Synthesis and Structure–Activity Relationship Study of 2-(amino)quinazolin-4(3H)-one Derivatives as Potential Inhibitors of Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
1. Introduction
2. Results and Discussion
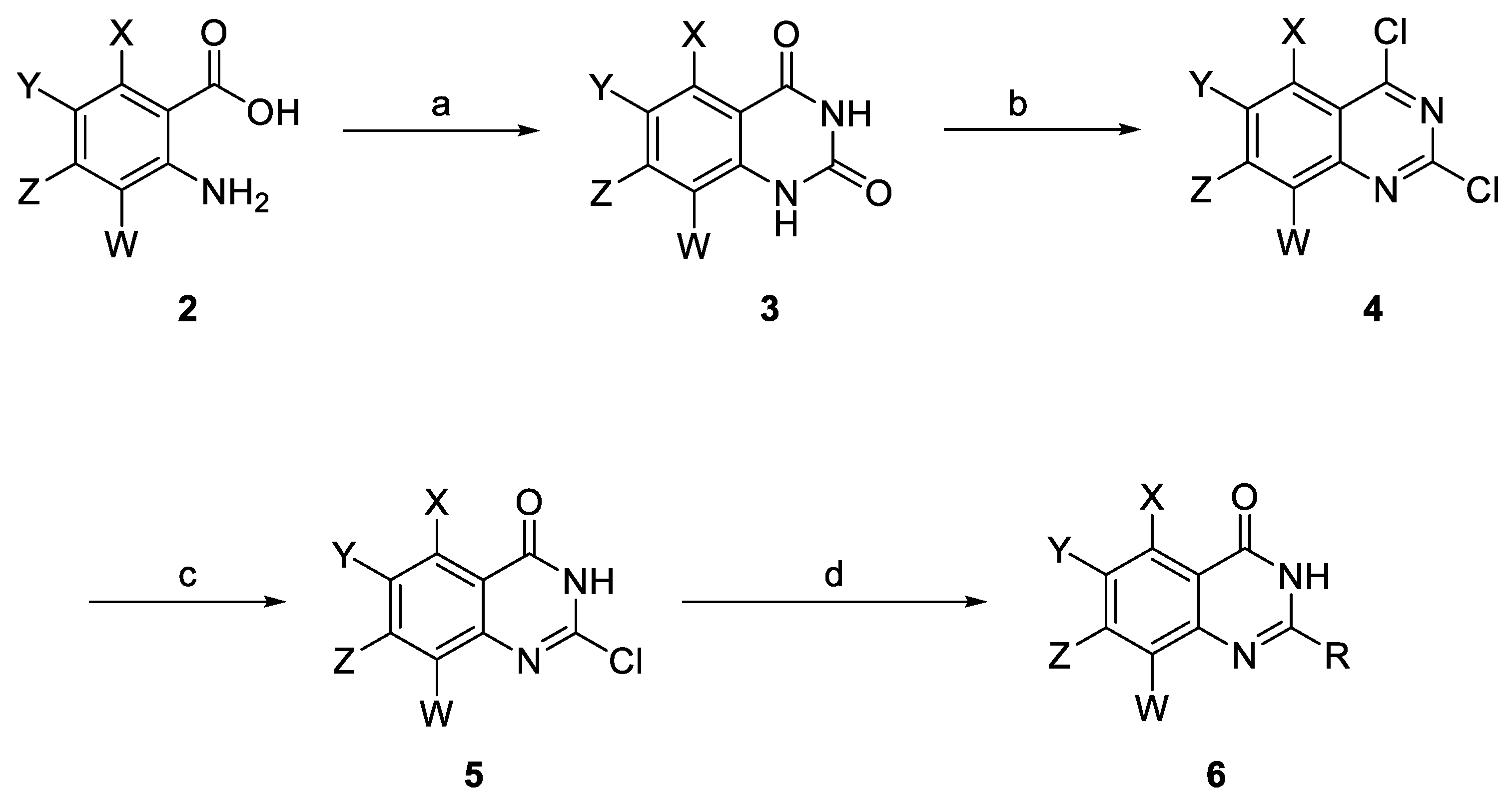
2.1. Synthesis of 2-(amino)quinazolin-4(3H)-one Derivatives
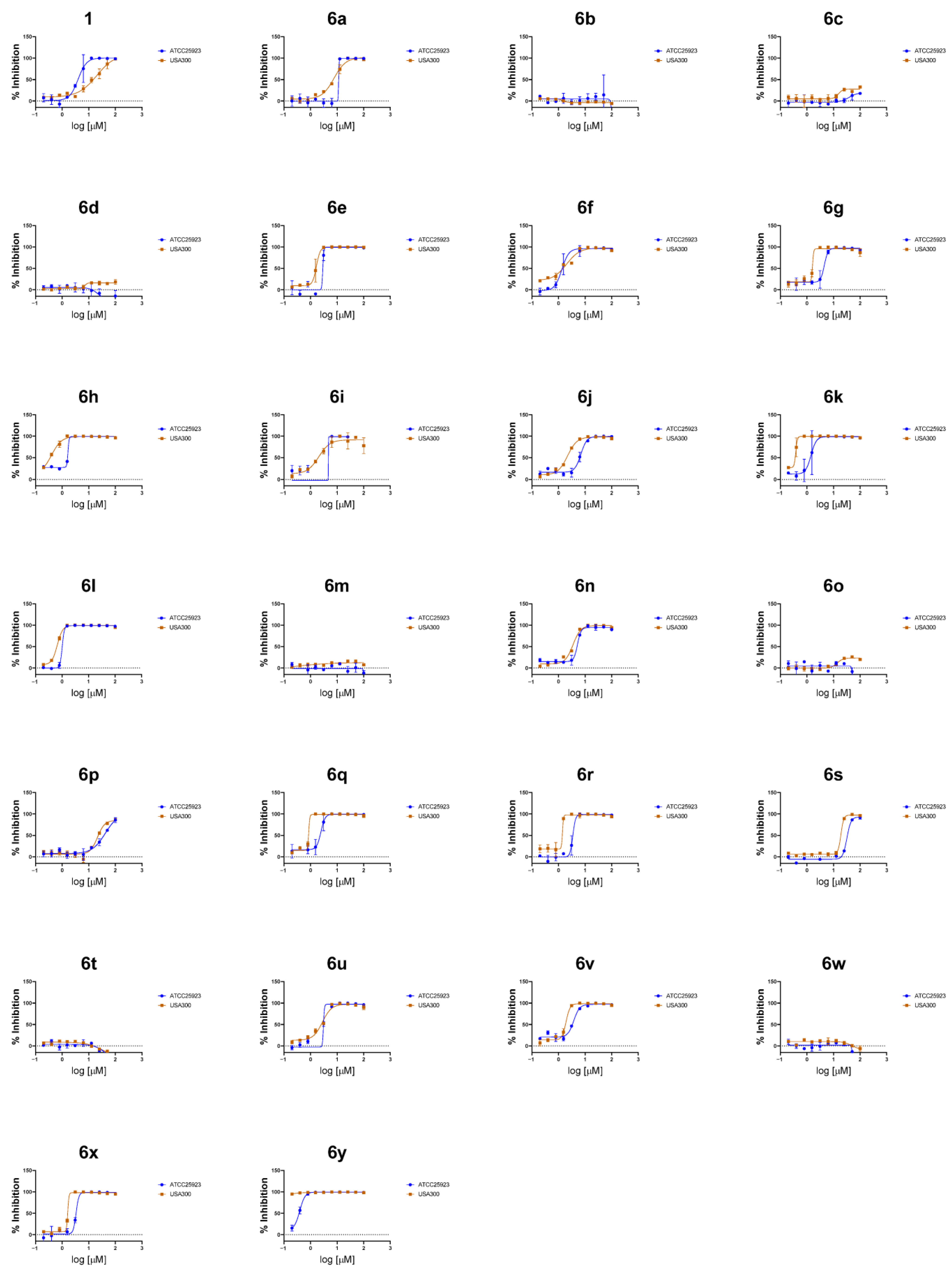
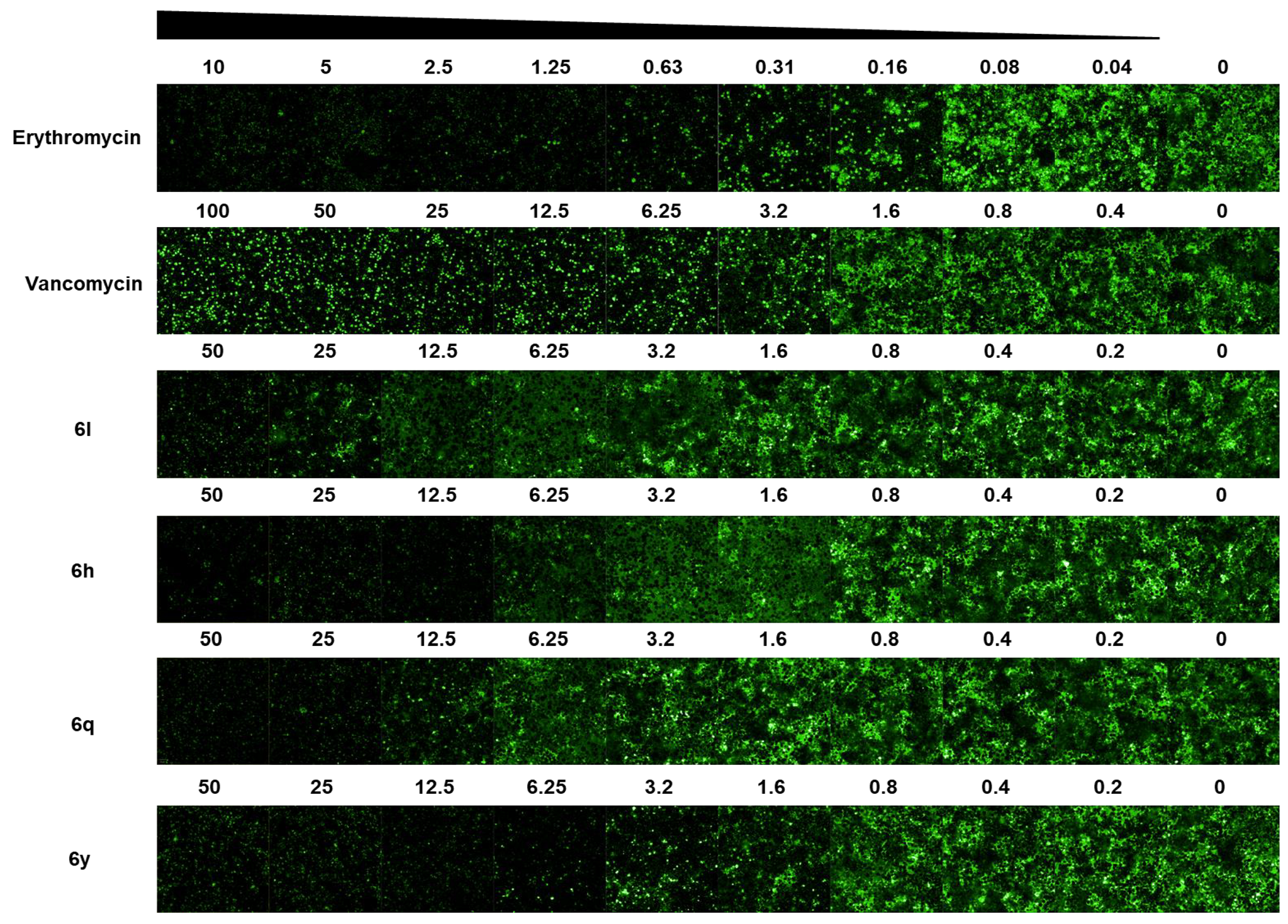
2.2. Antistaphylococcal Activities Screening and SAR Studies
2.3. In Vitro Cytotoxicity
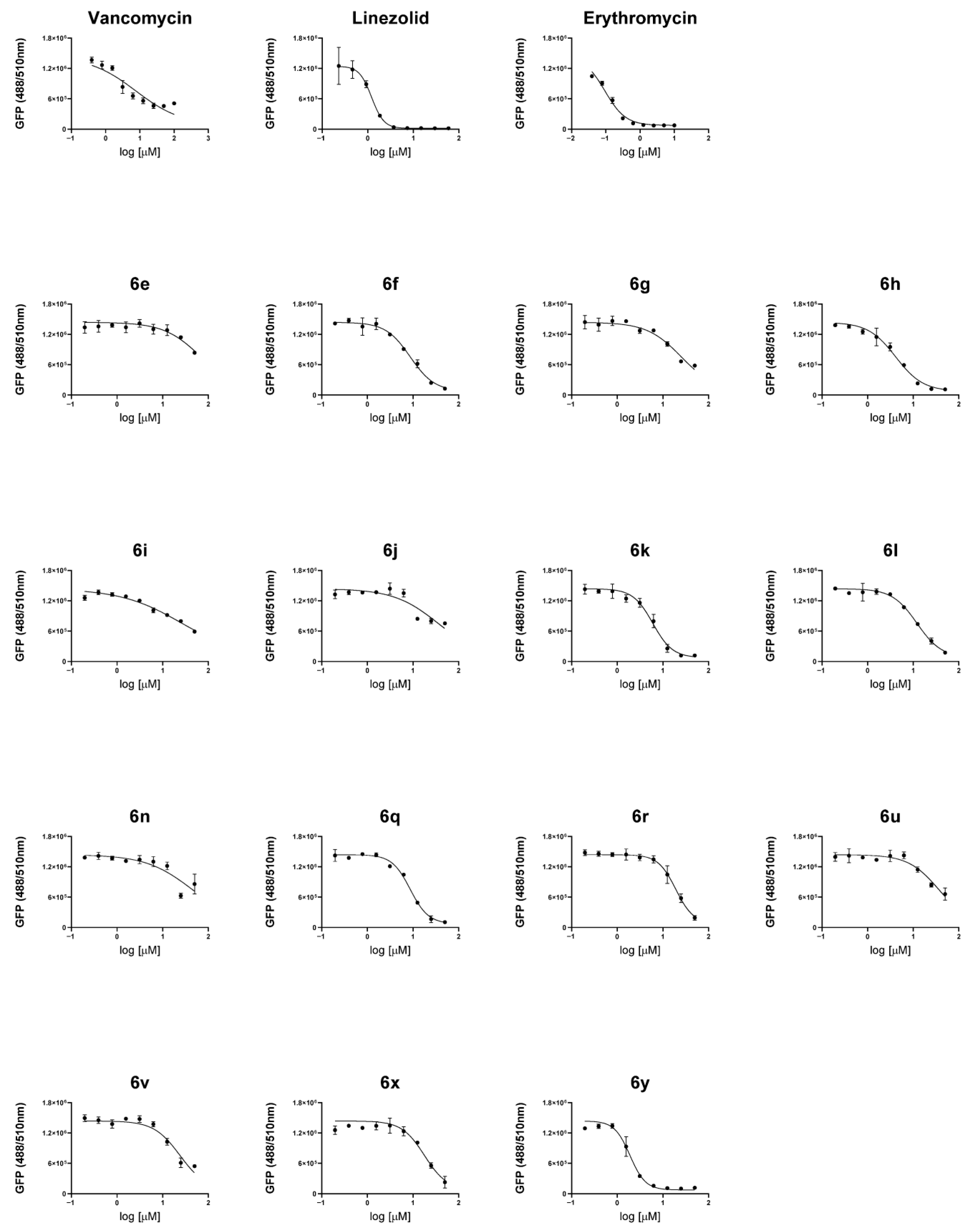
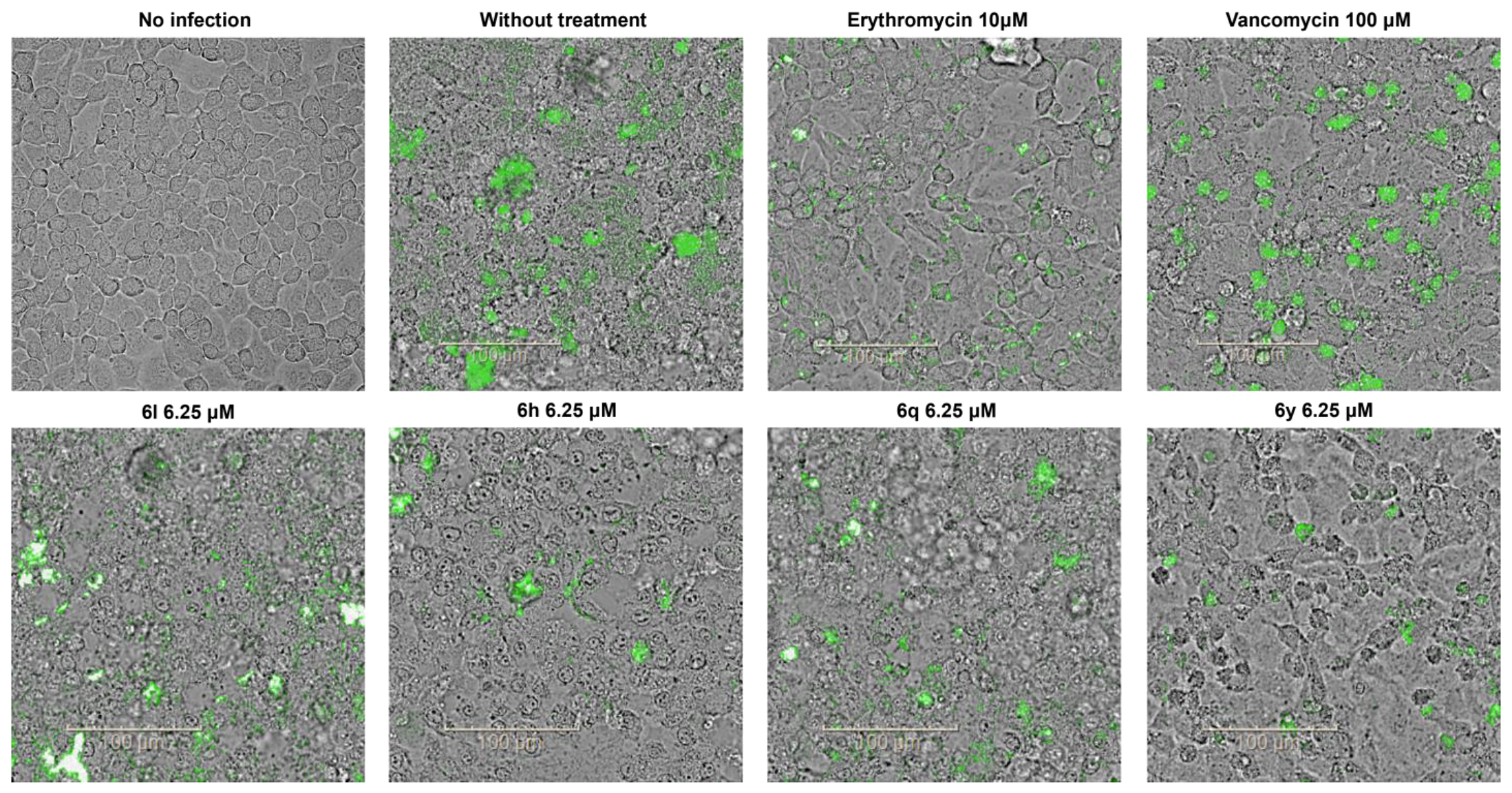
2.4. Antibacterial Activity in a Cell Infection Model
3. Materials and Methods
3.1. Materials
3.2. Chemistry
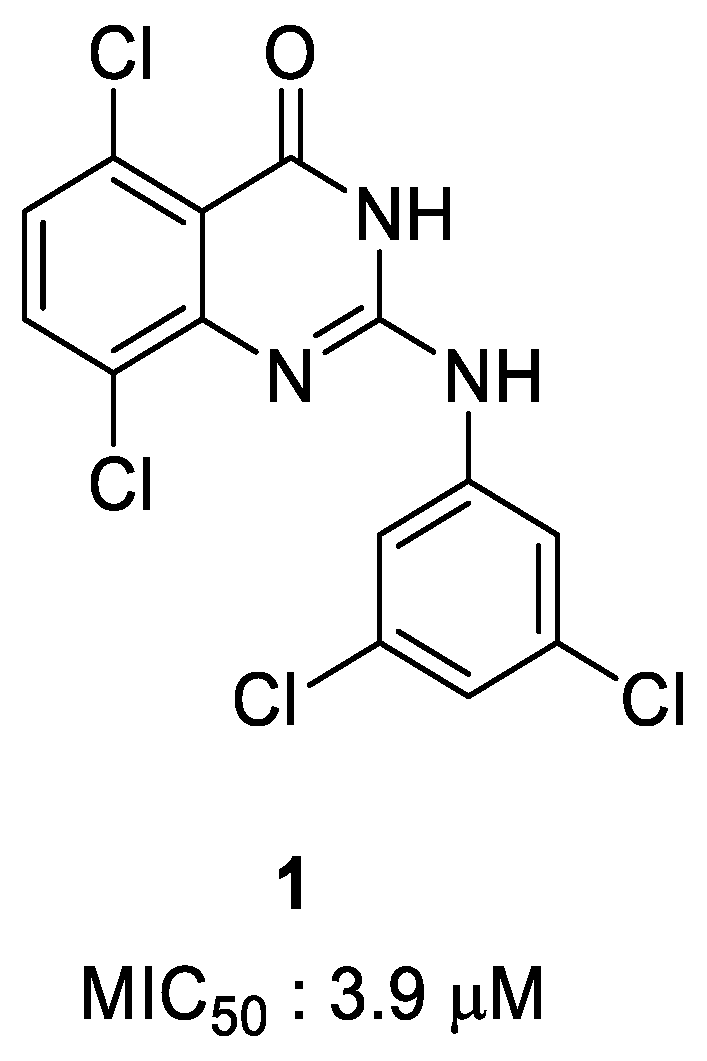
3.2.1. 5,8-Dichloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (1)
3.2.2. 7-Chloroquinazoline-2,4(1H,3H)-dione (3l)
3.2.3. 2,4,7-Trichloroquinazoline (4l)
3.2.4. 2,7-Dichloroquinazolin-4(3H)-one (5l)
3.2.5. 7,8-Dichloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (6a)
3.2.6. 2-((3,5-Dichlorophenyl)amino)-7-morpholinoquinazolin-4(3H)-one (6b)
3.2.7. 2-((3,5-Dichlorophenyl)amino)-6,7-dimethoxyquinazolin-4(3H)-one (6c)
3.2.8. 2-((3,5-Dichlorophenyl)amino)-7-methoxyquinazolin-4(3H)-one (6d)
3.2.9. 2-((3,5-Dichlorophenyl)amino)-7-nitroquinazolin-4(3H)-one (6e)
3.2.10. 6,8-Dibromo-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (6f)
3.2.11. 2-((3,5-Dichlorophenyl)amino)-6,7-difluoroquinazolin-4(3H)-one (6g)
3.2.12. 6,7-Dichloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (6h)
