New Antibiotics for Treating Infections Caused by Multidrug-Resistant Bacteria
Abstract
1. Introduction
2. New Antibiotics Simultaneously Covering CR-E, CR-PA and CR-AB
Cefiderocol: A New Siderophore Cephalosporin
3. New Antibiotics for CR-E
3.1. Plazomicin: A New Aminoglycoside
3.2. Aztreonam/Avibactam: A Revolutionary β-Lactam/β-Lactamase Inhibitor Combination
4. New Antibiotics for MRSA
Omadacycline: A New Generation Tetracycline
5. New Antibiotics for Pre-XDR Mycobacterium tuberculosis
Pretomanid
6. Some Comments on Other New Antibiotics
7. New Antibiotics: Problems
- Most have not been tested in clinical trials for use in less common severe infections (such as endocarditis, meningitis or osteomyelitis);
- There is a lack of data regarding their clinical use in special populations, such as children, the elderly, obese people and critically ill patients;
- Most of them belong to previously approved classes of antimicrobials, which increases the risk of rapid emergence and dissemination of bacterial strains harboring resistance mechanisms to those new recently approved antibiotics;
- They are expensive antibiotics.
8. New Therapeutic Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Schäberle, T.F.; Hack, I.M. Overcoming the current deadlock in antibiotic research. Trends Microbiol. 2014, 22, 165–167. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/WHO-EMP-IAU-2017.12 (accessed on 12 June 2025).
- Butler, M.S.; Gigante, V.; Sati, H.; Paulin, S.; Al-Sulaiman, L.; Rex, J.H.; Fernandes, P.; Arias, C.A.; Paul, M.; Thwaites, G.E.; et al. Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed. Antimicrob. Agents Chemother. 2022, 66, e0199121. [Google Scholar] [CrossRef]
- Kabbara, W.K.; Sadek, E.; Mansour, H. Sulbactam–Durlobactam: A Novel Antibiotic Combination for the Treatment of Acinetobacter baumannii–Calcoaceticus Complex (ABC) Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia. Can. J. Infect. Dis. Med. Microbiol. 2025, 2025, 2001136. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Bonomo, R.A. Sulbactam-durlobactam: A Step Forward in Treating Carbapenem-Resistant Acinetobacter baumannii (CRAB) Infections. Clin. Infect. Dis. 2023, 76, S163–S165. [Google Scholar] [CrossRef]
- Daikos, G.L.; Cisneros, J.M.; Carmeli, Y.; Wang, M.; Leong, C.L.; Pontikis, K.; Anderzhanova, A.; Florescu, S.; Kozlov, R.; Rodriguez-Noriega, E.; et al. Aztreonam–avibactam for the treatment of serious infections caused by metallo-β-lactamase-producing Gram-negative pathogens: A Phase 3 randomized trial (ASSEMBLE). JAC-Antimicrobial Resist. 2025, 7, dlaf131. [Google Scholar] [CrossRef]
- Bhowmick, T.; Cantón, R.; Pea, F.; Quevedo, J.; Henriksen, A.S.; Timsit, J.-F.; Kaye, K.S. Cefepime-enmetazobactam: First approved cefepime-β-lactamase inhibitor combination for multi-drug resistant Enterobacterales. Futur. Microbiol. 2025, 20, 277–286. [Google Scholar] [CrossRef]
- Mallalieu, N.L.; Winter, E.; Fettner, S.; Patel, K.; Zwanziger, E.; Attley, G.; Rodriguez, I.; Kano, A.; Salama, S.M.; Bentley, D.; et al. Safety and Pharmacokinetic Characterization of Nacubactam, a Novel β-Lactamase Inhibitor, Alone and in Combination with Meropenem, in Healthy Volunteers. Antimicrob. Agents Chemother. 2020, 64, e02229-19. [Google Scholar] [CrossRef]
- Barnes, M.D.; Taracila, M.A.; Good, C.E.; Bajaksouzian, S.; Rojas, L.J.; van Duin, D.; Kreiswirth, B.N.; Jacobs, M.R.; Haldimann, A.; Papp-Wallace, K.M.; et al. Nacubactam Enhances Meropenem Activity against Carbapenem-Resistant Klebsiella pneumoniae Producing KPC. Antimicrob. Agents Chemother. 2019, 63, e00432-19. [Google Scholar] [CrossRef]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam–β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef]
