Epidemiology, Pathogenesis, Clinical Features, and Management of Non-HACEK Gram-Negative Infective Endocarditis
Abstract
1. Introduction
2. Epidemiology and Etiology
3. Pathogenesis
3.1. Adhesion and Biofilm Formation in Gram-Negative Bacteria
3.2. Pseudomonas aeruginosa
3.3. Serratia marcescens
3.4. Klebsiella pneumoniae
3.5. Escherichia coli
| Pathogen | Entry and Source | Adhesion and Colonization | Biofilm Features | Other Pathogenesis Notes |
|---|---|---|---|---|
| Pseudomonas aeruginosa | Healthcare-associated infections Device-related infections | Type IV pili, flagella, adhesins Alginate enhances binding | Complex (flat or tower/mushroom) Alginate and rhamnolipids matrix QS-regulated (las, rhl systems) Architecture influenced by environmental factors | Toxins (exotoxin A, elastases, alkaline protease, hemolytic phospholipase C) Dispersal triggered by environment |
| Serratia marcescens | Injection drug use Healthcare-associated infections Device-related infections | Type I fimbriae (fimA, fimC) Extracellular polymeric substances aids device adhesion | Porous, filamentous QS-regulated Serrawettin-enhanced development Nutrient-dependent | QS controls proteases, lipases, hemolysins, prodigiosin and motility Injection equipment colonization in IDU |
| Klebsiella pneumoniae | Healthcare-associated infections | Type I/III fimbriae Capsular polysaccharides Adhesins | High prevalence of biofilm producers QS for intra- and interspecies communication | Multidrug resistance enzymes (ESBL, carbapenemases) Toxin production (hemolysins, cytotoxins) Antibiotic recalcitrance |
| Escherichia coli | Urinary tract infections | Poor cardiac endothelium adhesion Type I fimbriae ExPEC adherence proteins | High biofilm production in uropathogenic strains Increased in serum-resistant and MDR | ExPEC (especially B2 strains): siderophores, toxins Immune evasion |
4. Diagnosis
4.1. Evolution of Diagnostic Criteria for Infective Endocarditis
4.2. Advances in Microbiological and Molecular Diagnostics
4.2.1. Expanded List of Typical Pathogens
4.2.2. Diagnostic Criteria and Imaging Updates
4.2.3. New Molecular Techniques
4.3. Comparative Performance of Diagnostic Criteria: Sensitivity and Specificity
4.4. Focus on NHGNIE in Recent Studies
5. Clinical Features and Outcomes
5.1. Fever
5.2. Valvular Vegetations and Intra-Cardiac Abscesses
5.3. Heart Failure
5.4. Embolic Events
5.5. Septic Shock
5.6. Relapses and Recurrences
5.7. Mortality
5.8. Pathogen-Specific Profiles in NHGNIE
5.8.1. Pseudomonas spp.
5.8.2. Serratia spp.
5.8.3. Other Enterobacterales
6. Management
6.1. Antimicrobial Treatment
6.2. Surgical Treatment
7. Future Directions and Unmet Needs
7.1. Improved Epidemiological Understanding
7.2. Diagnostic Advances
7.3. Antimicrobial Strategies and Surgical Managment
- What should be considered as the first-line treatment of choice?
- Is there a role for combination therapy (especially in selected populations, e.g., patients with PVE or P. infections)?
- What is the most appropriate treatment duration?
- Is there a role for transition to oral therapy?
- Should patients with PVE not undergoing surgery receive suppressive antimicrobial treatment?
7.4. Translational Science and Novel Therapeutic Adjuncts
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NHGNIE | Non-HACEK Gram-Negative Infective Endocarditis |
| IE | Infective Endocarditis |
| PWID | people who inject drugs |
| PVE | prosthetic valve endocarditis |
| MDR | multidrug-resistant |
| EPS | extracellular polymeric substances |
| QS | quorum sensing |
| ExPEC | Extraintestinal Pathogenic Escherichia coli |
| UTI(s) | urinary tract infection(s) |
| ESC | European Society of Cardiology |
| ISCVID | International Society for Cardiovascular Infectious Diseases |
| CIED | cardiovascular implantable electronic device |
| PET/CT | positron emission tomography/computed tomography |
| 18F-FDG | Fluorine-18 Fluorodeoxyglucose (radiotracer used in PET scans) |
| WBC SPECT/CT | white blood cell single-photon emission-computed tomography/computed tomography |
| CT | computed tomography |
| BCNIE | blood-culture-negative infective endocarditis |
| PCR | polymerase chain reaction |
| ESBL(s) | extended-spectrum beta-lactamase(s) |
| NVE | native valve endocarditis |
| CNS | central nervous system |
| MRSA | methicillin-resistant Staphylococcus aureus |
| VRE | vancomycin-resistant Enterococcus |
| AHA | American Heart Association |
| BL | beta-lactams |
| AG | aminoglycosides |
| FQ | fluoroquinolones |
| p-value | p |
| aOR | Adjusted Odds Ratio |
| CI | Confidence Interval |
| IDU | injection drug use |
References
- Morpeth, S.; Murdoch, D.; Cabell, C.H.; Karchmer, A.W.; Pappas, P.; Levine, D.; Nacinovich, F.; Tattevin, P.; Fernández-Hidalgo, N.; Dickerman, S.; et al. Non-HACEK Gram-Negative Bacillus Endocarditis. Ann. Intern. Med. 2007, 147, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Clarke, L.G.; Shields, R.K. Epidemiology and Clinical Outcomes of Non-HACEK Gram-Negative Infective Endocarditis. Open Forum Infect. Dis. 2023, 10, ofad052. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, A.; Sobhanie, M.M.E.; Orzel, L.; Coe, K.; Wardlow, L. Clinical Outcomes of Combination versus Monotherapy for Gram Negative Non-HACEK Infective Endocarditis. Diagn. Microbiol. Infect. Dis. 2021, 101, 115504. [Google Scholar] [CrossRef] [PubMed]
- Veve, M.P.; McCurry, E.D.; Cooksey, G.E.; Shorman, M.A. Epidemiology and Outcomes of Non-HACEK Infective Endocarditis in the Southeast United States. PLoS ONE 2020, 15, e0230199. [Google Scholar] [CrossRef]
- de Sousa, L.P.; Fortes, C.Q.; Damasco, P.V.; Barbosa, G.I.F.; Golebiovski, W.F.; Weksler, C.; Garrido, R.Q.; Siciliano, R.F.; Lamas, C.d.C. Infective Endocarditis Due to Non-HACEK Gram-Negative Bacilli: Clinical Characteristics and Risk Factors from a Prospective Multicenter Brazilian Cohort. Trop. Med. Infect. Dis. 2023, 8, 283. [Google Scholar] [CrossRef]
- Burgos, L.M.; Oses, P.; Iribarren, A.C.; Pennini, M.; Merkt, M.; Vrancic, M.; Camporrotondo, M.; Ronderos, R.; Sucari, A.; Nacinovich, F. Endocarditis infecciosa por bacilos gram negativos no HACEK. Experiencia en un centro de alta complejidad de la República Argentina (1998–2016). Rev. Argent. Microbiol. 2019, 51, 136–139. [Google Scholar] [CrossRef]
- Dörfler, J.; Grubitzsch, H.; Schneider-Reigbert, M.; Pasic, M.; Pfäfflin, F.; Stegemann, M.; Sander, L.E.; Kurth, F.; Lingscheid, T. Non-HACEK Gram-Negative Bacilli Infective Endocarditis: Data from a Retrospective German Cohort Study. Infection 2025, 53, 405–413. [Google Scholar] [CrossRef]
- Al Janabi, J.; El Noaimi, M.; Sunnerhagen, T.; Snygg-Martin, U.; Rasmussen, M. Infective Endocarditis Caused by Non-HACEK Gram-Negative Bacteria, a Registry-Based Comparative Study. Open Forum Infect. Dis. 2025, 12, ofaf085. [Google Scholar] [CrossRef]
