Should Microhematuria Be Incorporated into the 2023 Duke-International Society for Cardiovascular Infectious Diseases Minor Immunological Criteria?
Abstract
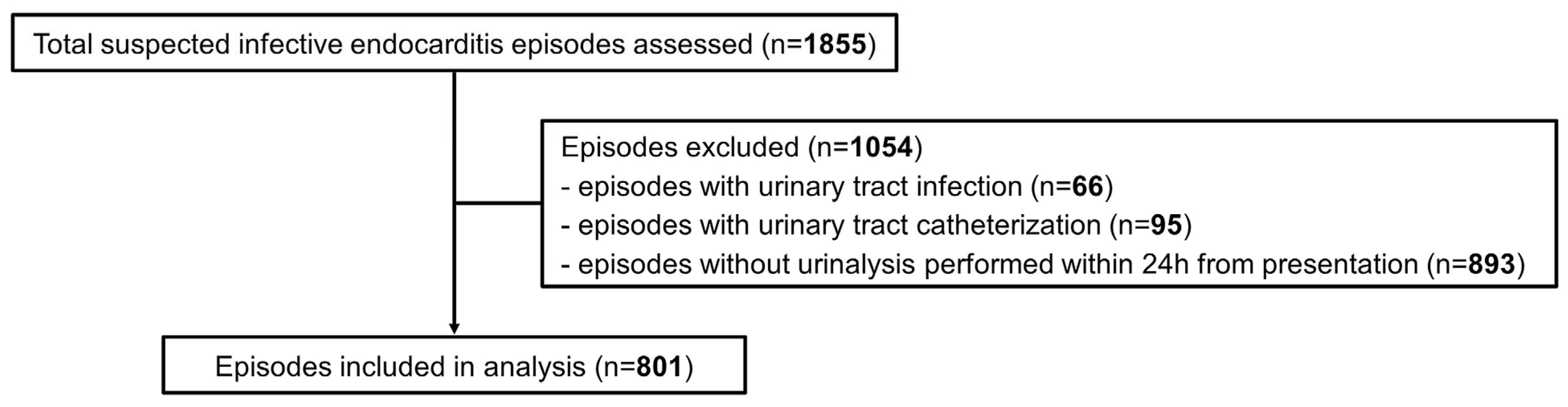
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van der Vaart, T.W.; Heerschop, L.L.; Bouma, B.J.; Freudenburg, W.; Bonten, M.J.M.; Prins, J.M.; van der Meer, J.T.M. Value of diagnosing immunological phenomena in patients with suspected endocarditis. Infection 2023, 51, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Lamas, C.C.; Eykyn, S.J. Suggested modifications to the Duke criteria for the clinical diagnosis of native valve and prosthetic valve endocarditis: Analysis of 118 pathologically proven cases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1997, 25, 713–719. [Google Scholar] [CrossRef] [PubMed]
- d’Almeida, S.; Reischmann, K.; Andress, S.; Felbel, D.; Stephan, T.; Hay, B.; Rohlmann, F.; Buckert, D.; Rottbauer, W.; Markovic, S. Evaluating the Duke Criteria for infectious endocarditis in a single-center retrospective study. Sci. Rep. 2024, 14, 19524. [Google Scholar] [CrossRef] [PubMed]
- Palepu, A.; Cheung, S.S.; Montessori, V.; Woods, R.; Thompson, C.R. Factors other than the Duke criteria associated with infective endocarditis among injection drug users. Clin. Investig. Med. 2002, 25, 118–125. [Google Scholar]
- Ghosh, S.; Sahoo, R.; Nath, R.K.; Duggal, N.; Gadpayle, A.K. A Study of Clinical, Microbiological, and Echocardiographic Profile of Patients of Infective Endocarditis. Int. Sch. Res. Not. 2014, 2014, 340601. [Google Scholar] [CrossRef]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; DiBernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 77, 518–526. [Google Scholar] [CrossRef]
- Delgado, V.; Ajmone Marsan, N.; de Waha, S.; Bonaros, N.; Brida, M.; Burri, H.; Caselli, S.; Doenst, T.; Ederhy, S.; Erba, P.A.; et al. 2023 ESC Guidelines for the management of endocarditis of the European Society of Cardiology (ESC). Eur. Heart. J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart. J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Corey, G.R.; Hoen, B.; Miro, J.M.; Fowler, V.G., Jr.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473. [Google Scholar] [CrossRef]
- van der Vaart, T.W.; Bossuyt, P.M.M.; Durack, D.T.; Baddour, L.M.; Bayer, A.S.; Durante-Mangoni, E.; Holland, T.L.; Karchmer, A.W.; Miro, J.M.; Moreillon, P.; et al. External Validation of the 2023 Duke-International Society for Cardiovascular Infectious Diseases Diagnostic Criteria for Infective Endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 78, 922–929. [Google Scholar] [CrossRef]
- Lindberg, H.; Berge, A.; Jovanovic-Stjernqvist, M.; Hagstrand Aldman, M.; Krus, D.; Oberg, J.; Kahn, F.; Blackberg, A.; Sunnerhagen, T.; Rasmussen, M. Performance of the 2023 Duke-ISCVID diagnostic criteria for infective endocarditis in relation to the modified Duke criteria and to clinical management-reanalysis of retrospective bacteremia cohorts. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 78, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Moisset, H.; Rio, J.; Benhard, J.; Arnoult, F.; Deconinck, L.; Grall, N.; Iung, B.; Lescure, X.; Rouzet, F.; Suc, G.; et al. Evaluation of the specificity of the 2023 Duke-International Society of Cardiovascular Infectious Diseases classification for infective endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 78, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Goehringer, F.; Lalloue, B.; Selton-Suty, C.; Alla, F.; Botelho-Nevers, E.; Chirouze, C.; Curlier, E.; El Hatimi, S.; Gagneux-Brunon, A.; le Moing, V.; et al. Compared Performance of the 2023 Duke-International Society for Cardiovascular Infectious Diseases, the 2000 Modified Duke, and the 2015 ESC Criteria for the Diagnosis of Infective Endocarditis in a French Multicenter Prospective Cohort. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 78, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Monney, P.; Frank, M.; Tzimas, G.; Tozzi, P.; Kirsch, M.; Van Hemelrijck, M.; Bauernschmitt, R.; Epprecht, J.; Guery, B.; et al. Evaluation of the 2023 Duke-ISCVID and 2023 Duke-ESC clinical criteria for the diagnosis of infective endocarditis in a multicenter cohort of patients with Staphylococcus aureus bacteremia. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 78, 655–662. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Monney, P.; Frank, M.; Tzimas, G.; Tozzi, P.; Kirsch, M.; Van Hemelrijck, M.; Bauernschmitt, R.; Epprecht, J.; Guery, B.; et al. Evaluation of the 2023 Duke-ISCVID criteria in a multicenter cohort of patients with suspected infective endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2024, 78, 949–955. [Google Scholar] [CrossRef]
- Majumdar, A.; Chowdhary, S.; Ferreira, M.A.; Hammond, L.A.; Howie, A.J.; Lipkin, G.W.; Littler, W.A. Renal pathological findings in infective endocarditis. Nephrol. Dial. Transplant. 2000, 15, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Westgeest, A.C.; Buis, D.T.P.; Sigaloff, K.C.E.; Ruffin, F.; Visser, L.G.; Yu, Y.; Schippers, E.F.; Lambregts, M.M.C.; Tong, S.Y.C.; de Boer, M.G.J.; et al. Global Differences in the Management of Staphylococcus aureus Bacteremia: No International Standard of Care. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 77, 1092–1101. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Monney, P.; Rotzinger, D.C.; Kamani, C.H.; Fahrni, G.; Prior, J.O.; Ianculescu, N.; Messaoudi, Y.; Tozzi, P.; Kirsch, M.; et al. Impact of thoracoabdominal imaging on diagnosis and management in patients with suspected infective endocarditis. Eur. J. Intern. Med. 2023, 116, 82–88. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Pouvaret, A.; Maillard, N.; Berthelot, P.; Lutz, M.F.; Cazorla, C.; Tulane, C.; Fuzellier, J.F.; Verhoeven, P.O.; Fresard, A.; et al. Acute kidney injury in infective endocarditis: A retrospective analysis. Med. Mal. Infect. 2019, 49, 527–533. [Google Scholar] [CrossRef]
- Zarbock, A.; Gomez, H.; Kellum, J.A. Sepsis-induced acute kidney injury revisited: Pathophysiology, prevention and future therapies. Curr. Opin. Crit. Care 2014, 20, 588–595. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Guery, B.; Ianculescu, N.; Dunet, V.; Messaoudi, Y.; Pistocchi, S.; Tozzi, P.; Kirsch, M.; Monney, P. Role of cerebral imaging on diagnosis and management in patients with suspected infective endocarditis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023, 77, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney. Int. Suppl. 2012, 2, 1–138. Available online: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf (accessed on 1 July 2025).