- Hoy, S.M. Contezolid: First Approval. Drugs 2021, 81, 1587–1591. [Google Scholar] [CrossRef] [PubMed]
- McLeod, S.M.; O’DOnnell, J.P.; Narayanan, N.; Mills, J.P.; Kaye, K.S. Sulbactam–durlobactam: A β-lactam/β-lactamase inhibitor combination targeting Acinetobacter baumannii. Futur. Microbiol. 2024, 19, 563–576. [Google Scholar] [CrossRef]
- Vrancianu, C.O.; Dobre, E.G.; Gheorghe, I.; Barbu, I.; Cristian, R.E.; Chifiriuc, M.C. Present and Future Perspectives on Therapeutic Options for Carbapenemase-Producing Enterobacterales Infections. Microorganisms 2021, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Cabañero-Navalón, M.D.; Lletí, M.S. Resistance to beta-lactams in Gram-negative bacilli: Relevance and potential therapeutic alternatives. Rev. Espanola de Quimioter. 2022, 35, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Yamano, Y.; Tone, K.; Kinoshita, M.; Sawada, T.; Nagata, T. Antimicrobial Resistance: Shionogi Advocates Policy Change to Address the Public Health Threat. Nature Portfolio’s Sponsor Features. 2020. Available online: https://www.nature.com/articles/d42473-020-00446-9 (accessed on 27 August 2025).
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef]
- Syed, Y.Y. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef]
- Rocha, D.M.G.C.; Viveiros, M.; Saraiva, M.; Osório, N.S. The Neglected Contribution of Streptomycin to the Tuberculosis Drug Resistance Problem. Genes 2021, 12, 2003. [Google Scholar] [CrossRef]
- Cox, G.; Ejim, L.; Stogios, P.J.; Koteva, K.; Bordeleau, E.; Evdokimova, E.; Sieron, A.O.; Savchenko, A.; Serio, A.W.; Krause, K.M.; et al. Plazomicin Retains Antibiotic Activity against Most Aminoglycoside Modifying Enzymes. ACS Infect. Dis. 2018, 4, 980–987. [Google Scholar] [CrossRef]
- Chambers, H.F. Omadacycline—The Newest Tetracycline. N. Engl. J. Med. 2019, 380, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Graber, E.M. Treating acne with the tetracycline class of antibiotics: A review. Dermatol. Rev. 2021, 2, 321–330. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Esquivel, J.; Zelenitsky, S.; Lawrence, C.K.; Adam, H.J.; Golden, A.; Hink, R.; Berry, L.; Schweizer, F.; Zhanel, M.A.; et al. Omadacycline: A Novel Oral and Intravenous Aminomethylcycline Antibiotic Agent. Drugs 2020, 80, 285–313. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Chen, C.; Cui, C.; Li, X.; Zhang, Y.; Liao, X.; Sun, J.; Liu, Y. Emerging High-Level Tigecycline Resistance: Novel Tetracycline Destructases Spread via the Mobile Tet(X). BioEssays 2020, 42, e2000014. [Google Scholar] [CrossRef]
- Bidell, M.R.; Lodise, T.P. Use of oral tetracyclines in the treatment of adult outpatients with skin and skin structure infections: Focus on doxycycline, minocycline, and omadacycline. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 915–931. [Google Scholar] [CrossRef]
- Markley, J.L.; Wencewicz, T.A. Tetracycline-Inactivating Enzymes. Front. Microbiol. 2018, 9, 1058. [Google Scholar] [CrossRef]
- Rusu, A.; Buta, E.L. The Development of Third-Generation Tetracycline Antibiotics and New Perspectives. Pharmaceutics 2021, 13, 2085. [Google Scholar] [CrossRef]
- Vena, A.; Castaldo, N.; Giacobbe, D.R.; Fantin, A.; Bassetti, M. Omadacycline for the treatment of skin and soft tissue infections. Curr. Opin. Infect. Dis. 2025, 38, 122–127. [Google Scholar] [CrossRef]
- Falzon, D.; Zignol, M.; Bastard, M.; Floyd, K.; Kasaeva, T. The impact of the COVID-19 pandemic on the global tuberculosis epidemic. Front. Immunol. 2023, 14, 1234785. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Meeting Report of the WHO Expert Consultation on the Definition of Extensively Drug-Resistant Tuberculosis, 27-29 October 2020; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240018662 (accessed on 20 May 2025).