- Sebillotte, M.; Boutoille, D.; Declerck, C.; Talarmin, J.-P.; Lemaignen, A.; Piau, C.; Revest, M.; Tattevin, P.; Gousseff, M.; Groupe d‘Epidémiologie et Recherche en Infectiologie Clinique du Centre et de l’Ouest (GERICCO). Non-HACEK Gram-Negative Bacilli Endocarditis: A Multicentre Retrospective Case-Control Study. Infect. Dis. 2023, 55, 599–606. [Google Scholar] [CrossRef]
- Loubet, P.; Lescure, F.-X.; Lepage, L.; Kirsch, M.; Armand-Lefevre, L.; Bouadma, L.; Lariven, S.; Duval, X.; Yazdanpanah, Y.; Joly, V. Endocarditis Due to Gram-Negative Bacilli at a French Teaching Hospital over a 6-Year Period: Clinical Characteristics and Outcome. Infect. Dis. 2015, 47, 889–895. [Google Scholar] [CrossRef]
- Calderón Parra, J.; De Castro-Campos, D.; Muñoz García, P.; Olmedo Samperio, M.; Marín Arriaza, M.; De Alarcón, A.; Gutierrez-Carretero, E.; Fariñas Alvarez, M.C.; Miró Meda, J.M.; Goneaga Sanchez, M.Á.; et al. Non-HACEK Gram Negative Bacilli Endocarditis: Analysis of a National Prospective Cohort. Eur. J. Intern. Med. 2021, 92, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, M.; de la Torre, J.; Ivanova, R.; Martínez, F.J.; Lomas, J.M.; Plata, A.; Gálvez, J.; Reguera, J.M.; Ruiz, J.; Hidalgo, C.; et al. Endocarditis sobre válvulas izquierdas por bacilos gran negativos: Epidemiología y características clínicas. Enfermedades Infecc. Microbiol. Clin. 2011, 29, 276–281. [Google Scholar] [CrossRef]
- Ertugrul Mercan, M.; Arslan, F.; Ozyavuz Alp, S.; Atilla, A.; Seyman, D.; Guliyeva, G.; Kayaaslan, B.; Sari, S.; Mutay Suntur, B.; Isik, B.; et al. Non-HACEK Gram-Negative Bacillus Endocarditis. Med. Mal. Infect. 2019, 49, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Falcone, M.; Tiseo, G.; Durante-Mangoni, E.; Ravasio, V.; Barbaro, F.; Ursi, M.P.; Pasticci, M.B.; Bassetti, M.; Grossi, P.; Venditti, M.; et al. Risk Factors and Outcomes of Endocarditis Due to Non-HACEK Gram-Negative Bacilli: Data from the Prospective Multicenter Italian Endocarditis Study Cohort. Antimicrob. Agents Chemother. 2018, 62, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Panda, P.K.; Cr, P.; Uppal, L.; Saroch, A.; Angrup, A.; Sharma, N.; Sharma, Y.P.; Vijayvergiya, R.; Rohit, M.K.; et al. Changing Spectrum of Infective Endocarditis in India: An 11-Year Experience from an Academic Hospital in North India. Indian Heart J. 2021, 73, 711–717. [Google Scholar] [CrossRef]
- Thomas, V.V.; Mishra, A.K.; Jasmine, S.; Sathyendra, S. Gram-Negative Infective Endocarditis: A Retrospective Analysis of 10 Years Data on Clinical Spectrum, Risk Factor and Outcome. Monaldi Arch. Chest Dis. 2020, 90, 614–619. [Google Scholar] [CrossRef]
- Tran, H.M.; Truong, V.T.; Ngo, T.M.N.; Bui, Q.P.V.; Nguyen, H.C.; Le, T.T.Q.; Mazur, W.; Chung, E.; Cafardi, J.M.; Pham, K.P.N.; et al. Microbiological Profile and Risk Factors for In-Hospital Mortality of Infective Endocarditis in Tertiary Care Hospitals of South Vietnam. PLoS ONE 2017, 12, e0189421. [Google Scholar] [CrossRef]
- Mondal, U.; Warren, E.; Bookstaver, P.B.; Kohn, J.; Al-Hasan, M.N. Incidence and Predictors of Complications in Gram-Negative Bloodstream Infection. Infection 2024, 52, 1725–1731. [Google Scholar] [CrossRef]
- Maskarinec, S.A.; Thaden, J.T.; Cyr, D.D.; Ruffin, F.; Souli, M.; Fowler, V.G. The Risk of Cardiac Device-Related Infection in Bacteremic Patients Is Species Specific: Results of a 12-Year Prospective Cohort. Open Forum Infect. Dis. 2017, 4, ofx132. [Google Scholar] [CrossRef]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, 77, 518–526. [Google Scholar] [CrossRef]
- Ma, L.; Ge, Y.; Ma, H.; Zhu, B.; Miao, Q. Infective Endocarditis at a Tertiary-Care Hospital in China. J. Cardiothorac. Surg. 2020, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Leviner, D.B.; Schultz, I.; Friedman, T.; Leizarowitz, A.; Orvin, K.; Itelman, E.; Bolotin, G.; Sharoni, E. Similar Outcomes in Males and Females Undergoing Surgery for Infective Endocarditis. J. Clin. Med. 2024, 13, 4984. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, J.H.; Lee, J.A.; Ahn, S.M.; Han, M.; Ahn, J.Y.; Jeong, S.J.; Choi, J.Y.; Yeom, J.-S.; Lee, S.H.; et al. Clinical Characteristics and Risk Factors for Right-Sided Infective Endocarditis in Korea: A 12-Year Retrospective Cohort Study. Sci. Rep. 2024, 14, 10466. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Antimicrobial Resistance Collaborators. Global Burden of Bacterial Antimicrobial Resistance 1990-2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Pitsikakis, K.; Skandalakis, M.; Fragkiadakis, K.; Baliou, S.; Ioannou, P. Infective Endocarditis by Carbapenem-Resistant Gram-Negative Bacteria—A Systematic Review. Germs 2024, 14, 149–161. [Google Scholar] [CrossRef]
- Budea, C.M.; Pricop, M.; Mot, I.C.; Horhat, F.G.; Hemaswini, K.; Akshay, R.; Negrean, R.A.; Oprisoni, A.L.; Citu, C.; Bumbu, B.A.; et al. The Assessment of Antimicrobial Resistance in Gram-Negative and Gram-Positive Infective Endocarditis: A Multicentric Retrospective Analysis. Medicina 2023, 59, 457. [Google Scholar] [CrossRef]
- Gould, K.; Ramirez-Ronda, C.H.; Holmes, R.K.; Sanford, J.P. Adherence of Bacteria to Heart Valves in Vitro. J. Clin. Investig. 1975, 56, 1364–1370. [Google Scholar] [CrossRef]
- Aubron, C.; Charpentier, J.; Trouillet, J.-L.; Offenstadt, G.; Mercat, A.; Bernardin, G.; Hyvernat, H.; Wolff, M. Native-Valve Infective Endocarditis Caused by Enterobacteriaceae: Report on 9 Cases and Literature Review. Scand. J. Infect. Dis. 2006, 38, 873–881. [Google Scholar] [CrossRef]
- Keren, I.; Shah, D.; Spoering, A.; Kaldalu, N.; Lewis, K. Specialized Persister Cells and the Mechanism of Multidrug Tolerance in Escherichia coli. J. Bacteriol. 2004, 186, 8172–8180. [Google Scholar] [CrossRef]
- Rice, S.A.; Koh, K.S.; Queck, S.Y.; Labbate, M.; Lam, K.W.; Kjelleberg, S. Biofilm Formation and Sloughing in Serratia Marcescens Are Controlled by Quorum Sensing and Nutrient Cues. J. Bacteriol. 2005, 187, 3477–3485. [Google Scholar] [CrossRef]
- Elkins, J.G.; Hassett, D.J.; Stewart, P.S.; Schweizer, H.P.; McDermott, T.R. Protective Role of Catalase in Pseudomonas Aeruginosa Biofilm Resistance to Hydrogen Peroxide. Appl. Environ. Microbiol. 1999, 65, 4594–4600. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as Weapons against Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.; Shenoy, A.T.; Orihuela, C.J.; González-Juarbe, N. Killing of Serratia Marcescens Biofilms with Chloramphenicol. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 19. [Google Scholar] [CrossRef]
- Høiby, N.; Krogh Johansen, H.; Moser, C.; Song, Z.; Ciofu, O.; Kharazmi, A. Pseudomonas Aeruginosa and the in Vitro and in Vivo Biofilm Mode of Growth. Microbes Infect. 2001, 3, 23–35. [Google Scholar] [CrossRef]
- Hentzer, M.; Teitzel, G.M.; Balzer, G.J.; Heydorn, A.; Molin, S.; Givskov, M.; Parsek, M.R. Alginate Overproduction Affects Pseudomonas Aeruginosa Biofilm Structure and Function. J. Bacteriol. 2001, 183, 5395–5401. [Google Scholar] [CrossRef]
