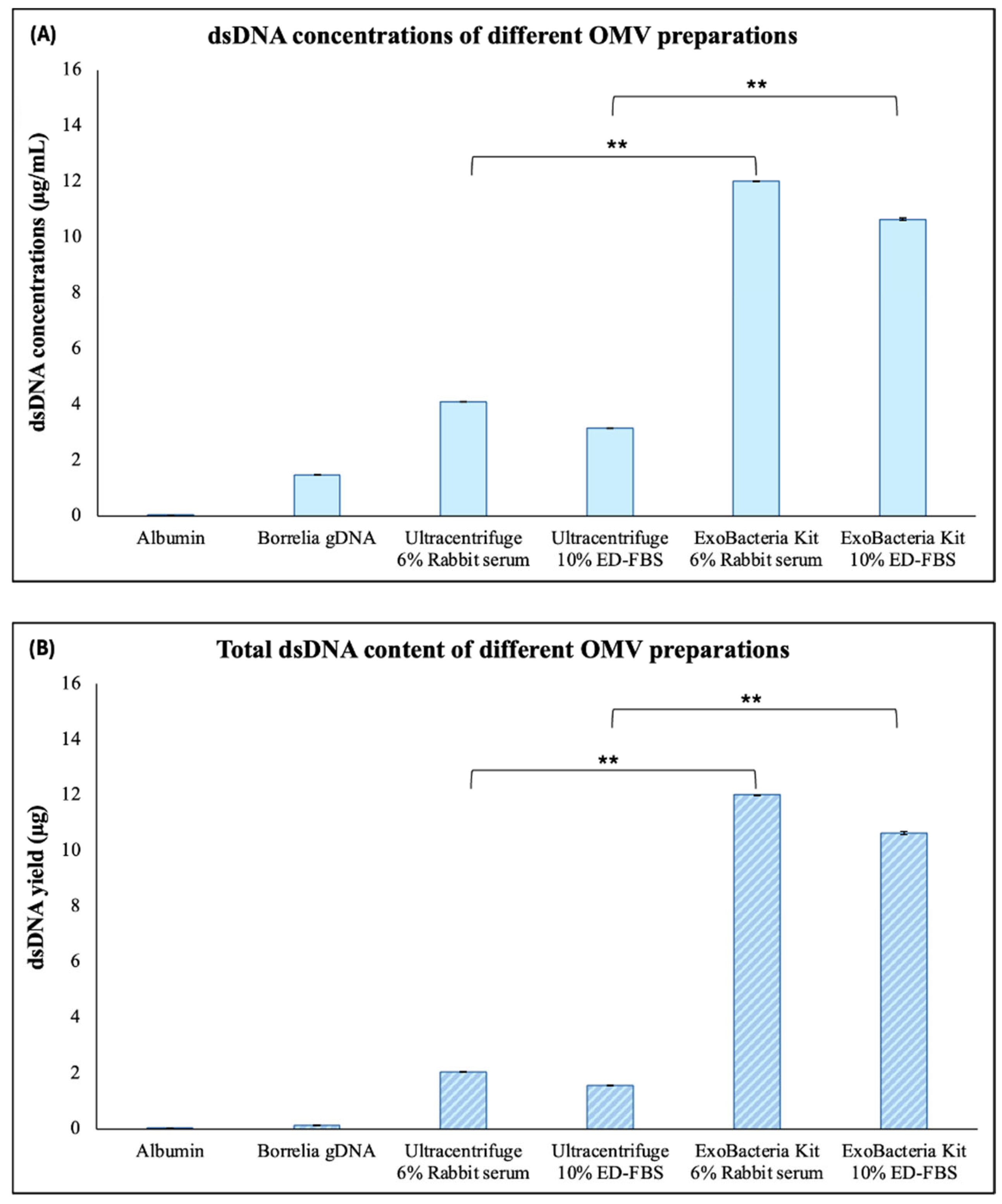
2.2. Characterization of OMVs Derived from B. burgdorferi
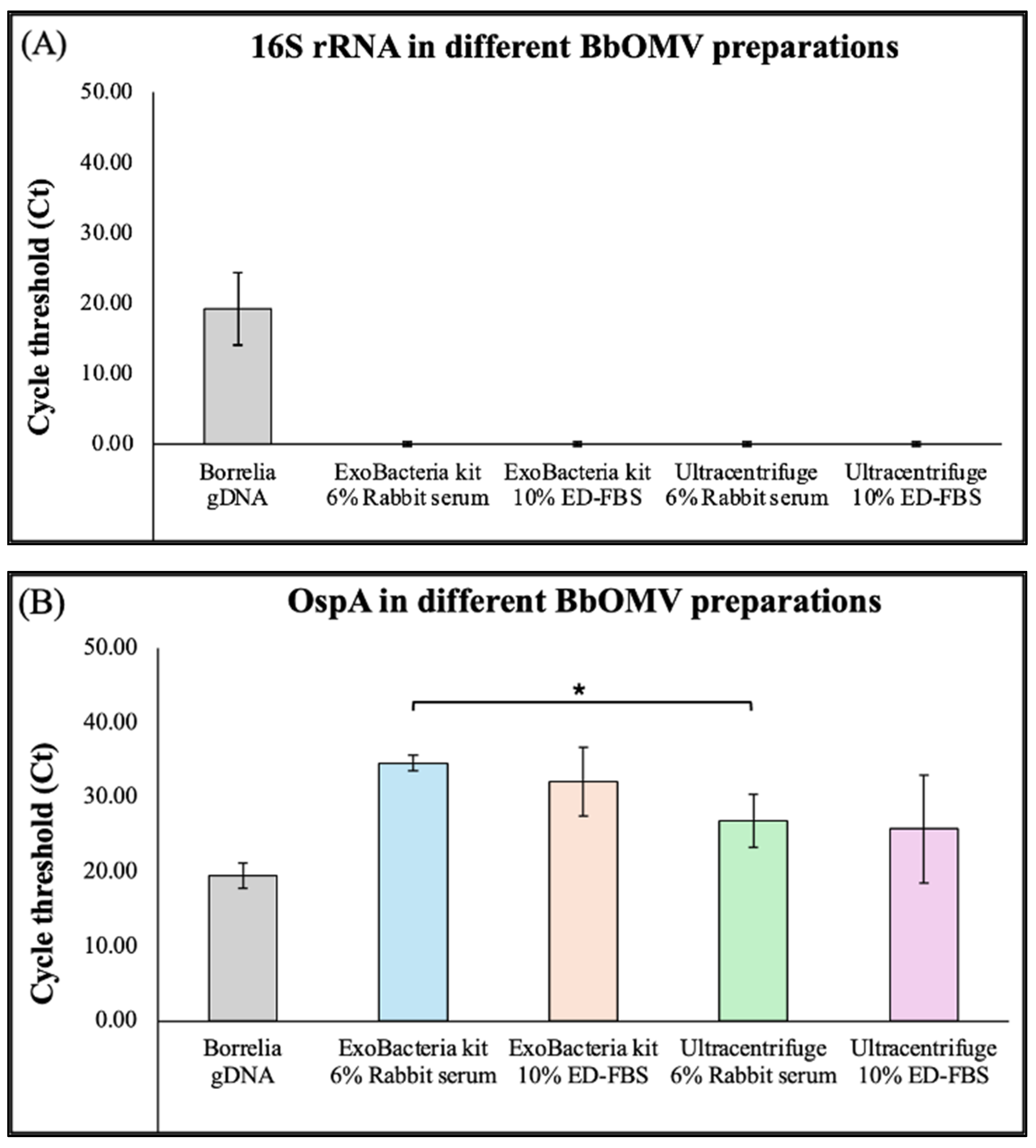
2.3. Analysis of Gene and Protein Expression Profiles of OMVs from B. burgdorferi Using RT-PCR and Western Blot Analyses Method
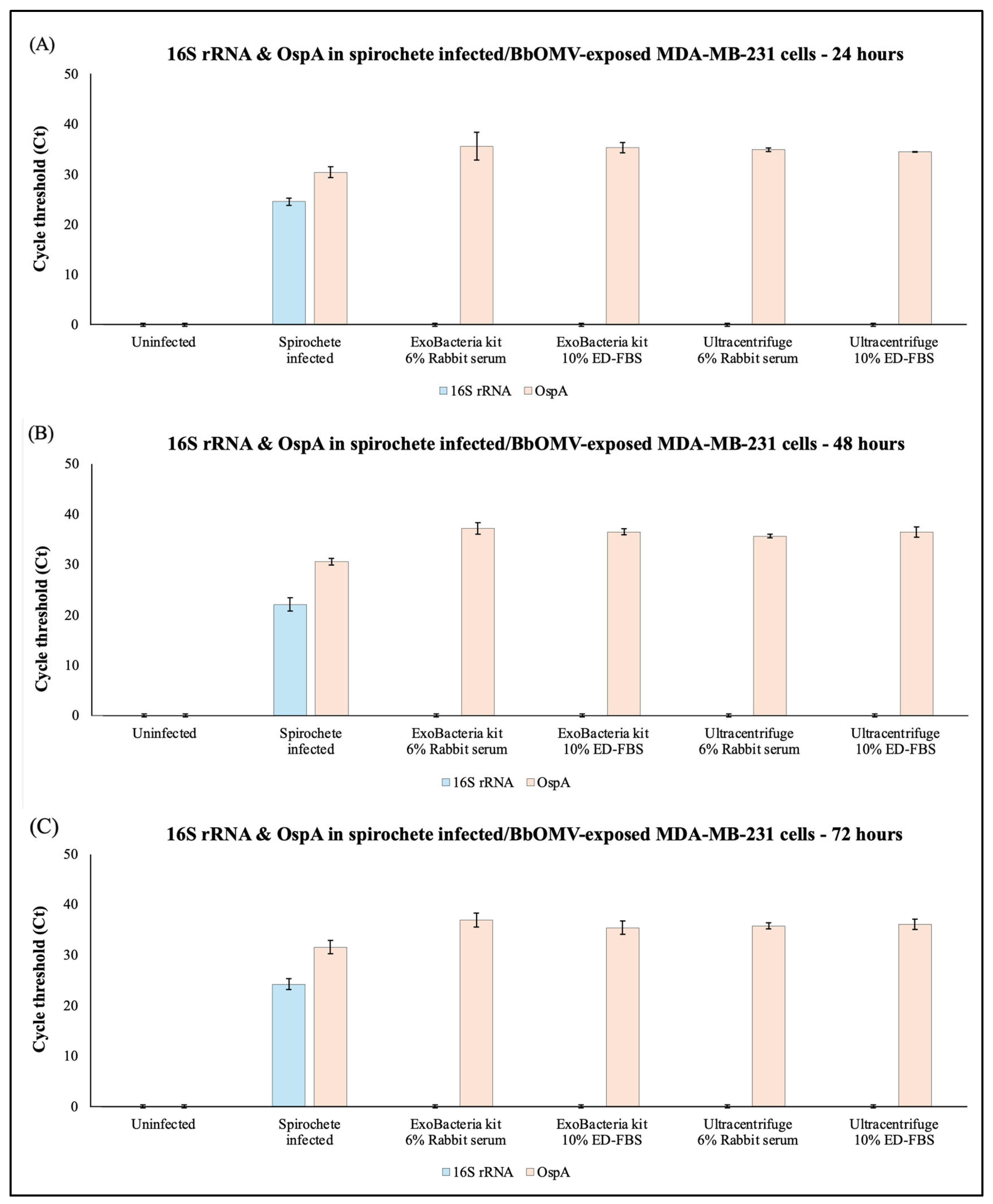
2.4. Functional Analyses of B. burgdorferi OMVs Presence in OMV-Exposed Breast Cancer Cells Using RT-PCR
3. Discussion
4. Materials and Methods
4.1. Bacterial Cell Culture
4.2. Mammalian Cell Culture
4.3. Isolation and Purification of Bacterial Outer Membrane Vesicles
4.4. Transmission Electron Microscopy
4.5. Western Blot
4.6. DNA and Protein Quantification
4.7. Infection of MDA-MB-231 with Spirochetes/OMVs
4.8. Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| B. burgdorferi | Borrelia burgdorferi |
| Bb-OMV | Borrelia burgdorferi outer membrane vesicle |
| BSA | Bovine Serum Albumin |
| BSK-H | Barbour-Stoenner-Kelly H medium |
| CO2 | Carbon Dioxide |
| dsDNA | Double-Stranded DNA |
| ECL | Enhanced Chemiluminescence |
| ED-FBS | Exosome-Depleted Fetal Bovine Serum |
| HRP | Horseradish Peroxidase |
| NP-40 | Nonidet P-40 Detergent |
| OMV | Outer Membrane Vesicle |
| OspA | Outer Surface Protein A |
| OspB | Outer Surface Protein B |
| OspC | Outer Surface Protein C |
| PBS | Phosphate-Buffered Saline |
| PCR | Polymerase Chain Reaction |
| p-value | Probability Value |
| PVDF | Polyvinylidene Difluoride |
| RT | Room Temperature |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SDS-PAGE | Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis |
| SEM | Standard Error of the Mean |
| TBST | Tris-Buffered Saline with Tween 20 |
| TEM | Transmission Electron Microscopy |
References
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and Origins of Bacterial Membrane Vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, M.G.; Pardue, E.J.; Feldman, M.F.; Haurat, M.F. Bacterial Outer Membrane Vesicles: From Discovery to Applications. Annu. Rev. Microbiol. 2021, 75, 609–630. [Google Scholar] [CrossRef] [PubMed]