3.2.13. 6,8-Dichloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (6i)
3.2.14. 2-((3,5-Dichlorophenyl)amino)quinazolin-4(3H)-one (6j)
3.2.15. 2-((3,5-Dichlorophenyl)amino)-7-(trifluoromethyl)quinazolin-4(3H)-one (6k)
3.2.16. 7-Chloro-2-((3,5-dichlorophenyl)amino)quinazolin-4(3H)-one (6l)
3.2.17. 7-Chloro-2-(phenylamino)quinazolin-4(3H)-one (6m)
3.2.18. 7-Chloro-2-((3-fluorophenyl)amino)quinazolin-4(3H)-one (6n)
3.2.19. 7-Chloro-2-((2,4-difluorophenyl)amino)quinazolin-4(3H)-one (6o)
3.2.20. 7-Chloro-2-((3-methoxyphenyl)amino)quinazolin-4(3H)-one (6p)
3.2.21. 7-Chloro-2-((3,5-difluorophenyl)amino)quinazolin-4(3H)-one (6q)
3.2.22. 7-Chloro-2-((3-(trifluoromethyl)phenyl)amino)quinazolin-4(3H)-one (6r)
3.2.23. 7-Chloro-2-((cyclohexylmethyl)amino)quinazolin-4(3H)-one (6s)
3.2.24. 7-Chloro-2-((2-chlorophenyl)amino)quinazolin-4(3H)-one (6t)
3.2.25. 7-Chloro-2-((3-chlorophenyl)amino)quinazolin-4(3H)-one (6u)
3.2.26. 7-Chloro-2-((4-chlorophenyl)amino)quinazolin-4(3H)-one (6v)
3.2.27. 7-Chloro-2-((3,5-difluorobenzyl)amino)quinazolin-4(3H)-one (6w)
3.2.28. 7-Chloro-2-((2,4-dichlorobenzyl)amino)quinazolin-4(3H)-one (6x)
3.2.29. 7-Chloro-2-((3,4-difluorobenzyl)amino)quinazolin-4(3H)-one (6y)
3.3. Biological Studies
3.3.1. Strains and Culture Conditions
3.3.2. Dose–Response Curve Assay
3.3.3. Cytotoxicity Test of Compounds
3.3.4. Effect of Compounds on a Cell Infection Model of S. aureus
3.3.5. Confocal Images
