Ampicillin- and Multidrug-Resistant Escherichia coli and Enterococcus spp. in Costa Rican Wastewater and Surface Water
Abstract
1. Introduction
2. Results
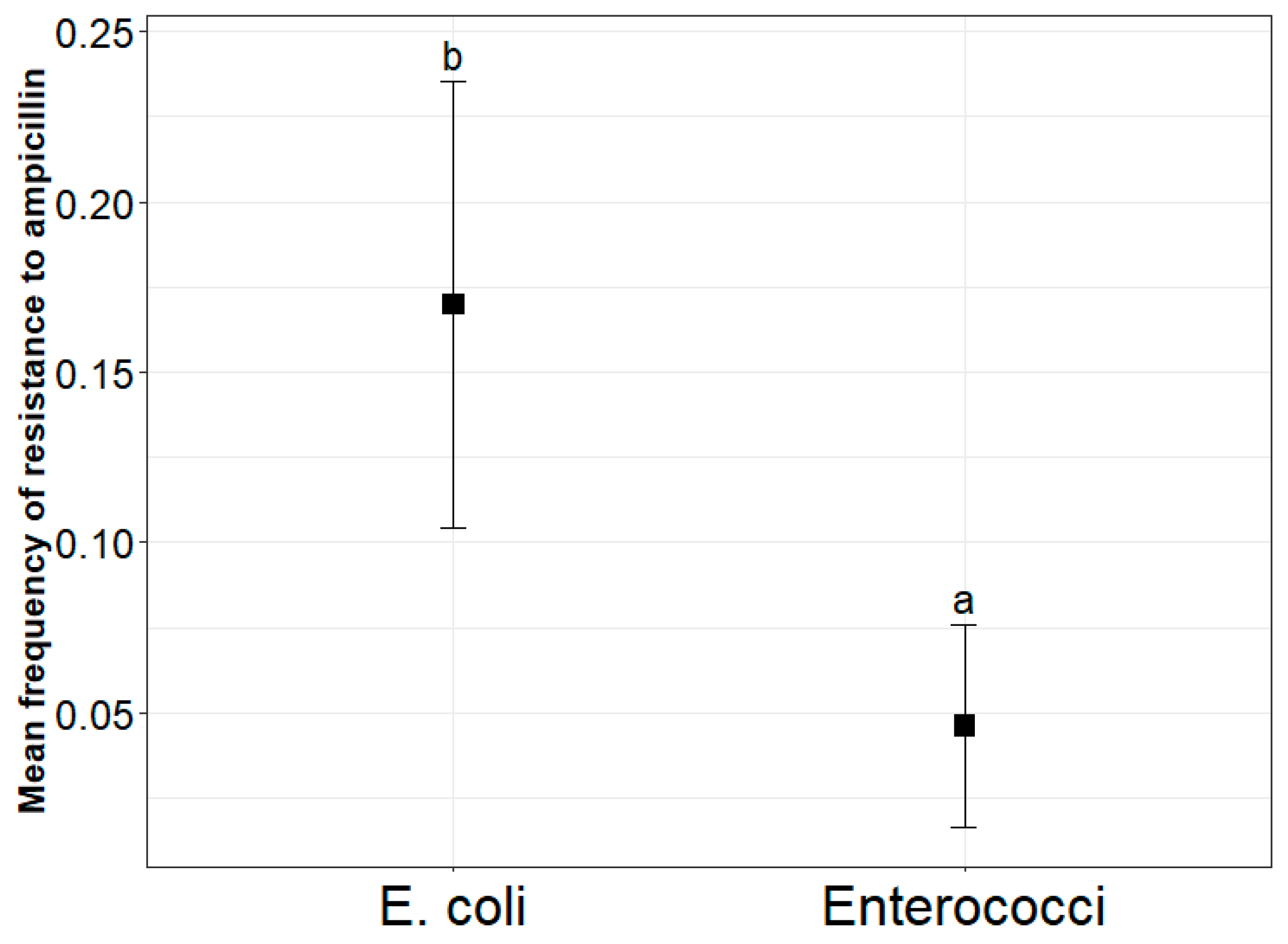
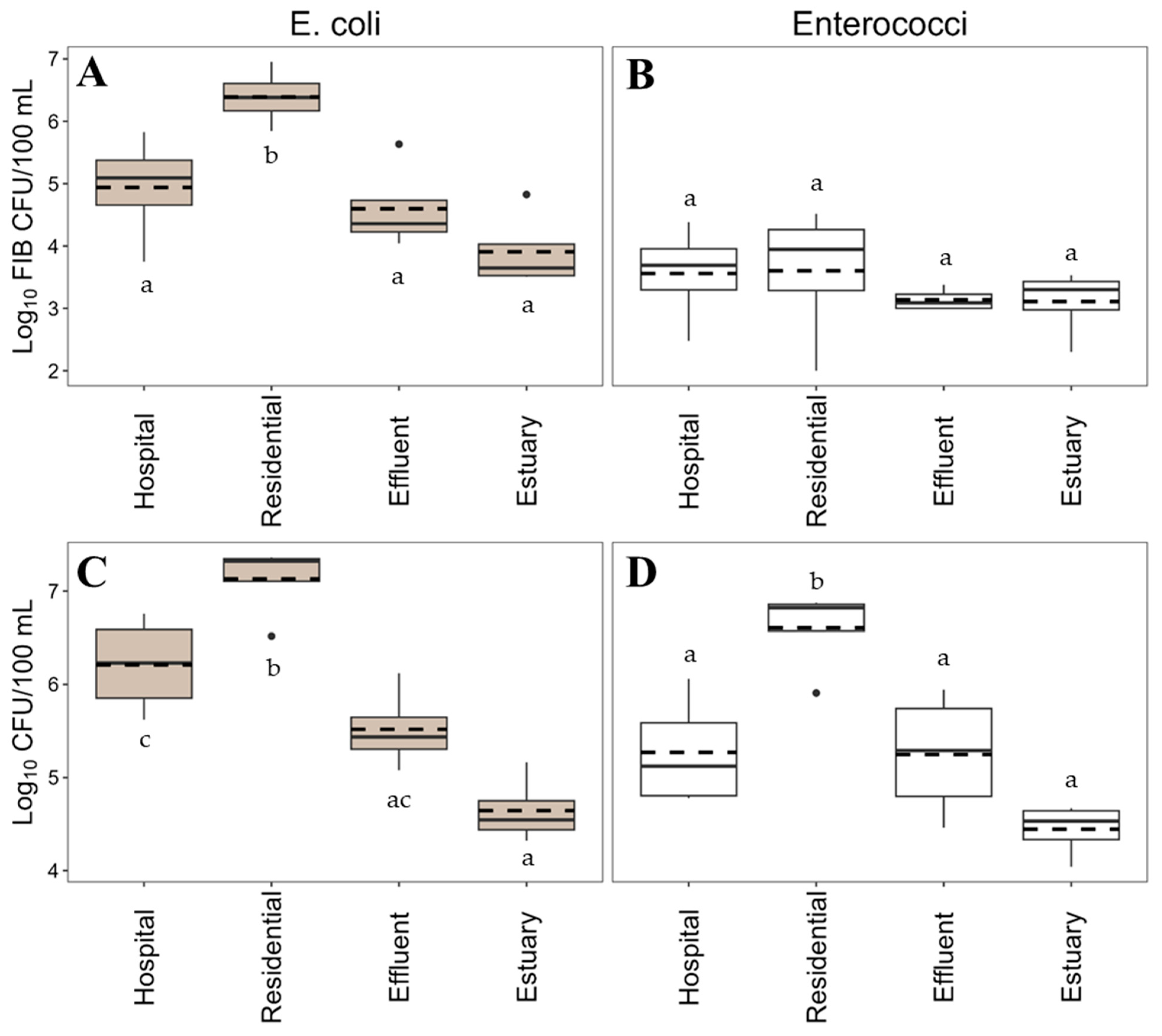
2.1. Frequency and Concentration of AmpR E. coli and Enterococci
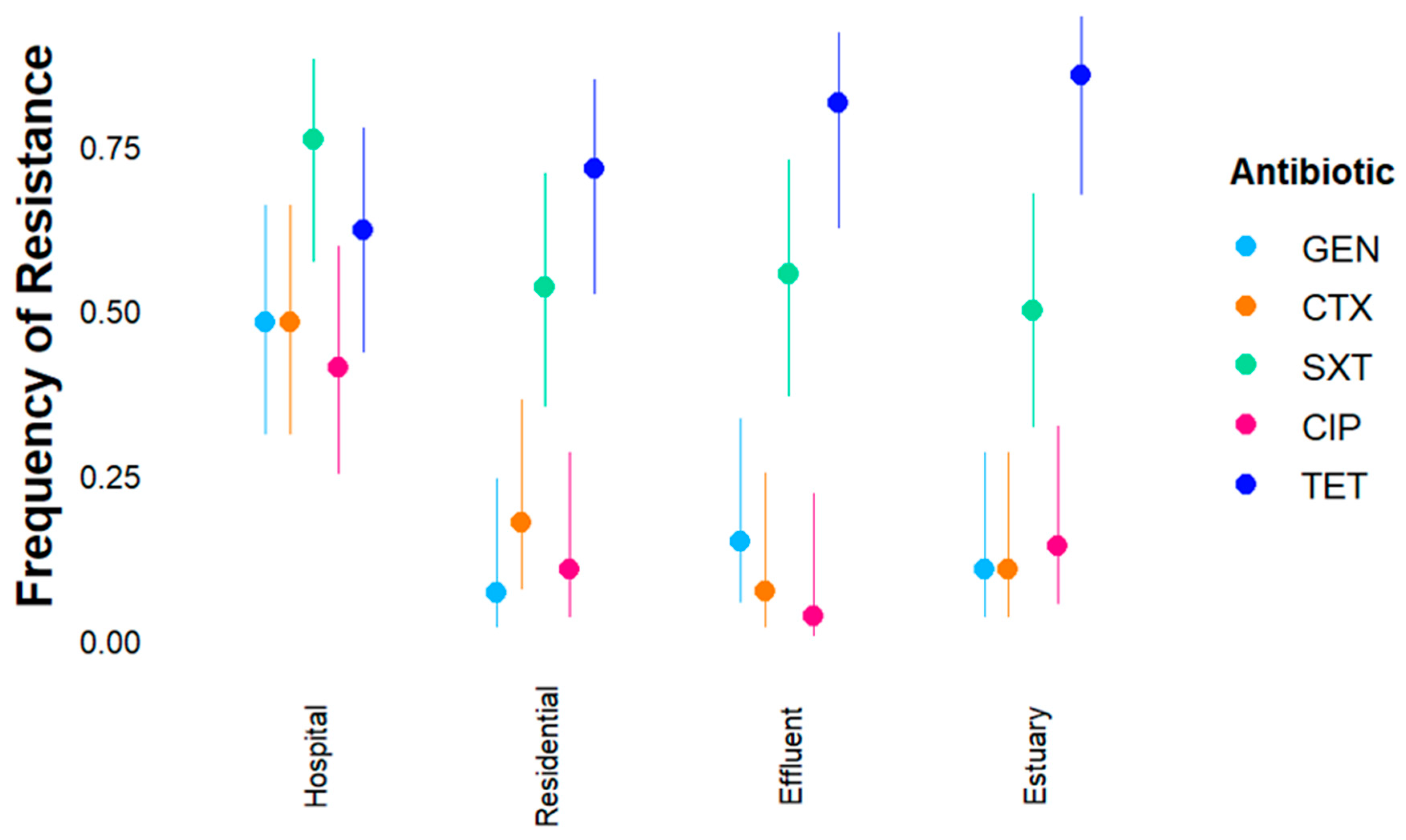
2.2. Susceptibility of Ampicillin-Resistant in E. coli and Enterococcus spp. to Other Antibiotics
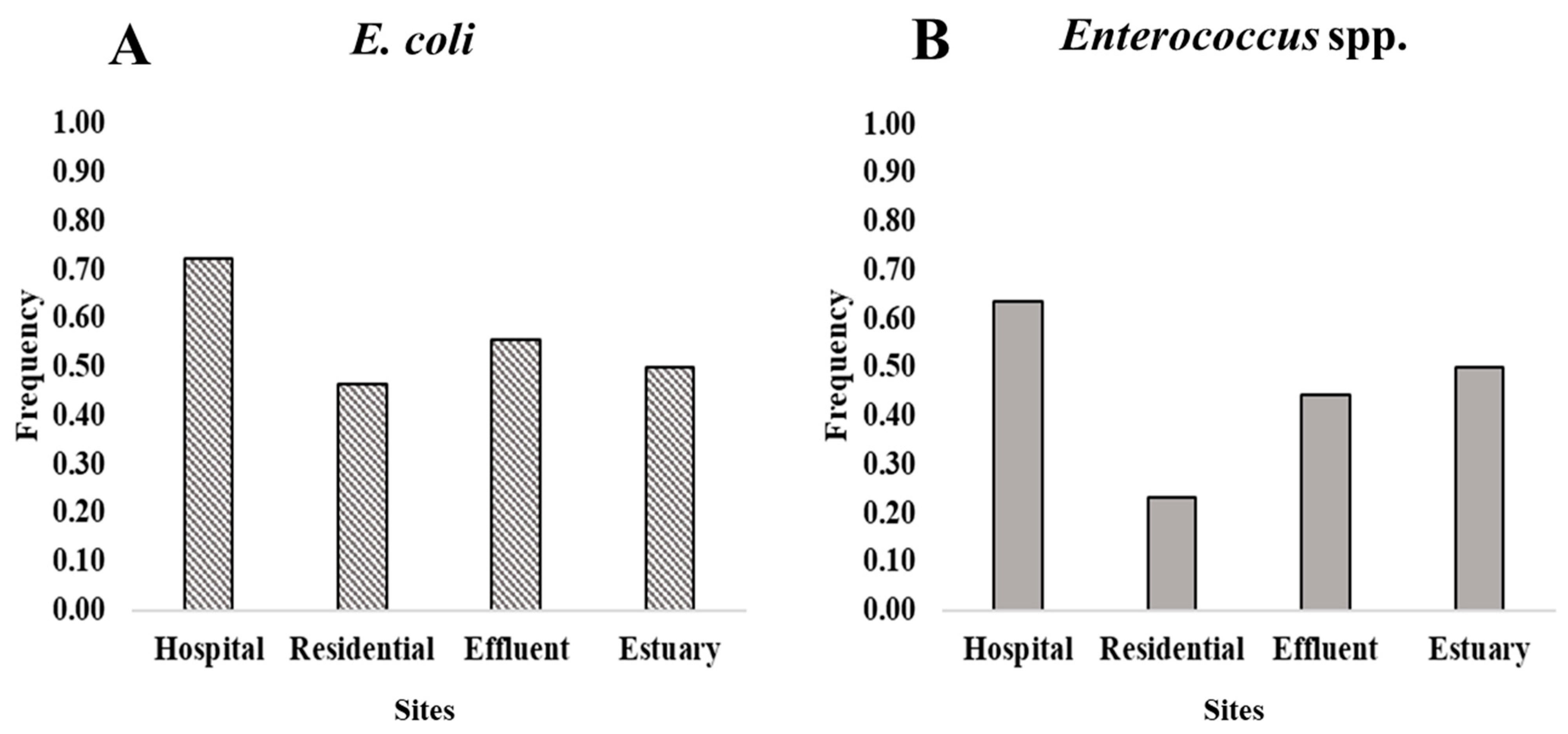
2.3. Multidrug Resistance in Wastewater, Effluent, and the Estuary
3. Discussion
3.1. Resistance of E. coli and Enterococci to Ampicillin in Wastewater, Effluent, and the Puntarenas Estuary
3.2. Susceptibility of Ampicillin-Resistant E. coli and Enterococcus to Other Antibiotics
3.3. Multidrug Resistance of E. coli and Enterococcus in Wastewater, Effluent, and the Estuary
4. Materials and Methods
4.1. Study Site
4.2. Sampling and Culture of FIB
4.3. Phylogenetic Confirmation of Ampicillin-Resistant Isolates
4.4. Multidrug Resistance Testing
4.5. Statistical Analysis
4.5.1. Frequency of Resistance of E. coli and Enterococci to Ampicillin