- Gervasoni, L.F.; Peixoto, I.C.; Imperador, A.C.; De Oliveira, L.B.; Correia, L.F.; de Oliveira Vieira, K.C.; Saeki, E.K.; da Silva Lima, P.E.; Mareco, E.A.; Pereira, V.C.; et al. Relationship between Antibiotic Resistance, Biofilm Formation, Virulence Factors and Source of Origin of Pseudomonas aeruginosa Environmental Isolates with Regard to the Presence of Metallo-β-Lactamase-Encoding Genes. Microb. Pathog. 2023, 182, 106223. [Google Scholar] [CrossRef]
- Barbosa, T.A.; Bentlin, M.R.; de Souza Rugolo, L.M.S.; Lyra, J.C.; Ferreira, A.M.; Santos, A.C.M.L.D.; Teixeira, N.B.; Medeiros Romero, L.C.; Castelo Branco Fortaleza, C.M.; Ribeiro de Souza da Cunha, M.d.L. Molecular Characterization of Gram-Negative Bacilli Isolated from a Neonatal Intensive Care Unit and Phenotypic and Molecular Detection of ESBL and Carbapenemase. Antibiotics 2025, 14, 342. [Google Scholar] [CrossRef]
- Giltner, C.L.; van Schaik, E.J.; Audette, G.F.; Kao, D.; Hodges, R.S.; Hassett, D.J.; Irvin, R.T. The Pseudomonas aeruginosa Type IV Pilin Receptor Binding Domain Functions as an Adhesin for Both Biotic and Abiotic Surfaces. Mol. Microbiol. 2006, 59, 1083–1096. [Google Scholar] [CrossRef]
- Jones, C.J.; Grotewold, N.; Wozniak, D.J.; Gloag, E.S. Pseudomonas aeruginosa Initiates a Rapid and Specific Transcriptional Response during Surface Attachment. J. Bacteriol. 2022, 204, e0008622. [Google Scholar] [CrossRef]
- Paulsson, M.; Su, Y.-C.; Ringwood, T.; Uddén, F.; Riesbeck, K. Pseudomonas Aeruginosa Uses Multiple Receptors for Adherence to Laminin during Infection of the Respiratory Tract and Skin Wounds. Sci. Rep. 2019, 9, 18168. [Google Scholar] [CrossRef]
- Sauer, K.; Cullen, M.C.; Rickard, A.H.; Zeef, L.A.H.; Davies, D.G.; Gilbert, P. Characterization of Nutrient-Induced Dispersion in Pseudomonas aeruginosa PAO1 Biofilm. J. Bacteriol. 2004, 186, 7312–7326. [Google Scholar] [CrossRef]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as Complex Differentiated Communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Whiteley, M.; Lee, K.M.; Greenberg, E.P. Identification of Genes Controlled by Quorum Sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 13904–13909. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.G.; Parsek, M.R.; Pearson, J.P.; Iglewski, B.H.; Costerton, J.W.; Greenberg, E.P. The Involvement of Cell-to-Cell Signals in the Development of a Bacterial Biofilm. Science 1998, 280, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Sauer, K. Biofilm Dispersion. Nat. Rev. Microbiol. 2020, 18, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-H.; Lai, H.-C.; Chen, S.-Y.; Yeh, M.-S.; Chang, J.-S. Biosurfactant Production by Serratia Marcescens SS-1 and Its Isogenic Strain SMdeltaR Defective in SpnR, a Quorum-Sensing LuxR Family Protein. Biotechnol. Lett. 2004, 26, 799–802. [Google Scholar] [CrossRef]
- Vieira, M.S.; da Silva, J.D.; Ferro, C.G.; Cunha, P.C.; Vidigal, P.M.P.; da Silva, C.C.; de Paula, S.O.; Dias, R.S. A Highly Specific Serratia-Infecting T7-like Phage Inhibits Biofilm Formation in Two Different Genera of the Enterobacteriaceae Family. Res. Microbiol. 2021, 172, 103869. [Google Scholar]
- Labbate, M.; Zhu, H.; Thung, L.; Bandara, R.; Larsen, M.R.; Willcox, M.D.P.; Givskov, M.; Rice, S.A.; Kjelleberg, S. Quorum-Sensing Regulation of Adhesion in Serratia Marcescens MG1 Is Surface Dependent. J. Bacteriol. 2007, 189, 2702–2711. [Google Scholar] [CrossRef]
- Van Houdt, R.; Givskov, M.; Michiels, C.W. Quorum Sensing in Serratia. FEMS Microbiol. Rev. 2007, 31, 407–424. [Google Scholar] [CrossRef]
- Hawver, L.A.; Jung, S.A.; Ng, W.-L. Specificity and Complexity in Bacterial Quorum-Sensing Systems. FEMS Microbiol. Rev. 2016, 40, 738–752. [Google Scholar] [CrossRef]
- Labbate, M.; Queck, S.Y.; Koh, K.S.; Rice, S.A.; Givskov, M.; Kjelleberg, S. Quorum Sensing-Controlled Biofilm Development in Serratia Liquefaciens MG1. J. Bacteriol. 2004, 186, 692–698. [Google Scholar] [CrossRef]
- Ramanathan, S.; Ravindran, D.; Arunachalam, K.; Arumugam, V.R. Inhibition of Quorum Sensing-Dependent Biofilm and Virulence Genes Expression in Environmental Pathogen Serratia Marcescens by Petroselinic Acid. Antonie Van Leeuwenhoek 2018, 111, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Eberl, L.; Molin, S.; Givskov, M. Surface Motility of Serratia Liquefaciens MG1. J. Bacteriol. 1999, 181, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Mohankumar, R.; Kannappan, A.; Karthick Raja, V.; Archunan, G.; Karutha Pandian, S.; Ruckmani, K.; Veera Ravi, A. Exploring the Anti-Quorum Sensing and Antibiofilm Efficacy of Phytol against Serratia Marcescens Associated Acute Pyelonephritis Infection in Wistar Rats. Front. Cell. Infect. Microbiol. 2017, 7, 498. [Google Scholar] [CrossRef] [PubMed]
- Luque Paz, D.; Lakbar, I.; Tattevin, P. A Review of Current Treatment Strategies for Infective Endocarditis. Expert Rev. Anti-Infect. Ther. 2021, 19, 297–307. [Google Scholar] [CrossRef]
- Wildenthal, J.A.; Schwartz, D.J.; Nolan, N.S.; Zhao, L.; Robinson, J.I.; Jones, E.; Jawa, R.; Henderson, J.P.; Marks, L.R. Everything but the Kitchen Sink: An Analysis of Bacterial and Chemical Contaminants Found in Syringe Residue from People Who Inject Drugs. Open Forum Infect. Dis. 2024, 11, ofad628. [Google Scholar] [CrossRef]
- Remuzgo-Martínez, S.; Lázaro-Díez, M.; Mayer, C.; Aranzamendi-Zaldumbide, M.; Padilla, D.; Calvo, J.; Marco, F.; Martínez-Martínez, L.; Icardo, J.M.; Otero, A.; et al. Biofilm Formation and Quorum-Sensing-Molecule Production by Clinical Isolates of Serratia Liquefaciens. Appl. Environ. Microbiol. 2015, 81, 3306–3315. [Google Scholar] [CrossRef]
- Schroll, C.; Barken, K.B.; Krogfelt, K.A.; Struve, C. Role of Type 1 and Type 3 Fimbriae in Klebsiella Pneumoniae Biofilm Formation. BMC Microbiol. 2010, 10, 179. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Chao, X.; Xie, L.; Wang, H. The Characteristic of Virulence, Biofilm and Antibiotic Resistance of Klebsiella Pneumoniae. Int. J. Environ. Res. Public Health 2020, 17, 6278. [Google Scholar] [CrossRef]
- Guerra, M.E.S.; Destro, G.; Vieira, B.; Lima, A.S.; Ferraz, L.F.C.; Hakansson, A.P.; Darrieux, M.; Converso, T.R. Klebsiella Pneumoniae Biofilms and Their Role in Disease Pathogenesis. Front. Cell. Infect. Microbiol. 2022, 12, 877995. [Google Scholar] [CrossRef] [PubMed]
- Anderl, J.N.; Franklin, M.J.; Stewart, P.S. Role of Antibiotic Penetration Limitation in Klebsiella Pneumoniae Biofilm Resistance to Ampicillin and Ciprofloxacin. Antimicrob. Agents Chemother. 2000, 44, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Sayed, M.G.; Mansy, M.S.; El Borhamy, M.I.; Elsherif, H.M. Exploring Virulence Factors, Virulome, and Multidrug Resistance of Klebsiella Pneumoniae Strains Isolated from Patients with Central Line-Associated Bloodstream Infections. Sci. Rep. 2025, 15, 20230. [Google Scholar] [CrossRef] [PubMed]