- Magaña, G.; Harvey, C.; Taggart, C.C.; Rodgers, A.M. Bacterial Outer Membrane Vesicles: Role in Pathogenesis and Host-Cell Interactions. Antibiotics 2023, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wen, Z.; Liu, X.; Zhang, T.; Liu, M.; Zhang, L.; Qiu, J.; Wang, M. Research Progress on Bacterial Outer Membrane Vesicles in Antibiotic Resistance and Clinical Anti-infective Therapy. Front. Microbiol. 2025, 16, 1670307. [Google Scholar] [CrossRef]
- Avila-Calderón, E.D.; Ruiz-Palma, M.D.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021, 12, 557902. [Google Scholar] [CrossRef]
- Kim, J.Y.; Suh, J.W.; Kang, J.S.; Kim, S.B.; Yoon, Y.K.; Sohn, J.W. Gram-Negative Bacteria’s Outer Membrane Vesicles. Infect. Chemother. 2023, 55, 1–9. [Google Scholar] [CrossRef]
- Abolhasani, F.S.; Vaghefinanekaran, N.; Yarahmadi, A.; Akrami, S.; Mirmahdavi, S.; Yousefi, M.H.; Afkhami, H.; Shafiei, M. Outer membrane Vesicles in Gram-negative Bacteria and Its Correlation with Pathogenesis. Front. Immunol. 2025, 16, 1541636. [Google Scholar] [CrossRef]
- Mozaheb, N.; Mingeot-Leclercq, M.P. Membrane Vesicle Production as a Bacterial Defense Against Stress. Front. Microbiol. 2020, 11, 600221. [Google Scholar] [CrossRef]
- Jeong, G.J.F.; Khan, F.; Tabassum, N.; Cho, K.J.; Kim, Y.M. Bacterial Extracellular Vesicles: Modulation of Biofilm and Virulence Properties. Acta Biomat. 2024, 178, 13–23. [Google Scholar] [CrossRef]
- Kadurugamuwa, J.L.; Beveridge, T.J. Virulence Factors Are Released from Pseudomonas aeruginosa in Association with Membrane Vesicles during Normal Growth and Exposure to Gentamicin: A Novel Mechanism of Enzyme Secretion. J. Bacteriol. 1995, 177, 3998–4008. [Google Scholar] [CrossRef]