4.5.2. Concentrations of Ampicillin-Resistant E. coli and Enterococci
4.5.3. Susceptibility of Ampicillin-Resistant FIB to Other Antibiotics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| ARB | Antimicrobial resistant bacteria |
| ARG | Antimicrobial resistance genes |
| CFU | Colony-forming units |
| FIB | Fecal indicator bacteria |
| MDR | Multidrug resistant |
| VRE | Vancomycin-resistant Enterococcus |
| WHO | World Health Organization |
| WWTP | Wastewater treatment plant |
References
- WHO. Antimicrobial Resistance. Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug resistant and extensively drug resistant bacteria: A study. J. Pathol. 2016, 2016, 4065603. [Google Scholar]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef]
- Sulis, G.; Sayood, S.; Gandra, S. Antimicrobial resistance in low- and middle-income countries: Current status and future directions. Expert Rev. Anti Infect. Ther. 2022, 20, 147–160. [Google Scholar]
- Zheng, D.; Yin, G.; Liu, M.; Chen, C.; Jiang, Y.; Hou, L.; Zheng, Y. A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total Environ. 2021, 777, 146009. [Google Scholar]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [PubMed]
- Costa Rica Gobierno del Bicentenario. Plan De Acción Nacional De Lucha Contra La Resistencia a Los Antimicrobianos Costa Rica 2018–2025; Costa Rica Gobierno del Bicentenario: San Jose, Costa Rica, 2018. [Google Scholar]
- Somarribas, C.M. Informe De Vigilancia Basada En Laboratorio; INCIENSA: Tres Rios, Costa Rica, 2022. [Google Scholar]
- Cordero Parra, M.; Parra, A. Falta De Competencia Mantiene Por Las Nubes El Precio De Los Medicamentos. 2019. Available online: https://semanariouniversidad.com/pais/falta-de-competencia-mantiene-por-las-nubes-el-precio-de-los-medicamentos/ (accessed on 7 July 2022).
- Decreto n.º 26984-S, Sistema Costarricense de Información Jurídica, Editor. Diario Oficial La Gaceta, 1998.
- Katherine. Farmacéuticos, Microbiólogos y Médicos De La CCSS Luchan Contra Mal Uso De Antimicrobianos-Periódico Mensaje Guanacaste. 2021. Available online: https://www.periodicomensaje.com/salud/7879-farmaceuticos-microbiologos-y-medicos-de-la-ccss-luchan-contra-mal-uso-de-antimicrobianos (accessed on 7 July 2022).