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, B.B.; Soman, K.C.; Bhoir, L.; Gadahire, M.; Patel, B.; Ahdal, J. The Burden of Methicillin Resistant Staphylococcus aureus in Surgical Site Infections: A Review. J. Clin. Diagn. Res. 2021, 15, E01–E05. [Google Scholar] [CrossRef]
- Hasanpour, A.H.; Sepidarkish, M.; Mollalo, A.; Ardekani, A.; Almukhtar, M.; Mechaal, A.; Hosseini, S.R.; Bayani, M.; Javanian, M.; Rostami, A. The global prevalence of methicillin-resistant Staphylococcus aureus colonization in residents of elderly care centers: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2023, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.J. Colonization and infection of the human host by staphylococci: Adhesion, survival and immune evasion. Vet. Dermatol. 2009, 20, 456–470. [Google Scholar] [CrossRef]
- Iwatsuki, K.; Yamasaki, O.; Morizane, S.; Oono, T. Staphylococcal cutaneous infections: Invasion, evasion and aggression. J. Dermatol. Sci. 2006, 42, 203–214. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Mattos-Graner, R.O.; Duncan, M.J. Two-component signal transduction systems in oral bacteria. J. Oral Microbiol. 2017, 9, 1400858. [Google Scholar] [CrossRef]
- Shoaib, M.; Aqib, A.I.; Muzammil, I.; Majeed, N.; Bhutta, Z.A.; Kulyar, M.F.-E.-A.; Fatima, M.; Zaheer, C.-N.F.; Muneer, A.; Murtaza, M.; et al. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 2023, 13, 1067284. [Google Scholar] [CrossRef]