- Pettit, R.K.; Judd, R.C. The Interaction of Naturally Elaborated Blebs from Serum-Susceptible and Serum-Resistant Strains of Neisseria gonorrhoeae with Normal Human Serum. Mol. Microbiol. 1992, 6, 729–734. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, Y.; Bu, Y.; Ren, X.; Dong, Z. Review on Bacterial Outer Membrane Vesicles: Structure, Vesicle Formation, Separation and Biotechnological Applications. Microbiol. Cell Factories 2025, 24, 27. [Google Scholar] [CrossRef]
- Shoberg, R.J.; Thomas, D.D. Specific Adherence of Borrelia burgdorferi Extracellular Vesicles to Human Endothelial Cells in Culture. Infect. Immun. 1993, 61, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Skare, J.T.; Shang, E.S.; Foley, D.M.; Blanco, D.R.; Champion, C.I.; Mirzabekov, T.; Sokolov, Y.; Kagan, B.L.; Miller, J.N.; Lovett, M.A. Virulent Strain Associated Outer Membrane Proteins of Borrelia burgdorferi. J. Clin. Investig. 1995, 96, 2380–2392. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.; Coleman, J.L.; Kuhlow, C.J.; Crowley, J.T.; Benach, J.L. The Enolase of Borrelia burgdorferi Is a Plasminogen Receptor Released in Outer Membrane Vesicles. Infect. Immun. 2012, 80, 359–368. [Google Scholar] [CrossRef]
- Wawrzeniak, K.; Gaur, G.; Sapi, E.; Senejani, A.G. Effect of Borrelia burgdorferi Outer Membrane Vesicles on Host Oxidative Stress Response. Antibiotics 2020, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, K.; Tammisto, H.; Nykky, J.; Gilbert, L. Borrelia burgdorferi Outer Membrane Vesicles Contain Antigenic Proteins, but Do Not Induce Cell Death in Human Cells. Microorganisms 2022, 10, 212. [Google Scholar] [CrossRef]
- Strnad, M.; Rudenko, N.; Rego, R.O.M. Pathogenicity and Virulence of Borrelia burgdorferi. Virulence 2023, 14, 2265015. [Google Scholar] [CrossRef]
- Tracy, K.E.; Baumgarth, N. Borrelia burgdorferi Manipulates Innate and Adaptive Immunity to Establish Persistence in Rodent Reservoir Hosts. Front. Immunol. 2017, 8, 116. [Google Scholar] [CrossRef]
- Ma, Y.; Sturrock, A.; Weiss, J.J. Intracellular Localization of Borrelia burgdorferi Within Human Endothelial Cells. Infect. Immun. 1991, 59, 671–678. [Google Scholar] [CrossRef]