- Suzuki, S.; Pruden, A.; Virta, M.; Zhang, T. Editorial: Antibiotic resistance in aquatic systems. Front. Microbiol. 2017, 8, 14. [Google Scholar] [CrossRef]
- Amarasiri, M.; Sano, D.; Suzuki, S. Understanding human health risks caused by antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARG) in water environments: Current knowledge and questions to be answered. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2016–2059. [Google Scholar] [CrossRef]
- Guo, J.; Li, J.; Chen, H.; Bond, P.L.; Yuan, Z. Metagenomic analysis reveals wastewater treatment plants as hotspots of antibiotic resistance genes and mobile genetic elements. Water Res. 2017, 123, 468–478. [Google Scholar] [CrossRef]
- Paulus, G.K.; Hornstra, L.M.; Alygizakis, N.; Slobodnik, J.; Thomaidis, N.; Medema, G. The impact of on-site hospital wastewater treatment on the downstream communal wastewater system in terms of antibiotics and antibiotic resistance genes. Int. J. Hyg. Environ. Health 2019, 222, 635–644. [Google Scholar]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Christou, A.; Agüera, A.; Bayona, J.M.; Cytryn, E.; Fotopoulos, V.; Lambropoulou, D.; Manaia, C.M.; Michael, C.; Revitt, M.; Schröder, P.; et al. The potential implications of reclaimed wastewater reuse for irrigation on the agricultural environment: The knowns and unknowns of the fate of antibiotics and antibiotic resistant bacteria and resistance genes—A review. Water Res. 2017, 123, 448–467. [Google Scholar] [CrossRef]
- Ghosh, S.; LaPara, T.M. The effects of subtherapeutic antibiotic use in farm animals on the proliferation and persistence of antibiotic resistance among soil bacteria. ISME J. 2007, 1, 191–203. [Google Scholar] [CrossRef]
- Hatosy, S.M.; Martiny, A.C. The ocean as a global reservoir of antibiotic resistance genes. Appl. Environ. Microbiol. 2015, 81, 7593–7599. [Google Scholar] [CrossRef]
- Leonard, A.F.; Morris, D.; Schmitt, H.; Gaze, W.H. Natural recreational waters and the risk that exposure to antibiotic resistant bacteria poses to human health. Curr. Opin. Microbiol. 2022, 65, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Nappier, S.P.; Liguori, K.; Ichida, A.M.; Stewart, J.R.; Jones, K.R. Antibiotic resistance in recreational waters: State of the science. Int. J. Environ. Res. Public Health 2020, 17, 8034. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, X.; Zheng, X. A descriptive study of open fractures contaminated by seawater: Infection, pathogens, and antibiotic resistance. BioMed Res. Int. 2017, 2017, 2796054. [Google Scholar] [CrossRef] [PubMed]
- Leonard, A.F.; Zhang, L.; Balfour, A.J.; Garside, R.; Gaze, W.H. Human recreational exposure to antibiotic resistant bacteria in coastal bathing waters. Environ. Int. 2015, 82, 92–100. [Google Scholar] [CrossRef]
- Schijven, J.F.; Blaak, H.; Schets, F.M.; de Roda Husman, A.M. Fate of extended-spectrum β-lactamase-producing Escherichia coli from faecal sources in surface water and probability of human exposure through swimming. Environ. Sci. Technol. 2015, 49, 11825–11833. [Google Scholar] [CrossRef]
- Laurens, C.; Jean-Pierre, H.; Licznar-Fajardo, P.; Hantova, S.; Godreuil, S.; Martinez, O.; Jumas-Bilak, E. Transmission of IMI-2 carbapenemase-producing Enterobacteriaceae from river water to human. J. Glob. Antimicrob. Resist. 2018, 15, 88–92. [Google Scholar] [CrossRef]
- Makkaew, P.; Kongprajug, A.; Chyerochana, N.; Sresung, M.; Precha, N.; Mongkolsuk, S.; Sirikanchana, K. Persisting antibiotic resistance gene pollution and its association with human sewage sources in tropical marine beach waters. Int. J. Hyg. Environ. Health 2021, 238, 113859. [Google Scholar] [CrossRef] [PubMed]
- Strauch, A.M.; Mackenzie, R.A.; Bruland, G.L.; Tingley, R.; Giardina, C.P. Climate Change and Land Use Drivers of Fecal Bacteria in Tropical Hawaiian Rivers. J. Environ. Qual. 2014, 43, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Pal, T. Update on antibacterial resistance in low-income countries: Factors favoring the emergence of resistance. Open Infect. Dis. J. 2010, 4, 38–54. [Google Scholar] [CrossRef]
- Morgan, D.J.; Okeke, I.N.; Laxminarayan, R.; Perencevich, E.N.; Weisenberg, S. Non-prescription antimicrobial use worldwide: A systematic review. Lancet Infect. Dis. 2011, 11, 692–701. [Google Scholar] [CrossRef]
- Murray, A.K.; Zhang, L.; Yin, X.; Zhang, T.; Buckling, A.; Snape, J.; Gaze, W.H. Novel insights into selection for antibiotic resistance in complex microbial communities. MBio 2018, 9, 10–1128. [Google Scholar]
- Stec, J.; Kosikowska, U.; Mendrycka, M.; Stępień-Pyśniak, D.; Niedźwiedzka-Rystwej, P.; Bębnowska, D.; Hrynkiewicz, R.; Ziętara-Wysocka, J.; Grywalska, E. Opportunistic Pathogens of Recreational Waters with Emphasis on Antimicrobial Resistance—A Possible Subject of Human Health Concern. Int. J. Environ. Res. Public Health 2022, 19, 7308. [Google Scholar]
- USEPA. Recreational Water Quality Criteria; U.S. Environmental Protection Agency: Washington, DC, USA, 2012.