- Mahjabeen, F.; Saha, U.; Mostafa, M.N.; Siddique, F.; Ahsan, E.; Fathma, S.; Tasnim, A.; Rahman, T.; Faruq, R.; Sakibuzzaman, M.; et al. An Update on Treatment Options for Methicillin-Resistant Staphylococcus aureus (MRSA) Bacteremia: A Systematic Review. Cureus 2022, 14, e31486. [Google Scholar] [CrossRef]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial treatment of Staphylococcus aureus biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef]
- Rossolini, G.M.; Arena, F.; Pecile, P.; Pollini, S. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014, 18, 56–60. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, N.; Shum, D.; Huddar, S.; Park, C.M.; Jang, S. Identification of antipneumococcal molecules effective against different Streptococcus pnueumoniae serotypes using a resazurin-based high-throughput screen. Assay Drug Dev. Technol. 2017, 15, 198–209. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lee, H.; Yun, S.H.; Jun, S.; Lee, Y.; Kim, W.; Park, E.C.; Baek, J.; Kwak, Y.; Noh, S.; et al. Application of multiomics technology for the elucidation of anti-pneumococcal activity of 3-acyl-2-phenylamino-1,4-dihydroquinolin-4-one (ADPQ) derivatives against Streptococcus pneumoniae. Sci. Rep. 2020, 10, 20685. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.; Gim, J.; Lee, Y.; Lee, H.; Lim, C.J.; Song, H.-S.; Park, H.-G.; Jang, S.; Park, C.M. Discovery of 3-acyl-2-anilino-1,4-dihydroquinolin-4-one derivatives as potential inhibitors of methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2025, 118, 130084. [Google Scholar] [CrossRef]