- Georgilis, K.; Peacocke, M.; Klempner, M.S. Fibroblasts Protect the Lyme Disease Spirochete, Borrelia burgdorferi, from Ceftriaxone In Vitro. J. Infect. Dis. 1992, 166, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Klempner, M.S.; Noring, R.; Rogers, R.A. Invasion of Human Skin Fibroblasts by the Lyme Disease Spirochete, Borrelia burgdorferi. J. Infect. Dis. 1993, 167, 1076–1081. [Google Scholar] [CrossRef]
- Livengood, J.A.; Gilmore, R.D., Jr. Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006, 8, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Hyde, J.A. Borrelia burgdorferi Keeps Moving and Carries On: A Review of Borrelial Dissemination and Invasion. Front. Immunol. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Gaur, G.; Sawant, J.Y.; Chavan, A.S.; Khatri, V.A.; Liu, Y.H.; Zhang, M.; Sapi, E. Effect of Invasion of Borrelia burgdorferi in Normal and Neoplastic Mammary Epithelial Cells. Antibiotics 2021, 10, 1295. [Google Scholar] [CrossRef]
- Khatri, V.A.; Paul, S.; Patel, N.J.; Thippani, S.; Sawant, J.Y.; Durkee, K.L.; Murphy, C.L.; Ortiz, G.A.; Valentino, J.A.; Jathan, J.; et al. Global Transcriptomic Analysis of Breast Cancer and Normal Mammary Epithelial Cells Infected with Borrelia burgdorferi. Eur. J. Microbiol. Immunol. 2023, 13, 63–76. [Google Scholar] [CrossRef]
- Malge, A.; Ghai, V.; Reddy, P.J.; Baxter, D.; Kim, T.; Moritz, R.L.; Wang, K. mRNA Transcript Distribution Bias between Borrelia burgdorferi Bacteria and Their Outer Membrane Vesicles. FEMS Microbiol. Lett. 2018, 365, fny135. [Google Scholar] [CrossRef]
- Crowley, J.T.; Toledo, A.M.; LaRocca, T.J.; Coleman, J.L.; London, E.; Benach, J.L. Lipid Exchange between Borrelia burgdorferi and Host Cells. PLoS Pathog. 2013, 9, e1003109. [Google Scholar] [CrossRef]
- Tammisto, H.; Karvonen, K. Purification of Borrelia burgdorferi Outer Membrane Vesicles. Method. Mol. Biol. 2024, 2742, 37–45. [Google Scholar]
- Klimentová, J.; Stulík, J. Methods of Isolation and Purification of Outer Membrane Vesicles from Gram-negative Bacteria. Microbiol. Res. 2015, 170, 1–9. [Google Scholar] [CrossRef]