- Griffith, J.F.; Cao, Y.; McGee, C.D.; Weisberg, S.B. Evaluation of rapid methods and novel indicators for assessing microbiological beach water quality. Water Res. 2009, 43, 4900–4907. [Google Scholar] [CrossRef]
- McQuaig, S.M.; Griffith, J.; Harwood, V.J. Association of fecal indicator bacteria with human viruses and microbial source tracking markers at coastal beaches impacted by nonpoint source pollution. Appl. Environ. Microbiol. 2012, 78, 6423–6432. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Moghaddam, M.J.M.; Mirbagheri, A.A.; Salehi, Z.; Habibzade, S.M. Prevalence of class 1 integrons and extended spectrum beta lactamases among multi-drug resistant Escherichia coli isolates from north of Iran. Iran. Biomed. J. 2015, 19, 233. [Google Scholar]
- VonBaum, H.; Marre, R. Antimicrobial resistance of Escherichia coli and therapeutic implications. Int. J. Med. Microbiol. 2005, 295, 503–511. [Google Scholar] [CrossRef]
- Hamiwe, T.; Kock, M.M.; Magwira, C.A.; Antiabong, J.F.; Ehlers, M.M. Occurrence of enterococci harbouring clinically important antibiotic resistance genes in the aquatic environment in Gauteng, South Africa. Environ. Pollut. 2019, 245, 1041–1049. [Google Scholar] [CrossRef]
- World Bank. Climate Risk Profile: Costa Rica. In Climate Risk Country Profiles; World Bank: Washington, WA, USA, 2021. [Google Scholar]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.
- USEPA. Method 1600: Enterococci in Water by Membrane Filtration Using Membrane-Enterococcus Indoxyl-B-D-Glucoside Agar (mEI); U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- Basu, S.; Copana, R.; Morales, R., Jr.; Anugulruengkitt, S.; Puthanakit, T.; Maramba-Lazarte, C.; Williams, P.; Musembi, J.; Boga, M.; Issack, M.; et al. Keeping it real: Antibiotic use problems and stewardship solutions in low-and middle-income countries. Pediatr. Infect. Dis. J. 2022, 41, S18. [Google Scholar] [CrossRef]
- Kaiser, R.A.; Taing, L.; Bhatia, H. Antimicrobial resistance and environmental health: A water stewardship framework for global and national action. Antibiotics 2022, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Apisarnthanarak, A.; Schellack, N.; Cornistein, W.; Maani, A.A.; Adnan, S.; Stevens, M.P. Global Antimicrobial Stewardship with a Focus on Low- and Middle-Income Countries. Int. J. Infect. Dis. 2020, 96, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Sugie, Y.; Yu, Z.; Okuno, Y.; Tanaka, H.; Ihara, M. Occurrence of E. coli and antibiotic-resistant E. coli in the southern watershed of Lake Biwa, including in wastewater treatment plant effluent and inflow rivers. Chemosphere 2022, 301, 134372. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Laird, E.; Gentry, T.J.; Brooks, J.P.; Karthikeyan, R. Increased Antimicrobial and Multidrug Resistance Downstream of Wastewater Treatment Plants in an Urban Watershed. Front. Microbiol. 2021, 12, 657353. [Google Scholar] [CrossRef]
- Łuczkiewicz, A.; Jankowska, K.; Fudala-Książek, S.; Olańczuk-Neyman, K. Antimicrobial resistance of fecal indicators in municipal wastewater treatment plant. Water Res. 2010, 44, 5089–5097. [Google Scholar] [CrossRef]
- Kolokotsa, A.; Leotsinidis, M.; Kalavrouziotis, I.; Sazakli, E. Effects of tourist flows on antibiotic resistance in wastewater of a Greek island. J. Appl. Microbiol. 2021, 130, 516–527. [Google Scholar] [CrossRef]
- Scheurer, M.; Heß, S.; Lüddeke, F.; Sacher, F.; Güde, H.; Löffler, H.; Gallert, C. Removal of micropollutants, facultative pathogenic and antibiotic resistant bacteria in a full-scale retention soil filter receiving combined sewer overflow. Environ. Sci. Process. Impacts 2015, 17, 186–196. [Google Scholar] [CrossRef]
- Taučer-Kapteijn, M.; Hoogenboezem, W.; Heiliegers, L.; de Bolster, D.; Medema, G. Screening municipal wastewater effluent and surface water used for drinking water production for the presence of ampicillin and vancomycin resistant enterococci. Int. J. Hyg. Environ. Health 2016, 219, 437–442. [Google Scholar]
- Tzoc, E.; Arias, M.L.; Valiente, C. Efecto de las aguas residuales hospitalarias sobre los patrones de resistencia a antibióticos de Escherichia coli y Aeromonas sp. Rev. Biomédica 2004, 15, 165–172. [Google Scholar]
- Gaşpar, C.M.; Cziszter, L.T.; Lăzărescu, C.F.; Ţibru, I.; Pentea, M.; Butnariu, M. Antibiotic resistance among Escherichia coli isolates from hospital wastewater compared to community wastewater. Water 2021, 13, 3449. [Google Scholar] [CrossRef]
- Leclercq, R.; Oberlé, K.; Galopin, S.; Cattoir, V.; Budzinski, H.; Petit, F. Changes in enterococcal populations and related antibiotic resistance along a medical center-wastewater treatment plant-river continuum. Appl. Environ. Microbiol. 2013, 79, 2428–2434. [Google Scholar]
- Garcia, S.; Wade, B.; Bauer, C.; Craig, C.; Nakaoka, K.; Lorowitz, W. The effect of wastewater treatment on antibiotic resistance in Escherichia coli and Enterococcus sp. Water Environ. Res. 2007, 79, 2387–2395. [Google Scholar]
- Tiwari, A.; Hokajärvi, A.M.; Santo Domingo, J.W.; Kauppinen, A.; Elk, M.; Ryu, H.; Jayaprakash, B.; Pitkänen, T. Categorical performance characteristics of method ISO 7899-2 and indicator value of intestinal enterococci for bathing water quality monitoring. J. Water Health 2018, 16, 711–723. [Google Scholar] [PubMed]
- Harris, S.; Morris, C.; Morris, D.; Cormican, M.; Cummins, E. Antimicrobial resistant Escherichia coli in the municipal wastewater system: Effect of hospital effluent and environmental fate. Sci. Total Environ. 2014, 468, 1078–1085. [Google Scholar] [PubMed]
- Silva, J.; Loyola, P.; Galleguillos, J.; Rodríguez, Y.; Colque-Navarro, P.; Möllby, R.; Kühn, I. Prevalencia de Enterococos resistentes a antibióticos en aguas servidas en el norte de Chile [Prevalence of antibiotic resistant Enterococcus spp. in wastewater in the north of Chile]. Rev. Medica Chile 2005, 133, 1201–1210. [Google Scholar]
- Baba, H.; Nishiyama, M.; Watanabe, T.; Kanamori, H. Review of Antimicrobial Resistance in Wastewater in Japan: Current Challenges and Future Perspectives. Antibiotics 2022, 11, 849. [Google Scholar] [CrossRef]
- Damani, A.; Klapsa, D.; Panopoulou, M.; Spiliopoulou, I.; Pantelidi, K.; Malli, E.; Kolonitsiou, F.; Grapsa, S.; Alepopoulou, E.; Frantzidou, F.; et al. A newly described vancomycin-resistant ST412 Enterococcus faecium predominant in Greek hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 29, 329–331. [Google Scholar] [CrossRef]
- USEPA. About NPDES. 2021. Available online: https://www.epa.gov/npdes/about-npdes (accessed on 30 June 2023).