- Nematpour, M.; Rezaee, E.; Nazari, M.; Hosseini, O.; Tabatabai, S.A. Targeting EGFR tyrosine kinase: Design, synthesis and biological evaluation of novel quinazolinone derivatives. Iran J. Pharm. Res. 2022, 21, e123826. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, Y.S.; Jeon, S.; Lee, S.I.; Noh, S.; Cho, J.-E.; Jang, M.S.; Kim, S.; Song, J.H.; Kim, H.R.; et al. Design, synthesis and biological evaluation of 2-aminoquinazolin-4(3H)-one derivatives as potential SARS-CoV-2 and MERS-CoV treatments. Bioorg. Med. Chem. Lett. 2021, 39, 127885. [Google Scholar] [CrossRef] [PubMed]
- DeRuiter, J.; Brubaker, A.N.; Millen, J.; Riley, T.N. Design and synthesis of 2-(arylamino)-4(3H)-quinazolinones as novel inhibitors of rat lens aldose reductase. J. Med. Chem. 1986, 29, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Shin, Y.S.; Jeon, S.; Lee, S.I.; Cho, J.-E.; Myung, S.; Jang, M.S.; Kim, S.; Song, J.H.; Kim, H.R.; et al. Synthesis and biological evaluation of 2-benzylaminoquinazolin-4(3H)-one derivatives as a potential treatment for SARS-CoV-2. Bull. Korean Chem. Soc. 2022, 43, 412. [Google Scholar] [CrossRef]
- French, G.L. Methods for screening for methicillin-resistant Staphylococcus aureus carriage. Clin. Microbiol. Infect. 2009, 15, 10. [Google Scholar] [CrossRef]
- Lowy, F.D. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 2000, 8, 341–343. [Google Scholar] [CrossRef]