- Horie, S.; Ito, S.; Okada, H.; Kikuchi, H.; Narita, I.; Nishiyama, T.; Hasegawa, T.; Mikami, H.; Yamagata, K.; Yuno, T.; et al. Japanese guidelines of the management of hematuria 2013. Clin. Exp. Nephrol. 2014, 18, 679–689. [Google Scholar] [CrossRef]
| No Infective Endocarditis (n = 538) | Infective Endocarditis (n = 263) | p | |
|---|---|---|---|
| Demographics | |||
| Male sex | 378 (70) | 208 (79) | 0.008 |
| Age (years) | 69 (57–79) | 66 (50–74) | 0.005 |
| Age > 60 years | 370 (69) | 163 (62) | 0.067 |
| Cardiac predisposing factors | |||
| Intravenous drug use | 21 (4) | 38 (14) | <0.001 |
| Congenital disease | 13 (2) | 38 (14) | <0.001 |
| Prosthetic valve including transcatheter aortic valve replacement | 50 (9) | 85 (32) | <0.001 |
| Prior endocarditis | 11 (2) | 34 (13) | <0.001 |
| Moderate/severe valve regurgitation/stenosis | 23 (4) | 13 (5) | 0.7117 |
| CIED | 47 (9) | 40 (15) | 0.008 |
| Microbiological data | |||
| Bacteremia/candidemia | 404 (75) | 247 (94) | <0.001 |
| S. aureus | 193 (36) | 111 (42) | 0.088 |
| Coagulase-negative staphylococci | 42 (8) | 14 (5) | 0.238 |
| Streptococcus spp. | 79 (15) | 70 (27) | <0.001 |
| Enterococcus spp. | 36 (7) | 32 (12) | 0.010 |
| Other Gram-positive | 16 (3) | 7 (3) | 1.000 |
| HACEK | 3 (0.6) | 3 (1) | 0.400 |
| Gram-negative other than HACEK | 60 (11) | 9 (3) | <0.001 |
| Candida spp. | 20 (4) | 4 (2) | 0.121 |
| Microorganisms that occasionally or rarely cause IE isolated from at least three blood culture sets | 15 (3) | 14 (5) | 0.105 |
| New typical microorganism in the presence of intracardiac prosthetic material | 80 (15) | 20 (8) | 0.003 |
| Positive serology for Coxiella burnetiid or Bartonella henselae/quintana | 1 (0.2) | 1 (0.4) | 0.549 |
| Imaging data | |||
| Positive echocardiography for vegetation, perforation, abscess, aneurysm, pseudoaneurysm, fistula | 7 (1) | 156 (59) | <0.001 |
| Abnormal metabolic activity in 18F-FDG PET/CT | 1 (0.2) | 39 (15) | <0.001 |
| Positive cardiac-CT for vegetation, perforation, abscess, aneurysm, pseudoaneurysm, fistula | 1 (0.2) | 20 (8) | <0.001 |
| Significant new valvular regurgitation on echocardiography as compared to previous imaging | 17 (3) | 93 (35) | <0.001 |
| Manifestations | |||
| Fever (temperature > 38 °C) | 439 (82) | 232 (88) | 0.019 |
| Immunological phenomena a | 14 (3) | 28 (11) | <0.001 |
| Glomerulonephritis a | 2 (0.4) | 9 (3) | 0.001 |
| Embolic events a | 65 (12) | 159 (61) | <0.001 |
| Hematogenous osteoarticular septic complications | 46 (9) | 45 (17) | 0.001 |
| Septic arthritis | 24 (5) | 26 (10) | 0.005 |
| Vertebral and non-vertebral osteomyelitis | 31 (6) | 23 (9) | 0.133 |
| Urinalysis results | |||
| Red blood cells (×106/L) | 10 (0–80) | 20 (0–80) | 0.190 |
| Microhematuria (red blood cells > 5/HPF) | 302 (56) | 160 (61) | 0.223 |
| Microhematuria (red blood cells > 17/HPF) | 231 (43) | 132 (50) | 0.059 |
| White blood cells (×106/L) | 1 (0–70) | 0 (0–25) | 0.018 |
| Pyuria (white blood cells > 10/HPF) | 233 (43) | 96 (37) | 0.067 |
| Proteinuria (g/L) | 0.25 (0–0.75) | 0.25 (0–0.75) | 0.650 |
| Proteinuria (>0.3 g/L) | 224 (42) | 112 (43) | 0.819 |
| Renal function upon presentation | |||
| Creatinine (μmol/L) | 111 (76–169) | 110 (81–167) | 0.868 |