- North, E.J.; Jackson, M.; Lee, R.E. New Approaches to Target the Mycolic Acid Biosynthesis Pathway for the Development of Tuberculosis Therapeutics. Curr. Pharm. Des. 2013, 20, 4357–4378. [Google Scholar] [CrossRef] [PubMed]
- Gils, T.; Lynen, L.; de Jong, B.C.; Van Deun, A.; Decroo, T. Pretomanid for tuberculosis: A systematic review. Clin. Microbiol. Infect. 2022, 28, 31–42. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2023; Available online: https://www.who.int/publications/i/item/9789240094000 (accessed on 28 September 2025).
- Tängdén, T.; Carrara, E.; Hellou, M.M.; Yahav, D.; Paul, M. Introducing new antibiotics for multidrug-resistant bacteria: Obstacles and the way forward. Clin. Microbiol. Infect. 2024, 31, 354–359. [Google Scholar] [CrossRef]
- Imran, M.; Ahmad, M.N.; Dasgupta, A.; Rana, P.; Srinivas, N.; Chopra, S. Novel Approaches for the Treatment of Infections Due to Multidrug-Resistant Bacterial Pathogens. Futur. Med. Chem. 2022, 14, 1133–1148. [Google Scholar] [CrossRef]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Dettori, S.; Di Bella, S.; Vena, A.; Granata, G.; Luzzati, R.; Petrosillo, N.; Bassetti, M. Bezlotoxumab for Preventing Recurrent Clostridioides difficile Infection: A Narrative Review from Pathophysiology to Clinical Studies. Infect. Dis. Ther. 2020, 9, 481–494. [Google Scholar] [CrossRef]
- Ray, K. Modifying recurrence of Clostridium difficile infection. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Schuch, R.; Cassino, C.; Vila-Farres, X. Direct Lytic Agents: Novel, Rapidly Acting Potential Antimicrobial Treatment Modalities for Systemic Use in the Era of Rising Antibiotic Resistance. Front. Microbiol. 2022, 13, 841905. [Google Scholar] [CrossRef]
- Fowler, V.G.; Das, A.F.; Lipka-Diamond, J.; Schuch, R.; Pomerantz, R.; Jáuregui-Peredo, L.; Bressler, A.; Evans, D.; Moran, G.J.; Rupp, M.E.; et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J. Clin. Investig. 2020, 130, 3750–3760. [Google Scholar] [CrossRef] [PubMed]
- Fowler, V.G.; Das, A.F.; Lipka-Diamond, J.; Ambler, J.E.; Schuch, R.; Pomerantz, R.; Cassino, C.; Jáuregui-Peredo, L.; Moran, G.J.; Rupp, M.E.; et al. Exebacase in Addition to Standard-of-Care Antibiotics for Staphylococcus aureus Bloodstream Infections and Right-Sided Infective Endocarditis: A Phase 3, Superiority-Design, Placebo-Controlled, Randomized Clinical Trial (DISRUPT). Clin. Infect. Dis. 2024, 78, 1473–1481. [Google Scholar] [CrossRef]