- Sabença, C.; Costa, E.; Sousa, S.; Barros, L.; Oliveira, A.; Ramos, S.; Igrejas, G.; Torres, C.; Poeta, P. Evaluation of the Ability to Form Biofilms in KPC-Producing and ESBL-Producing Klebsiella Pneumoniae Isolated from Clinical Samples. Antibiotics 2023, 12, 1143. [Google Scholar] [CrossRef]
- Akuzawa, N.; Kurabayashi, M. Native Valve Endocarditis Due to Escherichia coli Infection: A Case Report and Review of the Literature. BMC Cardiovasc. Disord. 2018, 18, 195. [Google Scholar] [CrossRef]
- Watanakunakorn, C.; Burkert, T. Infective Endocarditis at a Large Community Teaching Hospital, 1980–1990. Medicine 1993, 72, 90–102. [Google Scholar] [CrossRef]
- Reyes, M.P.; Reyes, K.C. Gram-Negative Endocarditis. Curr. Infect. Dis. Rep. 2008, 10, 267–274. [Google Scholar] [CrossRef]
- Russo, T.A.; Johnson, J.R. Proposal for a New Inclusive Designation for Extraintestinal Pathogenic Isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef]
- Micenková, L.; Bosák, J.; Vrba, M.; Ševčíková, A.; Šmajs, D. Human Extraintestinal Pathogenic Escherichia coli Strains Differ in Prevalence of Virulence Factors, Phylogroups, and Bacteriocin Determinants. BMC Microbiol. 2016, 16, 218. [Google Scholar] [CrossRef]
- Raza, S.S.; Sultan, O.W.; Sohail, M.R. Gram-Negative Bacterial Endocarditis in Adults: State-of-the-Heart. Expert Rev. Anti-Infect. Ther. 2010, 8, 879–885. [Google Scholar] [CrossRef]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular Bacterial Biofilm-like Pods in Urinary Tract Infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef]
- Reisner, A.; Krogfelt, K.A.; Klein, B.M.; Zechner, E.L.; Molin, S. In Vitro Biofilm Formation of Commensal and Pathogenic Escherichia coli Strains: Impact of Environmental and Genetic Factors. J. Bacteriol. 2006, 188, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Sharma, M.; Chaudhary, U. Biofilm and Multidrug Resistance in Uropathogenic Escherichia coli. Pathog. Glob. Health 2015, 109, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Pant, N.D.; Khatiwada, S.; Chaudhary, R.; Banjara, M.R. Correlation between Biofilm Formation and Resistance toward Different Commonly Used Antibiotics along with Extended Spectrum Beta Lactamase Production in Uropathogenic Escherichia coli Isolated from the Patients Suspected of Urinary Tract Infections Visiting Shree Birendra Hospital, Chhauni, Kathmandu, Nepal. Antimicrob. Resist. Infect. Control 2016, 5, 5. [Google Scholar] [PubMed]
- Schembri, M.A.; Klemm, P. Biofilm Formation in a Hydrodynamic Environment by Novel Fimh Variants and Ramifications for Virulence. Infect. Immun. 2001, 69, 1322–1328. [Google Scholar] [CrossRef]
- Durack, D.T.; Lukes, A.S.; Bright, D.K. New Criteria for Diagnosis of Infective Endocarditis: Utilization of Specific Echocardiographic Findings. Duke Endocarditis Service. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the Management of Endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Schmidt-Hellerau, K.; Camp, J.; Marmulla, P.A.; Rieg, S.; Jung, N.; DESTINi (German Network for Clinical Studies in Infectious Diseases). In Which Patients Do the 2023 Duke-ISCVID Criteria for Infective Endocarditis Increase the Diagnosis of “Definite Endocarditis”?-A Preliminary Analysis in the Prospectively Evaluated DERIVE Cohort. J. Clin. Med. 2024, 13, 4721. [Google Scholar] [CrossRef]
- Goehringer, F.; Lalloué, B.; Selton-Suty, C.; Alla, F.; Botelho-Nevers, E.; Chirouze, C.; Curlier, E.; El Hatimi, S.; Gagneux-Brunon, A.; le Moing, V.; et al. Compared Performance of the 2023 Duke-International Society for Cardiovascular Infectious Diseases, 2000 Modified Duke, and 2015 European Society of Cardiology Criteria for the Diagnosis of Infective Endocarditis in a French Multicenter Prospective Cohort. Clin. Infect. Dis. 2024, 78, 937–948. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Monney, P.; Frank, M.; Tzimas, G.; Tozzi, P.; Kirsch, M.; Van Hemelrijck, M.; Bauernschmitt, R.; Epprecht, J.; Guery, B.; et al. Evaluation of the 2023 Duke-International Society of Cardiovascular Infectious Diseases Criteria in a Multicenter Cohort of Patients with Suspected Infective Endocarditis. Clin. Infect. Dis. 2024, 78, 949–955. [Google Scholar] [CrossRef]
- van der Vaart, T.W.; Bossuyt, P.M.M.; Durack, D.T.; Baddour, L.M.; Bayer, A.S.; Durante-Mangoni, E.; Holland, T.L.; Karchmer, A.W.; Miro, J.M.; Moreillon, P.; et al. External Validation of the 2023 Duke-International Society for Cardiovascular Infectious Diseases Diagnostic Criteria for Infective Endocarditis. Clin. Infect. Dis. 2024, 78, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, H.; Berge, A.; Jovanovic-Stjernqvist, M.; Hagstrand Aldman, M.; Krus, D.; Öberg, J.; Kahn, F.; Bläckberg, A.; Sunnerhagen, T.; Rasmussen, M. Performance of the 2023 Duke-International Society of Cardiovascular Infectious Diseases Diagnostic Criteria for Infective Endocarditis in Relation to the Modified Duke Criteria and to Clinical Management-Reanalysis of Retrospective Bacteremia Cohorts. Clin. Infect. Dis. 2024, 78, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Fourré, N.; Zimmermann, V.; Senn, L.; Monney, P.; Tzimas, G.; Tagini, F.; Tozzi, P.; Kirsch, M.; Guery, B.; Papadimitriou-Olivgeris, M. Comparison of the 2023 ISCVID and ESC Duke Clinical Criteria for the Diagnosis of Infective Endocarditis among Patients with Positive Blood Cultures for New Typical Microorganisms. Infection 2025, 53, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical Presentation, Aetiology and Outcome of Infective Endocarditis. Results of the ESC-EORP EURO-ENDO (European Infective Endocarditis) Registry: A Prospective Cohort Study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miró, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- Trifunovic, D.; Vujisic-Tesic, B.; Obrenovic-Kircanski, B.; Ivanovic, B.; Kalimanovska-Ostric, D.; Petrovic, M.; Boricic-Kostic, M.; Matic, S.; Stevanovic, G.; Marinkovic, J.; et al. The Relationship between Causative Microorganisms and Cardiac Lesions Caused by Infective Endocarditis: New Perspectives from the Contemporary Cohort of Patients. J. Cardiol. 2018, 71, 291–298. [Google Scholar] [CrossRef]
- Pericàs, J.M.; Hernández-Meneses, M.; Muñoz, P.; Álvarez-Uría, A.; Pinilla-Llorente, B.; de Alarcón, A.; Reviejo, K.; Fariñas, M.C.; Falces, C.; Goikoetxea-Agirre, J.; et al. Outcomes and Risk Factors of Septic Shock in Patients with Infective Endocarditis: A Prospective Cohort Study. Open Forum Infect. Dis. 2021, 8, ofab119. [Google Scholar] [CrossRef]
- van der Vaart, T.W.; Stuifzand, M.; Boekholdt, S.M.; Cramer, M.J.; Bonten, M.J.M.; Prins, J.M.; van der Meer, J.T.M. The Prevalence of Persistent Bacteraemia in Patients with a Non-Staphylococcal Infective Endocarditis, a Retrospective Cohort Study. Int. J. Cardiol. 2022, 367, 49–54. [Google Scholar] [CrossRef]