- Liguori, K.; Calarco, J.; Maldonado Rivera, G.; Kurowski, A.; Keenum, I.; Davis, B.C.; Harwood, V.J.; Pruden, A. Comparison of Cefotaxime-Resistant Escherichia coli and sul1 and intI1 by qPCR for Monitoring of Antibiotic Resistance of Wastewater, Surface Water, and Recycled Water. Antibiotics 2023, 12, 1252. [Google Scholar]
- McQuaig, S.M.; Scott, T.M.; Lukasik, J.O.; Paul, J.H.; Harwood, V.J. Quantification of human polyomaviruses JC virus and BK virus by TaqMan quantitative PCR and comparison to other water quality indicators in water and fecal samples. Appl. Environ. Microbiol. 2009, 75, 3379–3388. [Google Scholar] [CrossRef]
- Zavaleta, E.; Ferrara, F.; Zovi, A.; Díaz-Madriz, J.P.; Fallas-Mora, A.; Serrano-Arias, B.; Valentino, F.; Arguedas-Chacón, S.; Langella, R.; Trama, U.; et al. Antibiotic Consumption in Primary Care in Costa Rica and Italy: A Retrospective Cross-Country Analysis. Cureus 2023, 15, e41414. [Google Scholar] [CrossRef]
- Gutiérrez, K.; Alfaro, M.; Granados, F.; Sánchez, J.; García, F.; Rodríguez, C. Detección de tetraciclinas en nueve lotes de alimentos para cerdos, tilapias y pollos producidos en Costa Rica: Incumplimiento de normativas y disconformidades con el etiquetado oficial de garantía. Agron. Costarric. 2010, 34, 145–151. [Google Scholar] [CrossRef]
- Salas-Vargas, A.V.; Boza-Cordero, R.; Bustamante-García, W.; García-Santamaria, F.; Barrantes-Valverde, E. Prevalencia e identificación genotípica de Enterococos Vancomicina resistentes en pacientes en un medio hospitalario. Acta Médica Costarric. 2004, 46, 19–26. [Google Scholar] [CrossRef]
- Bustamante, W.; Alpízar, A.; Hernández, S.; Pacheco, A.; Vargas, N.; Herrera, M.L.; Vargas, A.; Caballero, M.; García, F. Predominance of vanA genotype among vancomycin-resistant Enterococcus isolates from poultry and swine in Costa Rica. Appl. Environ. Microbiol. 2003, 69, 7414–7419. [Google Scholar]
- Aarestrup, F.M. The livestock reservoir for antimicrobial resistance: A personal view on changing patterns of risks, effects of interventions and the way forward. Philo Trans. R. Soc. B 2015, 370, 20140085. [Google Scholar] [CrossRef]
- PAHO. Informe Anual De La Red De Monitoreo/Vigilancia De La Resistencia a Los Antibióticos; Pan-American Health Organization: Washington, DC, USA, 2008. [Google Scholar]
- Low, D.E.; Keller, N.; Barth, A.; Jones, R.N. Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: Results from the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32 (Suppl. S2), S133–S145. [Google Scholar] [CrossRef]
- Ayobami, O.; Willrich, N.; Reuss, A.; Eckmanns, T.; Markwart, R. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: An epidemiological analysis of bloodstream infections. Emerg. Microbes Infect. 2020, 9, 1180–1193. [Google Scholar] [CrossRef]
- Markwart, R.; Willrich, N.; Haller, S.; Noll, I.; Koppe, U.; Werner, G.; Eckmanns, T.; Reuss, A. The rise in vancomycin-resistant Enterococcus faecium in Germany: Data from the German Antimicrobial Resistance Surveillance (ARS). Antimicrob. Resist. Infect. Control. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Ping, S.; Mayorga-Reyes, N.; Price, V.J.; Onuoha, M.; Bhardwaj, P.; Rodrigues, M.; Owen, J.; Araya, D.P.; Akins, R.L.; Palmer, K.L. Characterization of presumptive vancomycin-resistant enterococci recovered during infection control surveillance in Dallas, Texas, USA. Access Microbiol. 2021, 3, 000214. [Google Scholar] [CrossRef]
- Young, S.; Nayak, B.; Sun, S.; Badgley, B.D.; Rohr, J.R.; Harwood, V.J. Vancomycin-resistant enterococci and bacterial community structure following a sewage spill into an aquatic environment. Appl. Environ. Microbiol. 2016, 82, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Critchley, I.A.; Cotroneo, N.; Pucci, M.J.; Jain, A.; Mendes, R.E. Resistance among urinary tract pathogens collected in Europe during 2018. J. Glob. Antimicrob. Resist. 2020, 23, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Scholes, D.; Stamm, W.E. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. Jama 1999, 281, 736–738. [Google Scholar] [PubMed]
- Jimenez Pearson, A.; Chaverri Murillo, J.; Perez Corrales, C.; Ramírez Cardoce, M.; Bolaños Acuna, H.M. Informe Técnico: Estrategia Vigilancia de Laboratorio De La Resistencia a Los Antimicrobianos De Microorganismos De Importancia En Salud Pública; INCIENSA: Tres Ríos, Costa Rica, 2018. [Google Scholar]
- Murray, B.E.; Alvarado, T.; Kim, K.H.; Vorachit, M.; Jayanetra, P.; Prenzel, I.; Fling, M.; Elwell, L.; Mc Cracken, G.H.; Madrigal, G.; et al. Increasing resistance to trimethoprim-sulfamethoxazole among isolates of Escherichia coli in developing countries. J. Infect. Dis. 1985, 152, 1107–1113. [Google Scholar] [CrossRef]
- Huovinen, P.; Sundström, L.; Swedberg, G.; Sköld, O. Trimethoprim and sulfonamide resistance. Antimicrob. Agents Chemother. 1995, 39, 279–289. [Google Scholar] [CrossRef]
- ECDC. Surveillance of Antimicrobial Resistance in Europe 2018; EARS-Net: Stockholm, Sweden, 2019. [Google Scholar]
- WHO. Prioritization of Pathogens to Guide Discovery, Research, and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- WHO. Proportion of Bloodstream Infection Due to Escherichia coli Resistant to Third-Generation Cephalosporins (%). 2023. Available online: https://data.who.int/indicators/i/745F475 (accessed on 29 November 2023).