- Laotee, S.; Arunmanee, W. Genetically Surface-Modified Escherichia coli Outer Membrane Vesicles Targeting MUC1 Antigen in Cancer Cells. Biotechnol. Rep. 2024, 44, e00854. [Google Scholar] [CrossRef] [PubMed]
- McCaig, W.D.; Koller, A.; Thanassi, D.G. Production of Outer Membrane Vesicles and Outer Membrane Tubes by Francisella novicida. J. Bacteriol. 2013, 195, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Garon, C.F.; Dorward, D.W.; Corwin, M.D. Structural Features of Borrelia burgdorferi—The Lyme Disease Spirochete: Silver Staining for Nucleic Acids. Scanning Microsc. Suppl. 1989, 3, 109–115. [Google Scholar] [PubMed]
- Li, D.; Zhu, L.; Wang, Y.; Zhou, X.; Li, Y. Bacterial Outer Membrane Vesicles in Cancer: Biogenesis, Pathogenesis, and Clinical Application. Biomed. Pharmacother. 2023, 165, 115120. [Google Scholar] [CrossRef]
- Xiang, S.; Yao, Q.; Khan, A.; Wang, D. Recent Advances in Bacterial Outer Membrane Vesicles: Effects on the Immune System, Mechanisms and Their Usage for Tumor Treatment. J. Pharm. Anal. 2024, 14, 101049. [Google Scholar] [CrossRef]
- Zhang, X.; Ding, W.; Yang, J.; Gao, L.; Wang, Q.; Wang, J.; Luo, Y.; Yuan, X.; Sun, B.; Yang, J.; et al. Mechanisms of Outer Membrane Vesicles in Bacterial Drug Resistance: Insights and Implications. Biochimie 2025. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, J.; Wang, S.; Zhou, J.; Qin, J.; Jia, Z.; Wang, Y.; Wang, Z.; Zhang, Y.; Hao, H. Research Progress on Bacterial Membrane Vesicles and Antibiotic Resistance. Int. J. Mol. Sci. 2022, 23, 11553. [Google Scholar] [CrossRef]
- Kouokam, J.C.; Wai, S.N.; Fällman, M.; Dobrindt, U.; Hacker, J.; Uhlin, B.E. Active Cytotoxic Necrotizing Factor 1 Associated with Outer Membrane Vesicles from Uropathogenic Escherichia coli. Infect. Immun. 2006, 74, 2022–2030. [Google Scholar] [CrossRef]