- Sendi, P.; Proctor, R.A. Staphylococcus aureus as an intracellular pathogen: The role of small colony variants. Trends Microbiol. 2009, 17, 54–58. [Google Scholar] [CrossRef]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus aureus: Live-in and let die. Front. Cell. Infect. Microbiol. 2012, 2, 43. [Google Scholar] [CrossRef]
- Dombach, J.L.; Quintana, J.L.J.; Detweiler, C.S. Staphylococcal bacterial persister cells, biofilms, and intracellular infection are disrupted by JD1, a Membrane-damaging small molecule. mBio 2021, 12, e0180121. [Google Scholar] [CrossRef]
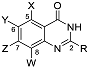 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No | Compound | X | Y | Z | W | R | ATCC25923 MIC50 (μM) a | USA300 MIC50 (μM) a | HepG2 IC50 (μM) b | Efficacy Window (IC50 HepG2/MIC50 USA300) |
| 1 | 1 | Cl | H | H | Cl | 3,5-Cl2-PhNH- | 3.9 | 12.9 | - | - |
| 2 | 6a | H | H | Cl | Cl | 3,5-Cl2-PhNH- | 10.8 | 6.9 | - | - |
| 3 | 6b | H | H | Morpholine | H | 3,5-Cl2-PhNH- | >100 | >100 | - | - |
| 4 | 6c | H | OMe | OMe | H | 3,5-Cl2-PhNH- | >100 | >100 | - | - |
| 5 | 6d | H | H | OMe | H | 3,5-Cl2-PhNH- | >100 | >100 | - | - |
| 6 | 6e | H | H | NO2 | H | 3,5-Cl2-PhNH- | 3.0 | 1.6 | 9.3 | 5.9 |
| 7 | 6f | H | Br | H | Br | 3,5-Cl2-PhNH- | 6.0 | 1.2 | 9.6 | 8.3 |
| 8 | 6g | H | F | F | H | 3,5-Cl2-PhNH- | 4.0 | 1.4 | 12.9 | 8.9 |
| 9 | 6h | H | Cl | Cl | H | 3,5-Cl2-PhNH- | 3.7 | 0.3 | 10.2 | 30.3 |
| 10 | 6i | H | Cl | H | Cl | 3,5-Cl2-PhNH- | 5.7 | 1.8 | 39.5 | 21.8 |
| 11 | 6j | H | H | H | H | 3,5-Cl2-PhNH- | 5.9 | 1.9 | 29.7 | 15.7 |
| 12 | 6k | H | H | CF3 | H | 3,5-Cl2-PhNH- | 1.3 | 0.3 | 10.5 | 39.0 |