- Shah, S.; Clarke, L.; Davis, M.W.; Topal, J.E.; Shields, R.K. Clinical Manifestations and Treatment Outcomes for Patients with Pseudomonas Endocarditis. J. Antimicrob. Chemother. 2024, 79, 2017–2021. [Google Scholar] [CrossRef]
- Walczak, A.; McCarthy, K.; Paterson, D.L. A Contemporary Case Series of Pseudomonas aeruginosa Infective Endocarditis. Medicine 2023, 102, e32662. [Google Scholar] [CrossRef] [PubMed]
- Meena, D.S.; Kumar, D.; Kumar, B.; Bohra, G.K.; Midha, N.; Garg, M.K. Clinical Characteristics and Outcomes in Pseudomonas Endocarditis: A Systematic Review of Individual Cases: Systematic Review of Pseudomonas Endocarditis. Infection 2024, 52, 2061–2069. [Google Scholar] [CrossRef] [PubMed]
- Lorson, W.C.; Heidel, R.E.; Shorman, M.A. Microbial Epidemiology of Infectious Endocarditis in the Intravenous Drug Abuse Population: A Retrospective Study. Infect. Dis. Ther. 2019, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- McCrary, L.M.; Slain, D.; Shah, S.; Stoner, B.J.; Marx, A.H.; Schranz, A.J. Emergence of Infective Endocarditis Due to Serratia Spp.: Results of a Multicenter Cohort. Open Forum Infect. Dis. 2025, 12, ofaf036. [Google Scholar] [CrossRef]
- Shah, S.; McCrary, M.; Schranz, A.J.; Clarke, L.; Davis, M.W.; Marx, A.; Slain, D.; Stoner, B.J.; Topal, J.; Shields, R.K. Serratia Endocarditis: Antimicrobial Management Strategies and Clinical Outcomes. J. Antimicrob. Chemother. 2023, 78, 2457–2461. [Google Scholar] [CrossRef]
- Sinner, G.J.; Annabathula, R.; Viquez, K.; Alnabelsi, T.S.; Leung, S.W. Infective Endocarditis in Pregnancy from 2009 to 2019: The Consequences of Injection Drug Use. Infect. Dis. 2021, 53, 633–639. [Google Scholar] [CrossRef]
- McCann, T.; Elabd, H.; Blatt, S.P.; Brandt, D.M. Intravenous Drug Use: A Significant Risk Factor for Serratia Bacteremia. Ther. Adv. Infect. Dis. 2022, 9, 20499361221078116. [Google Scholar] [CrossRef]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef]
- Bouza, E.; Muñoz, P.; Burillo, A. Gram-Negative Endocarditis: Disease Presentation, Diagnosis and Treatment. Curr. Opin. Infect. Dis. 2021, 34, 672–680. [Google Scholar] [CrossRef]
- Archer, G.; Fekety, F.R., Jr. Experimental Endocarditis Due to Pseudomonas Aeruginosa. II. Therapy with Carbenicillin and Gentamicin. J. Infect. Dis. 1977, 136, 327–335. [Google Scholar] [CrossRef]
- Andriole, V.T. Synergy of Carbenicillin and Gentamicin in Experimental Infection with Pseudomonas. J. Infect. Dis. 1971, 124, S46–S55. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.T.; Young, L.S.; Hewitt, W.L. Antimicrobial Synergism in the Therapy of Gram-Negative Rod Bacteremia. Chemotherapy 1978, 24, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.G.; Lewis, J.W. Synergistic Interaction between Ofloxacin and Cefotaxime against Common Clinical Pathogens. Infection 1995, 23, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Burgess, D.S.; Nathisuwan, S. Cefepime, Piperacillin/Tazobactam, Gentamicin, Ciprofloxacin, and Levofloxacin Alone and in Combination against Pseudomonas aeruginosa. Diagn. Microbiol. Infect. Dis. 2002, 44, 35–41. [Google Scholar] [CrossRef]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef]
- Fatsis-Kavalopoulos, N.; Roelofs, L.; Andersson, D.I. Potential Risks of Treating Bacterial Infections with a Combination of β-Lactam and Aminoglycoside Antibiotics: A Systematic Quantification of Antibiotic Interactions in E. coli Bloodstream Isolates. EBioMedicine 2022, 78, 103979. [Google Scholar] [CrossRef]
- Cha, M.K.; Kang, C.I.; Kim, S.H. In Vitro Activities of 21 Antimicrobial Agents Alone and in Combination with Aminoglycosides or Fluoroquinolones against ESBL-Producing Escherichia coli Isolates Causing Bacteremia. Antimicrob. Agents Chemother. 2015, 59, 5834–5837. [Google Scholar] [CrossRef]
- Tang, P.-C.; Sánchez-Hevia, D.L.; Westhoff, S.; Fatsis-Kavalopoulos, N.; Andersson, D.I. Within-Species Variability of Antibiotic Interactions in Gram-Negative Bacteria. mBio 2024, 15, e0019624. [Google Scholar] [CrossRef]
- Neu, H.C. Synergy and Antagonism of Combinations with Quinolones. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 255–261. [Google Scholar] [CrossRef]
- Mayer, I.; Nagy, E. Investigation of the Synergic Effects of Aminoglycoside-Fluoroquinolone and Third-Generation Cephalosporin Combinations against Clinical Isolates of Pseudomonas Spp. J. Antimicrob. Chemother. 1999, 43, 651–657. [Google Scholar] [CrossRef]
- Song, W.; Woo, H.J.; Kim, J.S.; Lee, K.M. In Vitro Activity of Beta-Lactams in Combination with Other Antimicrobial Agents against Resistant Strains of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 2003, 21, 8–12. [Google Scholar] [CrossRef]
- Slade-Vitković, M.; Batarilo, I.; Bielen, L.; Maravić-Vlahoviček, G.; Bedenić, B. In Vitro Antibiofilm Activity of Fosfomycin Alone and in Combination with Other Antibiotics against Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa. Pharmaceuticals 2024, 17, 769. [Google Scholar] [CrossRef]
- Soares, A.; Alexandre, K.; Lamoureux, F.; Lemée, L.; Caron, F.; Pestel-Caron, M.; Etienne, M. Efficacy of a Ciprofloxacin/Amikacin Combination against Planktonic and Biofilm Cultures of Susceptible and Low-Level Resistant Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2019, 74, 3252–3259. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Tripodi, M.-F.; Albisinni, R.; Utili, R. Management of Gram-Negative and Fungal Endocarditis. Int. J. Antimicrob. Agents 2010, 36, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Durante-Mangoni, E.; Andini, R.; Agrusta, F.; Iossa, D.; Mattucci, I.; Bernardo, M.; Utili, R. Infective Endocarditis Due to Multidrug Resistant Gram-Negative Bacilli: Single Centre Experience over 5 Years. Eur. J. Intern. Med. 2014, 25, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Alifragki, A.; Kontogianni, A.; Protopapa, I.; Baliou, S.; Ioannou, P. Infective Endocarditis by Pasteurella Species: A Systematic Review. J. Clin. Med. 2022, 11, 5037. [Google Scholar] [CrossRef]
- Satta, G.; Gorton, R.L.; Kandil, H. Prosthetic Valve Endocarditis Caused by Pasteurella in a Penicillin Allergic Patient: Challenges in Diagnosis and Treatment. Infect. Dis. Rep. 2012, 4, e32. [Google Scholar] [CrossRef]
- Tirmizi, A.; Butt, S.; Molitorisz, S. First Reported Case of Pasteurella Pneumotropica Tricuspid Valve Endocarditis. Int. J. Cardiol. 2012, 161, e44–e45. [Google Scholar] [CrossRef]
- Spentzouri, D.; Baliou, S.; Ioannou, P. Infective Endocarditis by Capnocytophaga Species-A Narrative Review. Medicina 2024, 60, 382. [Google Scholar] [CrossRef]
- Banjari, M.; Haddad, E.; Bonnet, I.; Legrand, L.; Cohen, F.; Caumes, E.; Pourcher, V. Infective Endocarditis Due to Neisseria elongata: A Case Report and Literature Review. Infect. Dis. Now 2021, 51, 622–626. [Google Scholar] [CrossRef] [PubMed]