- Sanderson, H.; Ortega-Polo, R.; McDermott, K.; Hall, G.; Zaheer, R.; Brown, R.S.; Majury, A.; McAllister, T.A.; Liss, S.N. Quantification and multidrug resistance profiles of vancomycin-resistant enterococci isolated from two wastewater treatment plants in the same municipality. Microorganisms 2019, 7, 626. [Google Scholar] [CrossRef]
- Molale-Tom, L.G.; Bezuidenhout, C.C. Prevalence, antibiotic resistance and virulence of Enterococcus spp. from wastewater treatment plant effluent and receiving waters in South Africa. J. Water Health 2020, 18, 753–765. [Google Scholar]
- Bojar, B.; Sheridan, J.; Beattie, R.; Cahak, C.; Liedhegner, E.; Munoz-Price, L.S.; Hristova, K.R.; Skwor, T. Antibiotic resistance patterns of Escherichia coli isolates from the clinic through the wastewater pathway. Int. J. Hyg. Environ. Health 2021, 238, 113863. [Google Scholar]
- Lien, L.T.Q.; Lan, P.T.; Chuc, N.T.K.; Hoa, N.Q.; Nhung, P.H.; Thoa, N.T.M.; Diwan, V.; Tamhankar, A.J.; Stålsby Lundborg, C. Antibiotic resistance and antibiotic resistance genes in Escherichia coli isolates from hospital wastewater in Vietnam. Int. J. Environ. Res. Public Health 2017, 14, 699. [Google Scholar]
- Alduhaidhawi, A.H.M.; AlHuchaimi, S.N.; Al-Mayah, T.A.; Al-Ouqaili, M.T.; Alkafaas, S.S.; Muthupandian, S.; Saki, M. Prevalence of CRISPR-cas systems and their possible association with antibiotic resistance in Enterococcus faecalis and Enterococcus faecium collected from hospital wastewater. Infect. Drug Resist. 2022, 15, 1143–1154. [Google Scholar] [CrossRef]
- Gotkowska-Płachta, A. The prevalence of virulent and multidrug-resistant enterococci in river water and in treated and untreated municipal and hospital wastewater. Int. J. Environ. Res. Public Health 2021, 18, 563. [Google Scholar] [PubMed]
- El País. Catorce Tuberías De Aguas Con Heces Caen En El Estero De Puntarenas. 2008. Available online: https://www.nacion.com/el-pais/14-tuberias-de-aguas-con-heces-caen-en-el-estero-de-puntarenas/3RTTZZ2BFBH6XBUQS7OAS6S7BA/story/ (accessed on 2 February 2020).
- Brinkhoff, T. City Population: Puntarenas, Costa Rica. 2023. Available online: https://www.citypopulation.de/en/costarica/admin/puntarenas/601__puntarenas/ (accessed on 29 November 2023).
- Mora-Alvarado, D.; Portuguez-Barquero, C.F. Cobertura de la disposición de excretas en Costa Rica en el periodo 2000–2014 y expectativas para el 2021 [Waterwater disposal coverage in Costa Rica from 2000 to 2014 and outlook for 2021]. Rev. Tecnol. En Marcha 2016, 29, 43. [Google Scholar]
- USEPA. Method 1603: Escherichia coli (E. coli) in Water by Membrane Filtration Using Modified Membrane-Thermotolerant Escherichia coli Agar; U.S. Environmental Protection Agency: Washington, DC, USA, 2009.
- USEPA. Method 1611: Enterococci in Water by TaqMan® Quantitative Polymerase Chain Reaction (qPCR) Assay; U.S. Environmental Protection Agency: Washington, DC, USA, 2012.
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. Am. Soc. Microbiol. 2009, 15, 55–63. [Google Scholar]
- Cribari-Neto, F.; Zeileis, A. Beta Regression in R. J. Stat. Softw. 2010, 34, 1–24. [Google Scholar] [CrossRef]
- Zeileis, A.; Hothorn, T. Diagnostic Checking in Regression Relationships. R News 2002, 2, 7–10. [Google Scholar]
- Tjur, T. Coefficients of determination in logistic regression models—A new proposal: The coefficient of discrimination. Am. Stat. 2009, 63, 366–372. [Google Scholar] [CrossRef]
- Hegewisch-Taylor, J.; Dreser-Mansilla, A.; Romero-Mónico, J.; Levy-Hara, G. Antimicrobial stewardship in hospitals in Latin America and the Caribbean: A scoping review. Rev. Panam. Salud Pública 2020, 44, e68. [Google Scholar]
- DECRETO EJECUTIVO No. 41385-S. Diario Oficial La Gaceta, 2019.

 and Monseñor Sanabria Hospital
and Monseñor Sanabria Hospital  appear on map. Sampling stations are designated: (A) hospital wastewater, (B) residential wastewater, (C) treated effluent, and (D) Puntarenas estuary
appear on map. Sampling stations are designated: (A) hospital wastewater, (B) residential wastewater, (C) treated effluent, and (D) Puntarenas estuary  where the WWTP effluent is discharged. A scale is provided at the bottom of the map.
where the WWTP effluent is discharged. A scale is provided at the bottom of the map.
 and Monseñor Sanabria Hospital
and Monseñor Sanabria Hospital  appear on map. Sampling stations are designated: (A) hospital wastewater, (B) residential wastewater, (C) treated effluent, and (D) Puntarenas estuary
appear on map. Sampling stations are designated: (A) hospital wastewater, (B) residential wastewater, (C) treated effluent, and (D) Puntarenas estuary  where the WWTP effluent is discharged. A scale is provided at the bottom of the map.