| 13 | 6l | H | H | Cl | H | 3,5-Cl2-PhNH- | 1.0 | 0.6 | 62.2 | 101.3 |
| 14 | 6m | H | H | Cl | H | PhNH- | >100 | >100 | - | - |
| 15 | 6n | H | H | Cl | H | 3-F-PhNH- | 4.5 | 3.0 | 19.7 | 6.6 |
| 16 | 6o | H | H | Cl | H | 2,4-F2-PhNH- | >100 | >100 | - | - |
| 17 | 6p | H | H | Cl | H | 3-MeO-PhNH- | 35.1 | 24.9 | - | - |
| 18 | 6q | H | H | Cl | H | 3,5-F2-PhNH- | 2.1 | 0.9 | 35.5 | 39.2 |
| 19 | 6r | H | H | Cl | H | 3-CF3-PhNH- | 3.1 | 2.2 | 15.1 | 6.9 |
| 20 | 6s | H | H | Cl | H | CyclohexymethylNH- | 35.5 | 18.1 | - | - |
| 21 | 6t | H | H | Cl | H | 2-Cl-PhNH- | >100 | >100 | - | - |
| 22 | 6u | H | H | Cl | H | 3-Cl-PhNH- | 3.5 | 1.1 | 4.3 | 4.0 |
| 23 | 6v | H | H | Cl | H | 4-Cl-PhNH- | 2.7 | 1.7 | 25.5 | 15.0 |
| 24 | 6w | H | H | Cl | H | 3,5-F2-PhCH2NH- | >100 | >100 | - | - |
| 25 | 6x | H | H | Cl | H | 2,4-Cl2-PhCH2NH- | 3.4 | 1.7 | 15.3 | 8.8 |
| 26 | 6y | H | H | Cl | H | 3,4-F2-PhCH2NH- | 0.36 | 0.02 | 20.2 | 885.2 |
| Erythromycin | 0.7 | 0.49 | >100 | - | ||||||
| Vancomycin | 0.3 | 0.35 | >100 | - | ||||||
| Compound | H460 Assay MIC50 (μM) a | Efficacy Window (IC50 HepG2/MIC50 USA300) |
|---|---|---|
| 6e | 68.8 | 0.1 |
| 6f | 8.7 | 1.1 |
| 6g | 25.3 | 0.5 |
| 6h | 4.1 | 2.5 |
| 6i | 26.5 | 1.5 |
| 6j | 35.4 | 0.8 |
| 6k | 6.0 | 1.7 |
| 6l | 12.1 | 5.2 |
| 6n | 43.8 | 0.4 |
| 6q | 8.7 | 4.1 |
| 6r | 35.8 | 0.4 |
| 6x | 17.8 | 0.9 |
| 6u | 19.2 | 0.2 |
| 6v | 23.9 | 1.1 |
| 6y | 1.9 | 10.6 |
| Vancomycin | 7.7 | |