| Acute kidney injury | 199 (37) | 110 (42) | 0.190 |
| Stage I | 139 (70) | 64 (58) | 0.049 |
| Stage II | 35 (18) | 20 (18) | |
| Stage III | 25 (13) | 26 (24) | |
| Data on surgery/CIED-extraction/histopathology | |||
| Valve surgery performed | 10 (2) | 97 (37) | <0.001 |
| CIED-extraction (among 87 patients with CIED) | 4 (9) | 18 (45) | <0.001 |
| Autopsy performed | 4 (0.7) | 8 (3) | 0.024 |
| Histopathology compatible for IE | 0 (0) | 50 (19) | <0.001 |
| Positive culture of vegetation, abscess | 0 (0) | 37 (14) | <0.001 |
| Positive nucleic acid-based tests | 0 (0) | 13 (5) | <0.001 |
| Macroscopic evidence of IE by inspection (surgery/autopsy) | 0 (0) | 66 (25) | <0.001 |
| No Infective Endocarditis (n = 538) | Infective Endocarditis (n = 263) | |
|---|---|---|
| Duke major clinical criteria | ||
| Major imaging criterion | 23 (4) | 194 (74) |
| Major surgery criterion | 0 (0) | 4 (2) |
| Major microbiological criterion | 228 (42) | 223 (89) |
| Duke minor clinical criteria | ||
| Minor microbiological criterion | 123 (19) | 8 (5) |
| Minor predisposition criterion | 145 (27) | 184 (70) |
| Minor vascular criterion | 65 (12) | 159 (41) |
| Minor fever criterion | 439 (82) | 232 (88) |
| Minor immunological criterion (without microhematuria; original version) | 14 (3) | 28 (11) |
| Minor immunological criterion (with microhematuria > 5/HPF) | 305 (57) | 168 (64) |
| Minor immunological criterion (with microhematuria > 17/HPF) | 235 (44) | 143 (55) |
| Classification according to 2023 ISCVID-Duke clinical criteria without microhematuria (original version) | ||
| Rejected | 282 (52) | 1 (0.4) |
| Possible | 241 (45) | 66 (25) |
| Definite | 15 (3) | 196 (75) |
| Classification according to 2023 ISCVID-Duke clinical criteria with microhematuria > 5/HPF | ||
| Rejected | 217 (40) | 0 (0) |
| Possible | 266 (49) | 38 (14) |
| Definite | 55 (10) | 225 (86) |
| Classification according to 2023 ISCVID-Duke clinical criteria with microhematuria > 17/HPF | ||
| Rejected | 231 (43) | 0 (0) |
| Possible | 263 (49) | 44 (17) |
| Definite | 44 (8) | 219 (83) |
| Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | Accuracy % (95% CI) | |
|---|---|---|---|---|---|
| Without microhematuria (original version) | 75 (69–80) | 52 (48–57) | 43 (41–46) | 81 (77–84) | 60 (56–63) |
| With microhematuria > 5/HPF | 86 (81–90) | 40 (36–45) | 41 (39–43) | 85 (81–89) | 55 (52–59) |
| With microhematuria > 17/HPF | 83 (78–88) | 43 (39–47) | 42 (39–44) | 84 (80–87) | 56 (53–60) |
| Without Microhematuria (n = 339) | With Microhematuria (n = 462) | p | |
|---|---|---|---|
| Demographics | |||
| Male sex | 259 (76) | 327 (71) | 0.090 |
| Age (years) | 66 (53–76) | 70 (55–79) | <0.001 |
| Age > 60 years | 211 (62) | 322 (70) | 0.028 |
| Comorbidities | |||
| Diabetes mellitus | 74 (22) | 123 (27) | 0.135 |
| Obesity (body mass index ≥ 30 kg/m2) | 67 (20) | 107 (23) | 0.261 |
| Chronic kidney disease (eGFR < 60 mL/min/1.73 m2) | 78 (23) | 120 (26) | 0.362 |
| Malignancy (solid organ or haematologic) | 77 (23) | 93 (20) | 0.383 |
| Chronic obstructive pulmonary disease | 41 (12) | 47 (10) | 0.424 |
| Cirrhosis | 28 (8) | 42 (9) | 0.706 |
| Congestive heart failure | 39 (12) | 47 (10) | 0.565 |
| Manifestations upon presentation | |||