- La Bella, G.; Salvato, F.; Minafra, G.A.; Bottalico, I.F.; Rollo, T.; Barbera, L.; Tullio, S.D.; Corso, G.; Caputo, S.L.; Nittis, R.D.; et al. Successful Treatment of Aortic Endocarditis by Achromobacter xylosoxidans with Cefiderocol Combination Therapy in a Non-Hodgkin Lymphoma Patient: Case Report and Literature Review. Antibiotics 2022, 11, 1686. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.G.; Rays, J.; Kanegae, M.Y. Native-Valve Endocarditis Caused by Achromobacter xylosoxidans: A Case Report and Review of Literature. Autops. Case Rep. 2017, 7, 50–55. [Google Scholar] [CrossRef]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial Oral versus Intravenous Antibiotic Treatment of Endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef]
- Street, A.C.; Durack, D.T. Experience with Trimethoprim-Sulfamethoxazole in Treatment of Infective Endocarditis. Rev. Infect. Dis. 1988, 10, 915–921. [Google Scholar] [CrossRef]
- Reyes, M.P.; Ali, A.; Mendes, R.E.; Biedenbach, D.J. Resurgence of Pseudomonas Endocarditis in Detroit, 2006–2008. Medicine 2009, 88, 294–301. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Marelli, C.; Cattardico, G.; Fanelli, C.; Signori, A.; Di Meco, G.; Di Pilato, V.; Mikulska, M.; Mazzitelli, M.; Cattelan, A.M.; et al. Mortality in KPC-Producing Klebsiella Pneumoniae Bloodstream Infections: A Changing Landscape. J. Antimicrob. Chemother. 2023, 78, 2505–2514. [Google Scholar] [CrossRef]
- Rasmussen, M.; Gilje, P.; Fagman, E.; Berge, A. Bacteraemia with Gram-Positive Bacteria-When and How Do I Need to Look for Endocarditis? Clin. Microbiol. Infect. 2024, 30, 306–311. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Sellmyer, M.A.; Gowrishankar, G.; Ruiz-Bedoya, C.A.; Tucker, E.W.; Palestro, C.J.; Hammoud, D.A.; Jain, S.K. Molecular Imaging of Bacterial Infections: Overcoming the Barriers to Clinical Translation. Sci. Transl. Med. 2019, 11, eaax8251. [Google Scholar] [CrossRef]
- Ordonez, A.A.; Weinstein, E.A.; Bambarger, L.E.; Saini, V.; Chang, Y.S.; DeMarco, V.P.; Klunk, M.H.; Urbanowski, M.E.; Moulton, K.L.; Murawski, A.M.; et al. A Systematic Approach for Developing Bacteria-Specific Imaging Tracers. J. Nucl. Med. 2017, 58, 144–150. [Google Scholar] [CrossRef]
- Wardak, M.; Gowrishankar, G.; Zhao, X.; Liu, Y.; Chang, E.; Namavari, M.; Haywood, T.; Gabr, M.T.; Neofytou, E.; Chour, T.; et al. Molecular Imaging of Infective Endocarditis with 6″-[18F]Fluoromaltotriose Positron Emission Tomography-Computed Tomography. Circulation 2020, 141, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Schulte, J.; Maurer, A.; Domogalla, L.-C.; Steinacker, N.; Wadle, C.; Kinzler, J.; Eder, M.; von Zur Mühlen, C.; Krohn-Grimberghe, M.; Eder, A.-C. 2-[18F]F-p-Aminobenzoic Acid Specifically Detects Infective Endocarditis in Positron Emission Tomography. J. Infect. Dis. 2025, 231, e536–e544. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chu, B.; Wang, J.; Song, B.; Su, Y.; Wang, H.; He, Y. Multifunctional Nanoagents for Ultrasensitive Imaging and Photoactive Killing of Gram-Negative and Gram-Positive Bacteria. Nat. Commun. 2019, 10, 4057. [Google Scholar] [CrossRef]
- Alaguvel, V.; Khetarpal, A.K.; Jankeel, A.; Tapia-Cano, W.A.; Martinez, G.; Lorenzana, A.; Hsiao, Z.; Rose, W.; Sakoulas, G.; Ulloa, E.R. Rapid Valve Sterilization with Meropenem plus Ceftolozane/Tazobactam Combination Therapy for Pseudomonas aeruginosa Prosthetic Valve Endocarditis. JAC-Antimicrob. Resist. 2025, 7, dlaf112. [Google Scholar] [CrossRef]
- Tascini, C.; Antonelli, A.; Pini, M.; De Vivo, S.; Aiezza, N.; Bernardo, M.; Di Luca, M.; Rossolini, G.M. Infective Endocarditis Associated with Implantable Cardiac Device by Metallo-β-Lactamase-Producing Pseudomonas aeruginosa, Successfully Treated with Source Control and Cefiderocol plus Imipenem. Antimicrob. Agents Chemother. 2023, 67, e0131322. [Google Scholar] [CrossRef]
- Lima, O.; Sousa, A.; Filgueira, A.; Otero, A.; Cabaleiro, A.; Martinez-Lamas, L.; Vasallo, F.; Pérez-Rodríguez, M.T. Successful Ceftazidime-Avibactam Therapy in a Patient with Multidrug-Resistant Pseudomonas aeruginosa Infective Endocarditis. Infection 2022, 50, 1039–1041. [Google Scholar] [CrossRef]
- Shah, S.; Bremmer, D.N.; Kline, E.G.; Nicolau, D.P.; Shields, R.K. Ceftolozane/Tazobactam for Refractory P. aeruginosa Endocarditis: A Case Report and Pharmacokinetic Analysis. J. Infect. Chemother. 2022, 28, 87–90. [Google Scholar] [CrossRef]
- Tilanus, A.; Rincon, F.M.; Rivera, A.M. Native Mitral Valve Endocarditis Associated with KPC Producing Serratia Marcescens Bacteremia Successfully Treated with Mitral Valve Replacement and Ceftazidime-Avibactam. IDCases 2021, 24, e01137. [Google Scholar] [CrossRef]
- Alghoribi, M.F.; Alqurashi, M.; Okdah, L.; Alalwan, B.; AlHebaishi, Y.S.; Almalki, A.; Alzayer, M.A.; Alswaji, A.A.; Doumith, M.; Barry, M. Successful Treatment of Infective Endocarditis Due to Pandrug-Resistant Klebsiella Pneumoniae with Ceftazidime-Avibactam and Aztreonam. Sci. Rep. 2021, 11, 9684. [Google Scholar] [CrossRef]
- Edgeworth, J.D.; Merante, D.; Patel, S.; Young, C.; Jones, P.; Vithlani, S.; Wyncoll, D.; Roberts, P.; Jones, A.; Den Nagata, T.; et al. Compassionate Use of Cefiderocol as Adjunctive Treatment of Native Aortic Valve Endocarditis Due to Extremely Drug-Resistant Pseudomonas aeruginosa. Clin. Infect. Dis. 2019, 68, 1932–1934. [Google Scholar] [CrossRef]
- Iaccarino, A.; Barbone, A.; Basciu, A.; Cuko, E.; Droandi, G.; Galbiati, D.; Romano, G.; Citterio, E.; Fumero, A.; Scarfò, I.; et al. Surgical Challenges in Infective Endocarditis: State of the Art. J. Clin. Med. 2023, 12, 5891. [Google Scholar] [CrossRef]
- Mack, M.J.; Lancellotti, P. Early Surgery in Infective Endocarditis: Can It Be Too Early? J. Am. Coll. Cardiol. 2020, 76, 41–42. [Google Scholar] [CrossRef]
- Arjomandi Rad, A.; Zubarevich, A.; Osswald, A.; Vardanyan, R.; Magouliotis, D.E.; Ansaripour, A.; Kourliouros, A.; Sá, M.P.; Rassaf, T.; Ruhparwar, A.; et al. The Surgical Treatment of Infective Endocarditis: A Comprehensive Review. Diagnostics 2024, 14, 464. [Google Scholar] [CrossRef]
- Lau, L.; Baddour, L.; Fernández Hidalgo, N.; Brothers, T.D.; Kong, W.K.F.; Borger, M.A.; Duval, X.; Tribouilloy, C.; Obadia, J.-F.; Golian, M.; et al. Infective Endocarditis: It Takes a Team. Eur. Heart J. 2025, 46, 2275–2288. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Luckett, J.; Dubern, J.-F.; Figueredo, G.P.; Ison, E.; Carabelli, A.M.; Scurr, D.J.; Hook, A.L.; Kammerling, L.; da Silva, A.C.; et al. Combinatorial Discovery of Microtopographical Landscapes That Resist Biofilm Formation through Quorum Sensing Mediated Autolubrication. Nat. Commun. 2025, 16, 5295. [Google Scholar] [CrossRef] [PubMed]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-Biofilm Activity as a Health Issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef]