| Erythromycin | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Lee, H.; Kim, S.; Gim, J.; Lee, Y.; Lim, C.J.; Song, H.-S.; Park, H.-g.; Jang, S.; Park, C.M. Synthesis and Structure–Activity Relationship Study of 2-(amino)quinazolin-4(3H)-one Derivatives as Potential Inhibitors of Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics 2025, 14, 967. https://doi.org/10.3390/antibiotics14100967
Lee JY, Lee H, Kim S, Gim J, Lee Y, Lim CJ, Song H-S, Park H-g, Jang S, Park CM. Synthesis and Structure–Activity Relationship Study of 2-(amino)quinazolin-4(3H)-one Derivatives as Potential Inhibitors of Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics. 2025; 14(10):967. https://doi.org/10.3390/antibiotics14100967
Chicago/Turabian StyleLee, Jun Young, Hyunjung Lee, Sungmin Kim, Jihwan Gim, Yunmi Lee, Chae Jo Lim, Hyun-Seob Song, Hyeung-geun Park, Soojin Jang, and Chul Min Park. 2025. "Synthesis and Structure–Activity Relationship Study of 2-(amino)quinazolin-4(3H)-one Derivatives as Potential Inhibitors of Methicillin-Resistant Staphylococcus aureus (MRSA)" Antibiotics 14, no. 10: 967. https://doi.org/10.3390/antibiotics14100967
APA StyleLee, J. Y., Lee, H., Kim, S., Gim, J., Lee, Y., Lim, C. J., Song, H.-S., Park, H.-g., Jang, S., & Park, C. M. (2025). Synthesis and Structure–Activity Relationship Study of 2-(amino)quinazolin-4(3H)-one Derivatives as Potential Inhibitors of Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics, 14(10), 967. https://doi.org/10.3390/antibiotics14100967