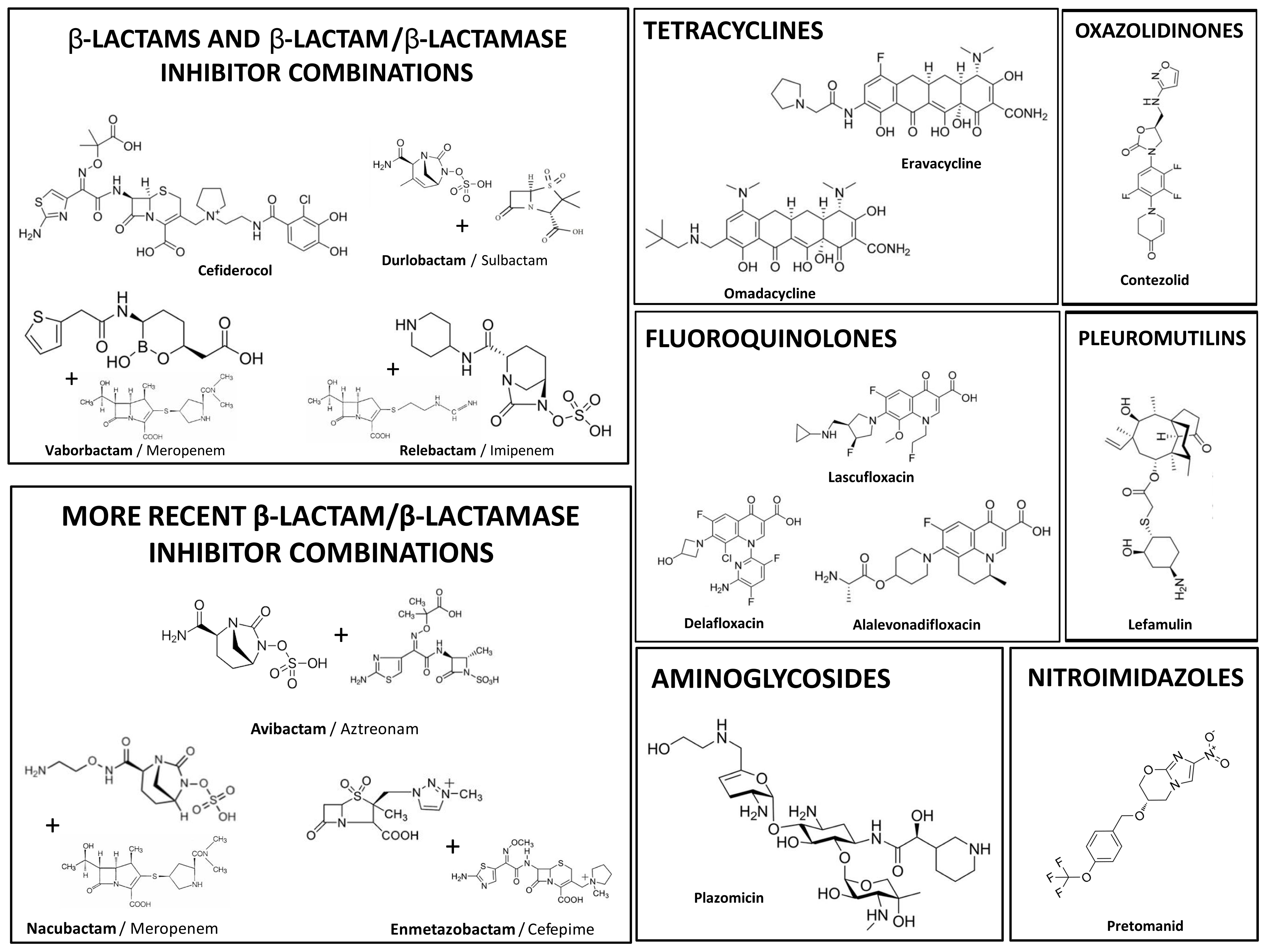
| Antibiotic/ Antibiotic Combination | Class | MDR Bacteria 2 | Year of Approval | Mechanism of Action 5 |
|---|---|---|---|---|
| Delafloxacin | Fluoroquinolone | MRSA | 2017 | Inhibition of bacterial DNA topoisomerase IV and DNA gyrase (topoisomerase II), disrupting DNA replication |
| Lascufloxacin | Fluoroquinolone | MRSA | 2019 | Inhibition of bacterial DNA topoisomerase IV and DNA gyrase (topoisomerase II), disrupting DNA replication |
| Alalevonadifloxacin | Fluoroquinolone | MRSA | 2020 | Inhibition of bacterial DNA topoisomerase IV and DNA gyrase (topoisomerase II), disrupting DNA replication |
| Meropenem/Vaborbactam 1a | β-Lactam (Carbapenem)/Boronate β-lactamase inhibitor | CR-E | 2017 | Inhibition of cell wall synthesis by blockage of PBPs (associated BLI protects from inactivation by class A β-lactamases) |
| Imipenem/Relebactam 1a | β-Lactam (Carbapenem)/Diazabicyclooctane β-lactamase inhibitor | CR-E | 2019 | Inhibition of cell wall synthesis by blockage of PBPs (associated BLI protects from inactivation by class A β-lactamases) |
| Aztreonam/Avibactam 1a | β-Lactam (Monobactam)/Diazabicyclooctane β-lactamase inhibitor | CR-E | 2025 | Inhibition of cell wall synthesis by blockage of PBPs without hydrolysis by class B β-lactamases (BLI protects from inactivation by class A and D β-lactamases) |
| Cefepime/Enmetazobactam 1a | β-Lactam (Cephalosporin)/Penicillanic acid sulfone β-lactamase inhibitor | ESBL-E | 2024 | Inhibition of cell wall synthesis by blockage of PBPs (associated BLI protects from inactivation by class A ESBL-type β-lactamases) |
| Meropenem/Nacubactam 1a | β-Lactam (Carbapenem)/Diazabicyclooctane β-lactamase inhibitor | CR-E | 2019 3 | Inhibition of cell wall synthesis by blockage of PBPs (associated BLI protects from inactivation by class A, C and some D β-lactamases) |
| Eravacycline | Tetracycline | CR-E | 2018 | Inhibition of protein synthesis at site A of 30S ribosomal subunit |
| Omadacycline | Tetracycline | MRSA, Streptococcus pneumoniae PNS | 2018 | Inhibition of protein synthesis at site A of 30S ribosomal subunit |
| Cefiderocol | β-Lactam (Cephalosporin) | CR-E, CR-PA, CR-AB | 2019 | Siderophore, entering through iron transport systems 6, further inhibiting cell wall synthesis by blockage of PBPs |