- Mondal, A.; Tapader, R.; Chatterjee, N.S.; Ghosh, A.; Sinha, R.; Koley, H.; Saha, D.R.; Chakrabarti, M.K.; Wai, S.N.; Pal, A. Cytotoxic and Inflammatory Responses Induced by Outer Membrane Vesicle-Associated Biologically Active Proteases from Vibrio cholerae. Infect. Immun. 2016, 84, 1478–1490. [Google Scholar] [CrossRef]
- Hellman, J.; Loiselle, P.M.; Zanzot, E.M.; Allaire, M.; Tehan, M.M.; Boyle, L.A.; Kurnick, J.T.; Warren, H.S. Release of Gram-Negative Outer-Membrane Proteins into Human Serum and Septic Rat Blood and Their Interactions with Immunoglobulin in Antiserum to Escherichia coli J5. J. Infect. Dis. 2000, 181, 1034–1043. [Google Scholar] [CrossRef]
- Keenan, J.; Day, T.; Neal, S.; Cook, B.; Perez-Perez, G.; Allardyce, R.; Bagshaw, P. A Role for the Bacterial Outer Membrane in the Pathogenesis of Helicobacter pylori Infection. FEMS Microbiol. Lett. 2000, 182, 259–264. [Google Scholar] [CrossRef]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of Extracellular Vesicles: Determining the Correct Approach. Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef]
- Sharif, E.; Eftekhari, Z.; Mohit, E. The Effect of Growth Stage and Isolation Method on Properties of ClearColi™ Outer Membrane Vesicles (OMVs). Curr. Microbiol. 2021, 78, 1602–1614. [Google Scholar] [CrossRef]
- Ahmed, A.A.Q.; Zheng, R.; Abdalla, A.M.E.; Bakadia, B.M.; Qi, F.; Xiao, L.; Atta, O.M.; Mao, L.; Yang, G. Heterogeneous Populations of Outer Membrane Vesicles Released from Helicobacter pylori SS1 with Distinct Biological Properties. Eng. Sci. 2021, 15, 148–165. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| 6% Rabbit Serum | 10% ED-FBS | |||||||
|---|---|---|---|---|---|---|---|---|
| Fold Change | Fold Change | |||||||
| Uninfected Control | ExoBacteria Kit | Ultracentrifuge | p-Value | Uninfected Control | ExoBacteria Kit | Ultracentrifuge | p-Value | |
| 24 h | 0.0 | 1.0 | 1.9 | 0.54 | 0.0 | 1.0 | 1.7 | 0.18 |
| 48 h | 0.0 | 1.0 | 4.0 | 0.01 (*) | 0.0 | 1.0 | 1.2 | 0.67 |
| 72 h | 0.0 | 1.0 | 1.8 | 0.22 | 0.0 | 1.0 | 0.5 | 0.22 |