- Karayamparambil, B.J.; Vadakkan, K.; Thomas, S. The Impact of Antibiofilm Strategies in Controlling Microbial Colonization. Curr. Microbiol. 2025, 82, 351. [Google Scholar] [CrossRef]
- Dicks, L.M.T.; Vermeulen, W. Bacteriophage-Host Interactions and the Therapeutic Potential of Bacteriophages. Viruses 2024, 16, 478. [Google Scholar] [CrossRef]
- Venturini, C.; Petrovic Fabijan, A.; Fajardo Lubian, A.; Barbirz, S.; Iredell, J. Biological Foundations of Successful Bacteriophage Therapy. EMBO Mol. Med. 2022, 14, e12435. [Google Scholar] [CrossRef] [PubMed]
- Glonti, T.; Chanishvili, N.; Taylor, P.W. Bacteriophage-Derived Enzyme That Depolymerizes the Alginic Acid Capsule Associated with Cystic Fibrosis Isolates of Pseudomonas aeruginosa. J. Appl. Microbiol. 2010, 108, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.D.R.; Veiga, P.; Cerca, N.; Kropinski, A.M.; Almeida, C.; Azeredo, J.; Sillankorva, S. Development of a Phage Cocktail to Control Proteus Mirabilis Catheter-Associated Urinary Tract Infections. Front. Microbiol. 2016, 7, 1024. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.; Abedon, S. Bacteriophages and Their Enzymes in Biofilm Control. Curr. Pharm. Des. 2014, 21, 85–99. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- El Ghali, A.; Stamper, K.; Kunz Coyne, A.J.; Holger, D.; Kebriaei, R.; Alexander, J.; Lehman, S.M.; Rybak, M.J. Ciprofloxacin in Combination with Bacteriophage Cocktails against Multi-Drug Resistant Pseudomonas aeruginosa in Ex Vivo Simulated Endocardial Vegetation Models. Antimicrob. Agents Chemother. 2023, 67, e0072823. [Google Scholar] [CrossRef]
- Oechslin, F.; Piccardi, P.; Mancini, S.; Gabard, J.; Moreillon, P.; Entenza, J.M.; Resch, G.; Que, Y.-A. Synergistic Interaction between Phage Therapy and Antibiotics Clears Pseudomonas aeruginosa Infection in Endocarditis and Reduces Virulence. J. Infect. Dis. 2017, 215, 703–712. [Google Scholar] [CrossRef]
- Eiferman, V.; Vion, P.-A.; Bleibtreu, A. Phage Therapy as a Rescue Treatment for Recurrent Pseudomonas aeruginosa Bentall Infection. Viruses 2025, 17, 123. [Google Scholar] [CrossRef]
- Pedersen, E.C.; Lerche, C.J.; Schwartz, F.A.; Ciofu, O.; Azeredo, J.; Thomsen, K.; Moser, C. Bacteriophage Therapy and Infective Endocarditis—Is It Realistic? APMIS 2024, 132, 675–687. [Google Scholar] [CrossRef]
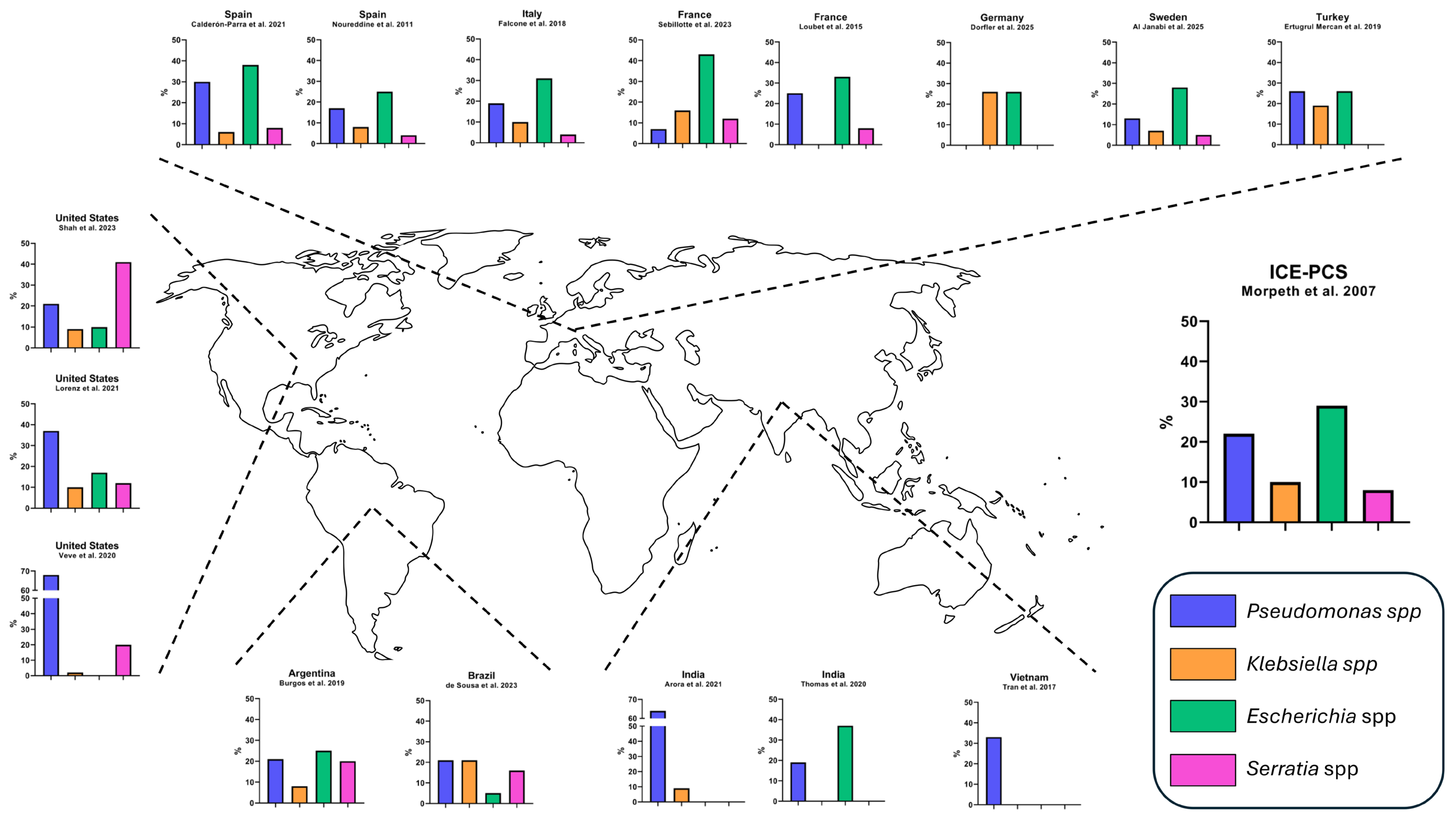
| Study | Year | Country, Setting | Cases | Median Age | Median Charlson | Previous IE | Nosocomial or Healthcare-Associated Acquisition | PWID | Source of Infection | Right-Sided IE | PVE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| International | |||||||||||
| Morpeth et al. 2007 [1] | 2000–2005 | International, multicenter | 49/2761 (1.8%) | 63 | 8% | Nosocomial 39% Healthcare-associated 17% | 4% | Genitourinary 22% Gastrointestinal 13% Skin 9% | 12% | 59% | |
| North America | |||||||||||
| Shah et al. 2023 [2] | 2010–2021 | USA, multicenter | 123 | 49 | 1 | 23% | 52% | 23% | 17% | ||
| Lorenz et al. 2021 [3] | 2011–2019 | USA, monocenter | 60/1036 (5.8%) | 50 | 3 | 40% | IVDU-related 35% Gastrointestinal 18% Genitourinary 17% CVC 5% | 25% | |||
| Veve et al. 2020 [4] | 2011–2019 | USA, monocenter | 43 | 40 | 67% | Healthcare-associated 49% | 93% | 63% | 30% | ||
| South America | |||||||||||
| de Sousa et al. 2023 [5] | 2006–2019 | Brazil, multicenter | 38/1154 (3.3%) | 57 | 8% | Nosocomial 53% Healthcare-associated 26% | 0% | CVC 29% Genitourinary 5% Gastrointestinal 5% | 11% | 47% | |
| Burgos et al. 2019 [6] | 1998–2016 | Argentina, monocenter | 31/452 (6.9%) | 72 | Healthcare-associated 70% | 0% | 46% | ||||
| Europe | |||||||||||
| Dorfler et al. 2025 [7] | 2015–2021 | Germany, monocenter | 19/1093 (1.7%) | 69 | 4 | 15% | Nosocomial 42% | 0% | Genitourinary 50% CVC 31% Gastrointestinal 25% | 0% | 37% |
| Al Janabi et al. 2025 [8] | 2008–2023 | Sweden, multicenter | 114/7426 (1.5%) | 69 | 16% | Nosocomial 9% Healthcare-associated 5% | 20% | 9% | 21% | ||
| Sebillotte et al. 2023 [9] | 2007–2020 | France, multicenter | 77/3230 (2.4%) | 69 | 2 | 8% | Healthcare-associated 36% | 8% | Genitourinary 33% Gastrointestinal 17% Skin 13% | 8% | 23% |
| Loubet et al. 2015 [10] | 2009–2014 | France, monocenter | 12 (4%) | 51 | 33% | Healthcare-associated 33% | 17% | Genitourinary 25% Gastrointestinal 25% | 33% | 67% | |
| Calderón-Parra et al. 2021 [11] | 2008–2018 | Spain, multicenter | 104/3910 (2.6%) | 71 | 5 | Nosocomial 48% Healthcare-associated 5% | 4% | Venous catheter 26% Genitourinary 22% Gastrointestinal 6% | 12% | 33% | |
| Noureddine et al. 2011 [12] | 1984–2008 | Spain, multicenter | 24/961 (2.5%) | 63 | 4 | Nosocomial 29% | 0% | 4% | 17% | ||
| Ertugrul Mercan et al. 2019 [13] | 2007–2016 | Turkey, multicenter | 26 | 53 | 4% | 0% | 19% | 19% | |||
| Falcone et al. 2018 [14] | 2004–2011 | Italy, multicenter | 58/1722 (3.4%) | 70 | Healthcare-associated 41% Nosocomial 3% | 9% | Genitourinary 28% Skin 14% Gastrointestinal 16% | 28% | |||
| Asia | |||||||||||
| Arora et al. 2021 [15] | 2010–2020 | India, monocenter | 11/199 (5.5%) | 55% | 45% | 18% | |||||
| Thomas et al. 2020 [16] | 2006–2016 | India, monocenter | 27/256 (10.7%) | 49 | 35% | ||||||
| Tran et al. 2017 [17] | 2005–2014 | Vietnam, monocenter | 6/189 (3.2%) | 17% | 33% | ||||||
| Study | Fever | Complications | Vegetations | Heart Failure | Cardiac Abscesses | Embolic Events | Septic Shock | Recurrence/Relapse | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Dorfler et al. 2025 [7] | 74% | 32% | 32% | 11% | In hospital 21% 1 year 44% | ||||
| Al Janabi et al. 2025 [8] | 31% | In hospital 17% | |||||||
| Shah et al. 2023 [2] | 61% | >10 mm 60% | 34% | 66% | 9 months 15% | In hospital 14% 90 days 20% | |||
| de Sousa et al. 2023 [5] | 58% | Median size 11 mm | 47% | 3% | 55% | 50% | |||
| Sebillotte et al. 2023 [9] | 88% | 16% | 5% | 31% | 20% | 5% | 1 year 36% | ||
| Arora et al. 2021 [15] | In hospital 36% | ||||||||
| Calderón-Parra et al. 2021 [11] | 76% | 69% | 33% | 17% | 20% | 21% | 1% | In hospital 37% 1 year 42% | |
| Lorenz et al. 2021 [3] | 72% >10 mm 38% | 42% | In hospital 6% 60 days 20% | ||||||
| Thomas et al. 2020 [16] | 100% | 51% | In hospital 30% | ||||||
| Veve et al. 2020 [4] | 65% | 40% | In hospital 5% 1 year 30% | ||||||
| Burgos et al. 2019 [6] | 84% | 21% | 19% | 3% | 10% | In hospital 21% | |||
| Ertugrul Mercan et al. 2019 [13] | 77% | 88% Median size 11 mm | 38% | 23% | In hospital 23% | ||||
| Falcone et al. 2018 [14] | 86% Median size 14 mm | 26% | 5% | 24% | 3% | In hospital 14% 1 year 31% | |||
| Loubet et al. 2015 [10] | 92% | 58% | 92% | 8% | 42% | 58% | 8% | In hospital 0% 1 year 8% | |
| Noureddine et al. 2011 [12] | 91% | 92% | 46% | 50% | 20% | 0% | In hospital 41% | ||
| Morpeth et al. 2007 [1] | 92% | 80% | 37% | 25% | 33% | In hospital 24% |