| Plazomicin | Aminoglycoside | CR-E | 2018 | Distortion of 30S ribosomal subunit, leading to production of abnormal proteins that modify cytoplasmic membrane permeability |
| Sulbactam/Durlobactam 1b | β-lactam-β-lactamase inhibitor/Diazabicyclooctane β-lactamase inhibitor | CR-AB | 2023 | Inhibition of cell wall synthesis by blockage of PBP3 and protection from inactivation by class A, C and D β-lactamases |
| Pretomanid | Nitroimidazole | Pre-XDR Mycobacterium tuberculosis | 2019 | Inhibition of mycolic acids synthesis and toxic action on the respiratory chain reducing intracellular ATP levels |
| Contezolid | Oxazolidinone | MRSA | 2021 3,4 | Inhibition of protein synthesis at 50S ribosomal subunit, by preventing the formation of the 70S initiation complex |
| Lefamulin | Pleuromutilin | S. pneumoniae PNS, Haemophilus influenzae AR | 2019 | Inhibition of protein synthesis at peptidyl transferase center of 50S ribosomal subunit, by preventing elongation |
| Category | Description |
|---|---|
| Traditional antibiotic approaches | New antibiotics from previously approved classes of antimicrobials |
| Non-traditional antibiotic approaches | New antibiotics with new mechanisms of action and/or bacterial targets |
| Non-antibiotic approaches | Molecules with different modes of action compared to the direct-acting antibiotics (e.g., by inhibiting virulence, boosting the immune system, restoring gut microbiome) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, E.; Sousa, J.C. New Antibiotics for Treating Infections Caused by Multidrug-Resistant Bacteria. Antibiotics 2025, 14, 997. https://doi.org/10.3390/antibiotics14100997
Machado E, Sousa JC. New Antibiotics for Treating Infections Caused by Multidrug-Resistant Bacteria. Antibiotics. 2025; 14(10):997. https://doi.org/10.3390/antibiotics14100997
Chicago/Turabian StyleMachado, Elisabete, and João Carlos Sousa. 2025. "New Antibiotics for Treating Infections Caused by Multidrug-Resistant Bacteria" Antibiotics 14, no. 10: 997. https://doi.org/10.3390/antibiotics14100997
APA StyleMachado, E., & Sousa, J. C. (2025). New Antibiotics for Treating Infections Caused by Multidrug-Resistant Bacteria. Antibiotics, 14(10), 997. https://doi.org/10.3390/antibiotics14100997