| Fever (temperature > 38 °C) | 276 (81) | 395 (86) | 0.146 |
| Sepsis or septic shock | 103 (30) | 219 (47) | <0.001 |
| Embolic events upon presentation a | 39 (19) | 150 (25) | 0.124 |
| Cerebral embolic events | 20 (10) | 63 (11) | 0.894 |
| Non-cerebral embolic events | 24 (12) | 116 (19) | 0.018 |
| Renal function upon presentation | |||
| Creatinine (μmol/L) | 95 (69–133) | 133 (87–190) | <0.001 |
| Acute kidney injury | 99 (29) | 210 (46) | <0.001 |
| Stage I | 81 (82) | 122 (58) | <0.001 |
| Stage II | 16 (16) | 39 (18) | |
| Stage III | 2 (2) | 49 (23) | |
| Diagnosis | |||
| Non-infectious diagnosis | 48 (14) | 31 (7) | 0.001 |
| Bacteremia/candidemia of unknown origin | 22 (7) | 44 (10) | 0.152 |
| Catheter-related | 59 (17) | 49 (11) | 0.006 |
| Low-respiratory tract infection | 21 (6) | 29 (6) | 1.000 |
| Abdominal infection | 15 (4) | 9 (2) | 0.057 |
| Skin and soft tissue infection | 32 (9) | 38 (8) | 0.613 |
| Bone and joint infections b | 37 (11) | 99 (21) | <0.001 |
| Septic arthritis | 9 (3) | 41 (9) | <0.001 |
| Vertebral and non-vertebral osteomyelitis | 16 (5) | 38 (8) | 0.063 |
| Osteoarticular implant-associated infection | 10 (3) | 22 (5) | 0.208 |
| Infective endocarditis | 103 (30) | 160 (35) | 0.223 |
| Other infection | 47 (14) | 88 (19) | 0.056 |
| Bacteremia/candidemia | 253 (75) | 398 (86) | <0.001 |
| S. aureus | 112 (33) | 192 (42) | 0.015 |
| Coagulase-negative staphylococci | 27 (8) | 29 (6) | 0.401 |
| Streptococcus spp. | 73 (22) | 76 (17) | 0.081 |
| Enterococcus spp. | 18 (5) | 50 (11) | 0.007 |
| Other Gram-positive | 13 (4) | 10 (2) | 0.199 |
| HACEK | 1 (0.3) | 5 (1) | 0.410 |
| Gram-negative other than HACEK | 31 (9) | 38 (8) | 0.703 |
| Candida spp. | 7 (2) | 17 (4) | 0.213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regina, J.; Stavart, L.; Guery, B.; Tzimas, G.; Monney, P.; Niclauss, L.; Kirsch, M.; Golshayan, D.; Papadimitriou-Olivgeris, M. Should Microhematuria Be Incorporated into the 2023 Duke-International Society for Cardiovascular Infectious Diseases Minor Immunological Criteria? Antibiotics 2025, 14, 687. https://doi.org/10.3390/antibiotics14070687
Regina J, Stavart L, Guery B, Tzimas G, Monney P, Niclauss L, Kirsch M, Golshayan D, Papadimitriou-Olivgeris M. Should Microhematuria Be Incorporated into the 2023 Duke-International Society for Cardiovascular Infectious Diseases Minor Immunological Criteria? Antibiotics. 2025; 14(7):687. https://doi.org/10.3390/antibiotics14070687
Chicago/Turabian StyleRegina, Jean, Louis Stavart, Benoit Guery, Georgios Tzimas, Pierre Monney, Lars Niclauss, Matthias Kirsch, Dela Golshayan, and Matthaios Papadimitriou-Olivgeris. 2025. "Should Microhematuria Be Incorporated into the 2023 Duke-International Society for Cardiovascular Infectious Diseases Minor Immunological Criteria?" Antibiotics 14, no. 7: 687. https://doi.org/10.3390/antibiotics14070687
APA StyleRegina, J., Stavart, L., Guery, B., Tzimas, G., Monney, P., Niclauss, L., Kirsch, M., Golshayan, D., & Papadimitriou-Olivgeris, M. (2025). Should Microhematuria Be Incorporated into the 2023 Duke-International Society for Cardiovascular Infectious Diseases Minor Immunological Criteria? Antibiotics, 14(7), 687. https://doi.org/10.3390/antibiotics14070687