| European Society of Cardiology Guidelines [79] | American Heart Association Guidelines [99] | |
|---|---|---|
| Antibiotic therapy | β-lactams in combination with aminoglycoside, sometimes with additional fluoroquinolones or cotrimoxazole. | Combination antibiotic therapy with β-lactams and either aminoglycosides or fluoroquinolones. |
| Duration of antibiotic therapy | 6 weeks | 6 weeks |
| Surgical therapy | Always recommended, as early as possible. | Cardiac surgery is reasonable for most patients, particularly when left-side valves are involved. |
| Other recommendations | Consultation with endocarditis team when available. In vitro bactericidal tests and monitoring of serum antibiotic concentrations. | Consultation with infectious diseases specialist is recommended. In vitro susceptibility testing. |
| Study | Number of Patients | Pathogen | Combination Therapy | Therapy | Outcomes of Combination vs. Monotherapy |
|---|---|---|---|---|---|
| Shah et al. 2023 [2] | 123 | Serratia marcescens 41% Pseudomonas aeruginosa 21% Escherichia coli 10% Klebsiella pneumoniae 9% | 43% | BL + AG 45% BL + FQ 42% Other 13% | No overall benefit of combination therapy Serratia and Enterobacterales: Clinical failure 10% vs. 26% (p = 0.09) 90-day mortality 15% vs. 41% (p = 0.037) Pseudomonas aeruginosa: Clinical failure 37% vs. 0% (p = 0.134) 90-day mortality 21% vs. 0% |
| Calderon-Parra et al. 2021 [11] | 104 | Escherichia coli 38% Pseudomonas aeruginosa 30% Serratia spp. 8% Klebsiella spp. 7% | 79% | BL + AG 45% BL + FQ 30% Other 25% | In-hospital mortality (BL + FQ) aOR 0.29, CI 0.09–0.96 |
| Lorenz et al. 2021 [3] | 60 | Pseudomonas aeruginosa 37% Escherichia coli 17% Serratia spp. 12% Klebsiella pneumoniae 10% | 43% | BL + AG 38% BL + FQ 58% Other 4% | Composite outcome (60-day mortality/readmission/recurrence) aOR 0.45 (CI 0.13–1.6) Bacteremia recurrence 19% vs. 9% (p = 0.28) Readmission 31% vs. 41% (p = 0.36) Mortality 19% vs. 21% (p = 0.9) Adverse events 19% vs. 0% (p = 0.012) |
| Veve et al. 2020 [4] | 43 | Pseudomonas aeruginosa 68% Serratia marcescens 20% Klebsiella spp. 2% | 76% | BL + AG 50% BL + FQ 34% Other 16% | No overall benefit of combination therapy BL + FQ: 90-day mortality/readmission aOR 6.7 (CI 1.4–30.4) 12-month readmission aOR 33.9 (CI 2.7–429.9) |
| Falcone et al. 2018 [14] | 58 | Escherichia coli 31% Pseudomonas aeruginosa 19% Klebsiella pneumoniae 10% Serratia marcescens 4% | 86% | Non-carbapenem BL + AG 28% Non-carbapenem BL + FQ 16% Carbapenem + AG or FQ 8% Non-carbapenem BL + carbapenems ± AG or FQ 14% | Higher survival with penicillin/cephalosporin-based vs. carbapenem-based regimens (univariate analysis) |
| Morpeth et al. 2007 [1] | 49 | Escherichia coli 29% Pseudomonas aeruginosa 22% Klebsiella spp. 10% Serratia spp. 8% | 63% | BL + AG 57% BL + FQ 30% BL + AG and FQ 7% Other 6% | In-hospital mortality 27% vs. 22% (p = 0.73) |
| Meena et al. 2024 [93] | 218 (systematic review) | Pseudomonas aeruginosa | 77% | Penicillin/cephalosporins + AG 54% Carbapenem + AG 13% FQ-based combination 15% Polymyxin-based combination 11% Other 7% | Mortality OR 0.64 (CI 0.34–1.20) |
| Shah et al. 2024 [91] | 48 | Pseudomonas aeruginosa | 69% | BL + AG 61% BL + FQ 33% Other 6% | No benefit in response rate or survival Adverse events leading to discontinuation 21% vs. 0% (p = 0.08) |
| Walczak et al. 2023 [92] | 15 | Pseudomonas aeruginosa | 60% | BL + AG 67% BL + FQ 33% | 1-year mortality 36% vs. 75% (p = 0.282) |
| McCrary et al. 2025 [95] | 159 | Serratia spp. | 43% | BL + AG 43% BL + FQ 54% Other 1% | In-hospital mortality aOR 0.15 (CI 0.03–0.74) |
| Shah et al. 2023 [96] | 75 | Serratia spp. | 48% | BL + AG 33% BL + FQ 56% Other 11% | Clinical failure aOR 0.17 (CI 0.03–0.86) Microbiological failure 0% vs. 15% (p = 0.026) 90-day all-cause mortality 11% vs. 31% (p = 0.049) Adverse events leading to discontinuation 36% vs. 8% (p = 0.058) |
| Study | Number of Patients | Indication for Surgery | Surgery | Outcomes of Surgery vs. No Surgery |
|---|---|---|---|---|
| Dorfler et al. 2025 [7] | 19 | 10 (53%) | 8 (42%) | In-hospital mortality 25% vs. 18% (p = 1.0) 1-year mortality 25% vs. 45% (p = 0.633) |
| Shah et al. 2023 [2] | 123 | 84 (68%) | 49 (40%) | No surgery despite indication: Clinical failure aOR 7.44 (CI 2.37–23.4) 90-day mortality aOR 14.6 (CI 4.62–45.9) |
| De Sousa et al. 2023 [5] | 38 | 8 (21%) | Mortality 42% vs. 58% (not significant) | |
| Sebillotte et al. 2023 [9] | 77 | 12 (16%) | No difference in 1-year mortality (p = 0.29) | |
| Calderón Parra et al. 2021 [11] | 104 | 64 (62%) | 47 (45%) | No surgery despite indication: In-hospital mortality aOR 3.60 (CI 1.17–11.05) |
| Veve et al. 2020 [4] | 43 | 10 (23%) | No difference in 90-day or 1-year mortality (p = 0.29) | |
| Burgos et al. 2019 [6] | 24 | 9 (38%) | No difference in in-hospital mortality | |
| Ertugrul Mercan et al. 2019 [13] | 26 | 10 (38%) | In-hospital mortality 20% vs. 25% (not significant) | |
| Falcone et al. 2018 [14] | 58 | 25 (43%) | Patients with complications (cardiac abscess, fistula, dehiscence, valve perforation, heart failure): mortality 18% vs. 60% (p = 0.049) | |
| Morpeth et al. 2007 [1] | 49 | 25 (51%) | In-hospital mortality 24% vs. 25% (p = 0.94) | |
| McCrary et al. 2025 [95] | 159 (Serratia spp.) | 57 (36%) | Mortality aOR 0.14 (CI 0.03–0.64) | |
| Shah et al. 2023 [96] | 75 (Serratia spp.) | 58 (77%) | 34 (45%) | No surgery despite indication: Clinical failure aOR 3.84 (CI 4.5–105) |
| Shah et al. 2024 [91] | 48 (Pseudomonas spp.) | 30 (63%) | 17 (35%) | No difference in clinical failure |
| Meena et al. 2024 [93] | 218 (Pseudomonas spp, systematic review) | 125 (57%) | Mortality aOR 0.41 (CI 0.20–0.82) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monardo, R.; Papaioannu Borjesson, R.; Ponta, G.; Castagna, A.; Ripa, M. Epidemiology, Pathogenesis, Clinical Features, and Management of Non-HACEK Gram-Negative Infective Endocarditis. Antibiotics 2025, 14, 980. https://doi.org/10.3390/antibiotics14100980
Monardo R, Papaioannu Borjesson R, Ponta G, Castagna A, Ripa M. Epidemiology, Pathogenesis, Clinical Features, and Management of Non-HACEK Gram-Negative Infective Endocarditis. Antibiotics. 2025; 14(10):980. https://doi.org/10.3390/antibiotics14100980
Chicago/Turabian StyleMonardo, Roberta, Rebecka Papaioannu Borjesson, Giacomo Ponta, Antonella Castagna, and Marco Ripa. 2025. "Epidemiology, Pathogenesis, Clinical Features, and Management of Non-HACEK Gram-Negative Infective Endocarditis" Antibiotics 14, no. 10: 980. https://doi.org/10.3390/antibiotics14100980
APA StyleMonardo, R., Papaioannu Borjesson, R., Ponta, G., Castagna, A., & Ripa, M. (2025). Epidemiology, Pathogenesis, Clinical Features, and Management of Non-HACEK Gram-Negative Infective Endocarditis. Antibiotics, 14(10), 980. https://doi.org/10.3390/antibiotics14100980