where the WWTP effluent is discharged. A scale is provided at the bottom of the map.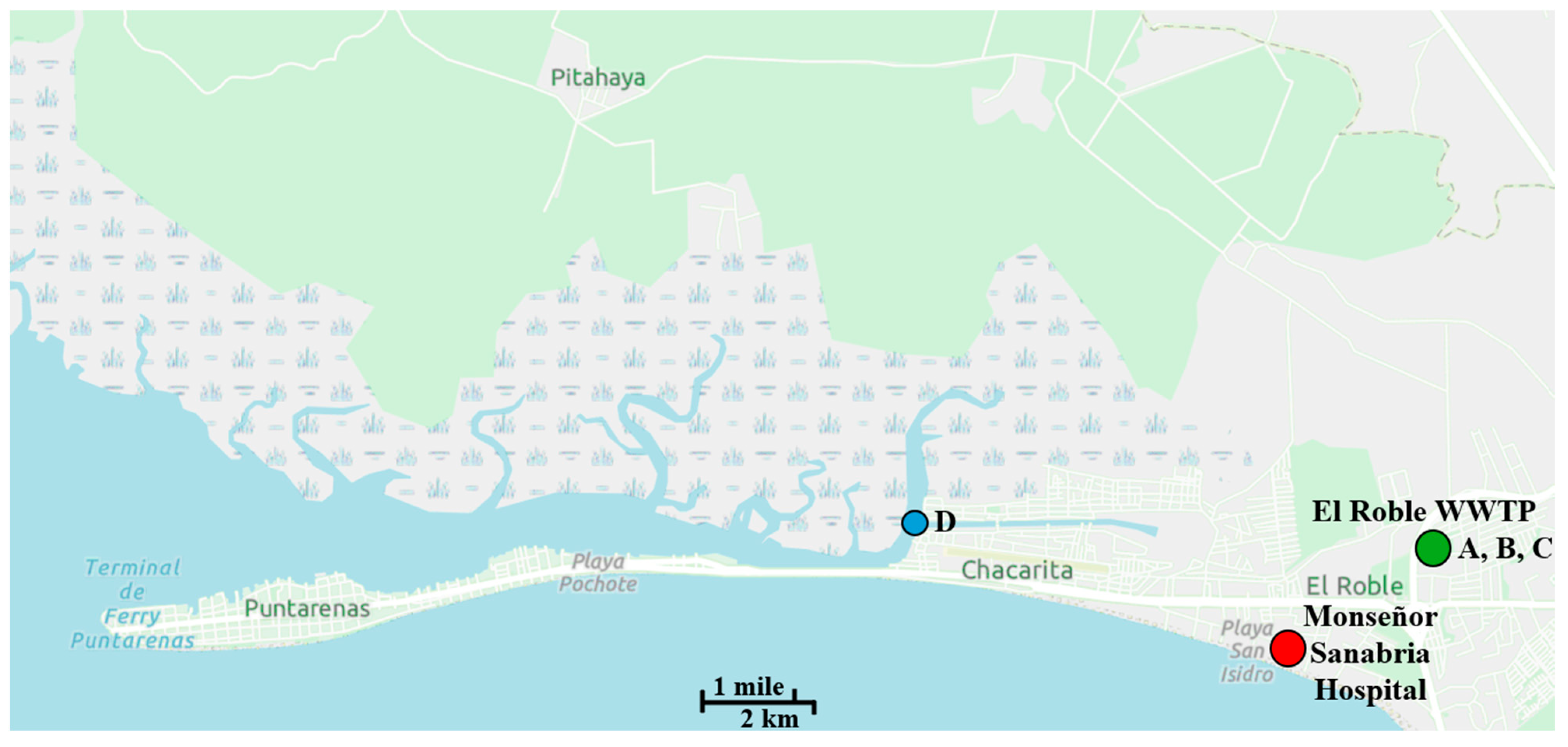
| (A) | |||
| Model | FIB | Chisq (χ2) | p-Value |
| Site | E. coli | 29.076 | <0.001 |
| Enterococcus | 9.862 | 0.020 | |
| Antibiotic | E. coli | 141.95 | <0.001 |
| Enterococcus | 71.301 | <0.001 | |
| Site: Antibiotic | E. coli | 30.312 | 0.003 |
| Enterococcus | 7.495 | 0.586 | |
| (B) | |||
| Model | Chisq (χ2) | p-Value | |
| Site | 7.8561 | 0.04928 | |
| FIB | 2.0838 | 0.14887 | |
| Multidrug Resistance | |||
|---|---|---|---|
| Predictors | Log Odds | CI | p |
| (Intercept) | 0.74 | 0.38–1.42 | 0.373 |
| Site [Hospital] | 3.63 | 1.46–9.43 | 0.006 |
| Site [Effluent] | 1.71 | 0.72–4.12 | 0.224 |
| Site [Estuary] | 1.76 | 0.74–4.26 | 0.201 |
| FIB [Enterococcus] | 0.62 | 0.32–1.19 | 0.150 |
| Observations | 170 | ||
| R2 Nagelkerke | 0.078 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brodrick, E.A.; González-Fernández, A.; Kramer, A.M.; Harwood, V.J. Ampicillin- and Multidrug-Resistant Escherichia coli and Enterococcus spp. in Costa Rican Wastewater and Surface Water. Antibiotics 2025, 14, 1024. https://doi.org/10.3390/antibiotics14101024
Brodrick EA, González-Fernández A, Kramer AM, Harwood VJ. Ampicillin- and Multidrug-Resistant Escherichia coli and Enterococcus spp. in Costa Rican Wastewater and Surface Water. Antibiotics. 2025; 14(10):1024. https://doi.org/10.3390/antibiotics14101024
Chicago/Turabian StyleBrodrick, Eleanor A., Adriana González-Fernández, Andrew M. Kramer, and Valerie J. Harwood. 2025. "Ampicillin- and Multidrug-Resistant Escherichia coli and Enterococcus spp. in Costa Rican Wastewater and Surface Water" Antibiotics 14, no. 10: 1024. https://doi.org/10.3390/antibiotics14101024
APA StyleBrodrick, E. A., González-Fernández, A., Kramer, A. M., & Harwood, V. J. (2025). Ampicillin- and Multidrug-Resistant Escherichia coli and Enterococcus spp. in Costa Rican Wastewater and Surface Water. Antibiotics, 14(10), 1024. https://doi.org/10.3390/antibiotics14101024