| Method of Isolation | Accessibility | Stimulation Serum | Conditions for OMV Stimulation |
|---|---|---|---|
| Ultracentrifuge | Less accessible, specialized equipment | 6% Rabbit serum | 2 h at 37 °C with 5% CO2 |
| 10% Exosome-depleted FBS | |||
| ExoBacteriaTM kit | More accessible, commercially available, user-friendly | 6% Rabbit serum | |
| 10% Exosome-depleted FBS |
| Target Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Annealing Temperature |
|---|---|---|---|
| 16S rRNA | CCTGGCTTAGAACTAACG | CCTACAAAGCTTAATTCCTCAT | 52 °C |
| OspA | GAACCAGACT-GAATACACAGGA | TTCAGCAGTTAGAGTTCCTTCA | 60 °C |
| GAPDH | GGAGCGAGATCCCTCCAAAAT | GGCTGTTGTCATACTTCTCATGG | 52 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jathan, J.; Pandya, J.M.; Jain, M.; Kaithalapuram, T.; Cherukuri, D.; Sapi, E. Evaluating Outer Membrane Vesicle Isolation Techniques for Borrelia burgdorferi and Their Impact on Vesicle Composition, Gene Expression Profile and Uptake. Antibiotics 2025, 14, 1079. https://doi.org/10.3390/antibiotics14111079
Jathan J, Pandya JM, Jain M, Kaithalapuram T, Cherukuri D, Sapi E. Evaluating Outer Membrane Vesicle Isolation Techniques for Borrelia burgdorferi and Their Impact on Vesicle Composition, Gene Expression Profile and Uptake. Antibiotics. 2025; 14(11):1079. https://doi.org/10.3390/antibiotics14111079
Chicago/Turabian StyleJathan, Jasmine, Jay M. Pandya, Mahima Jain, Tejasri Kaithalapuram, Dhara Cherukuri, and Eva Sapi. 2025. "Evaluating Outer Membrane Vesicle Isolation Techniques for Borrelia burgdorferi and Their Impact on Vesicle Composition, Gene Expression Profile and Uptake" Antibiotics 14, no. 11: 1079. https://doi.org/10.3390/antibiotics14111079
APA StyleJathan, J., Pandya, J. M., Jain, M., Kaithalapuram, T., Cherukuri, D., & Sapi, E. (2025). Evaluating Outer Membrane Vesicle Isolation Techniques for Borrelia burgdorferi and Their Impact on Vesicle Composition, Gene Expression Profile and Uptake. Antibiotics, 14(11), 1079. https://doi.org/10.3390/antibiotics14111079







