Impact of Complying with a Procalcitonin-Guided Stopping Rule on the Duration of Antibiotic Therapy in Critically Ill Patients: A Real-Life Study
Abstract
1. Background
2. Results
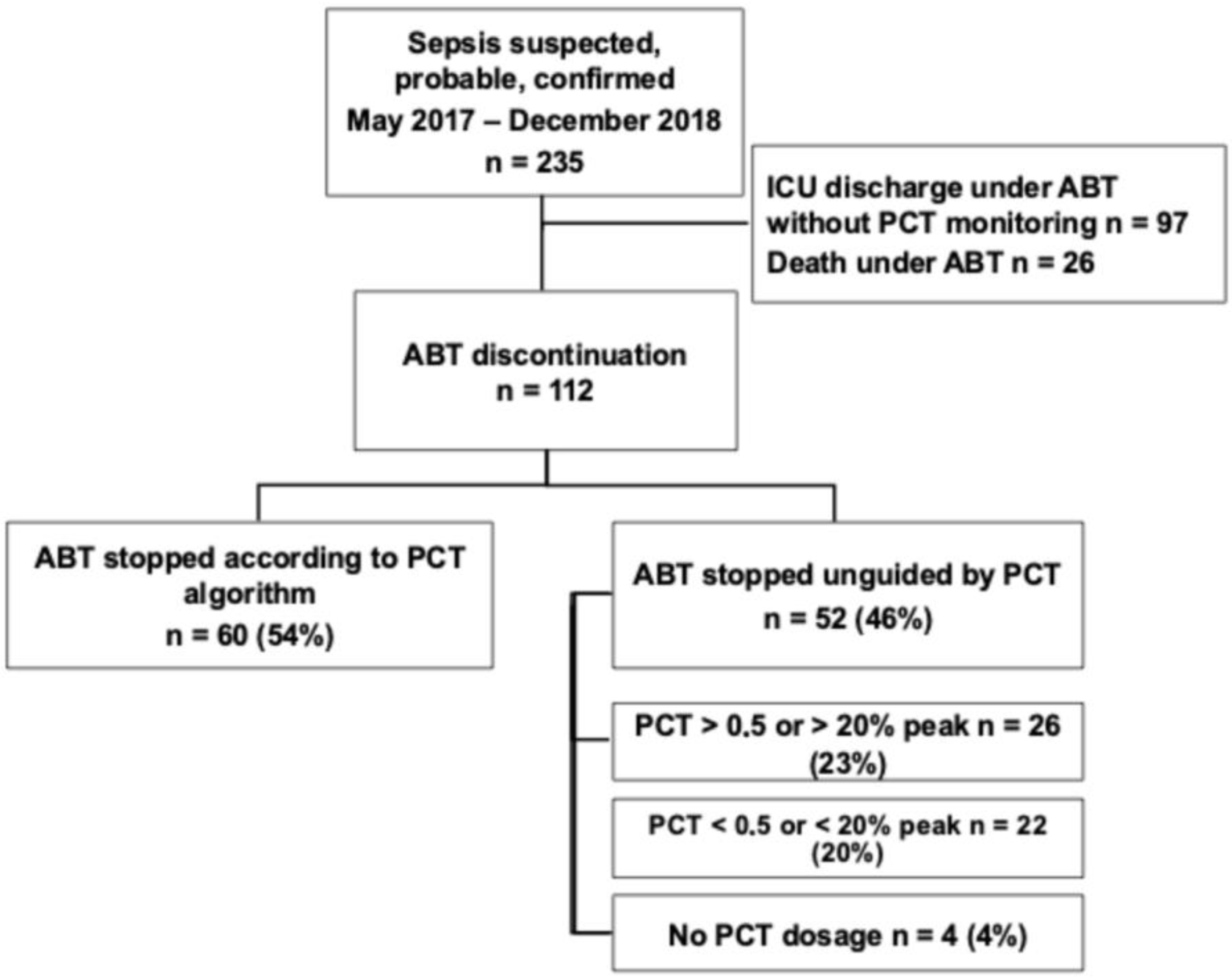
2.1. Study Population and Septic Episode Description
2.2. Antibiotics Exposure and Compliance to the PCT-Guided Stopping Rule
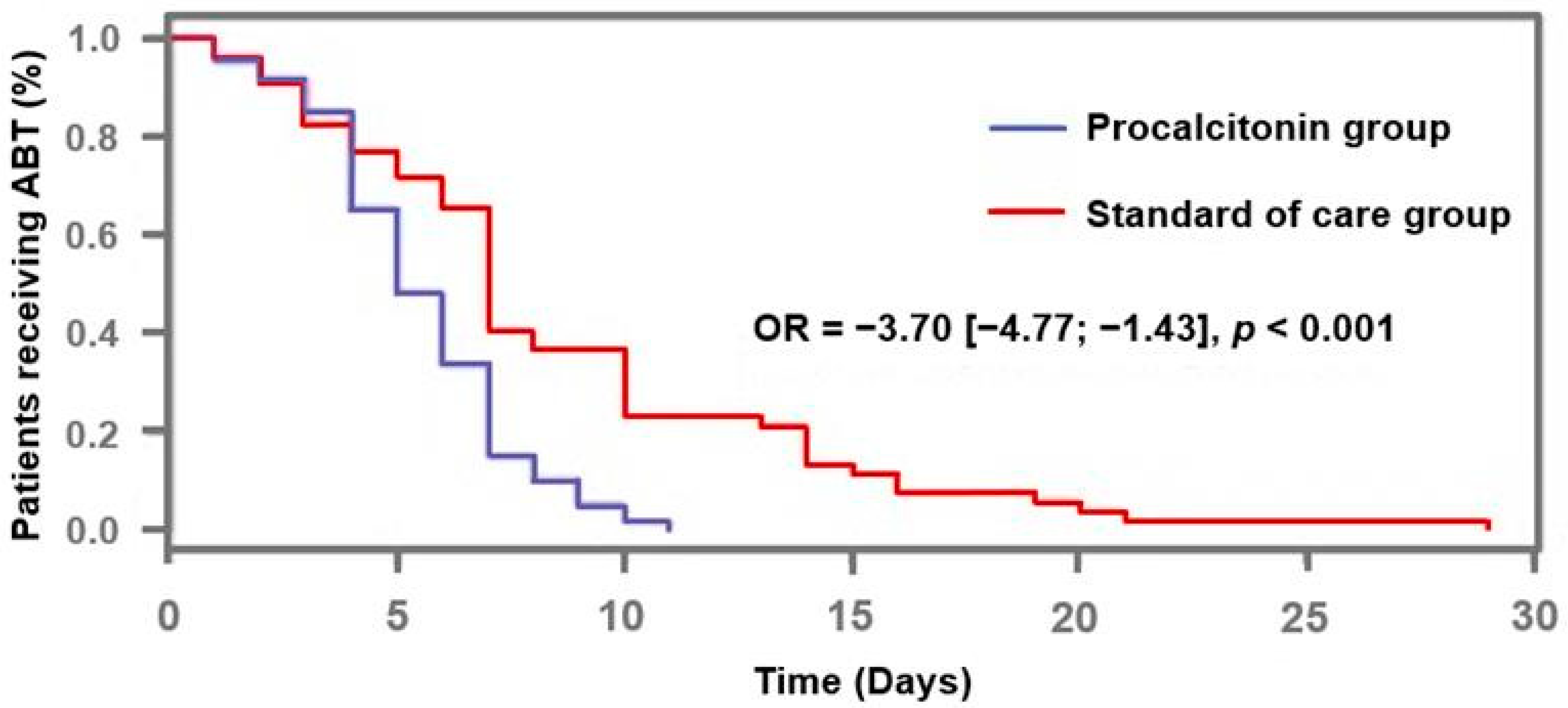
2.3. Outcome
3. Discussion
4. Methods
4.1. Patients and Setting
4.2. Definitions
4.3. Data Collections
4.4. Management of PCT-Guided ABT
4.5. Clinical Endpoints
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Versporten, A.; Zarb, P.; Caniaux, I.; Gros, M.-F.; Drapier, N.; Miller, M.; Jarlier, V.; Nathwani, D.; Goossens, H.; Koraqi, A.; et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. 2018, 6, e619–e629. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Neubeiser, A.; Bonsignore, M.; Tafelski, S.; Alefelder, C.; Schwegmann, K.; Rüden, H.; Geffers, C.; Nachtigall, I. Mortality attributable to hospital acquired infections with multidrug-resistant bacteria in a large group of German hospitals. J. Infect. Public Health 2020, 13, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Wintenberger, C.; Guery, B.; Bonnet, E.; Castan, B.; Cohen, R.; Diamantis, S.; Lesprit, P.; Maulin, L.; Péan, Y.; Peju, E.; et al. Proposal for shorter antibiotic therapies. Med. Mal. Infect. 2017, 47, 92–141. [Google Scholar] [CrossRef]
- Payne, L.E.; Gagnon, D.J.; Riker, R.R.; Seder, D.B.; Glisic, E.K.; Morris, J.G.; Fraser, G.L. Cefepime-induced neurotoxicity: A systematic review. Crit. Care 2017, 21, 276. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Lo, J.; Leung, V.; Brown, K.; Schwartz, K.L.; Daneman, N.; Garber, G.; Wu, J.H.; Langford, B.J. Estimating daily antibiotic harms: An umbrella review with individual study meta-analysis. Clin. Microbiol. Infect. 2022, 28, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J.; The Geneva Sepsis Network. Diagnostic Value of Procalcitonin, Interleukin-6, and Interleukin-8 in Critically Ill Patients Admitted with Suspected Sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef]
- Charles, P.E.; Tinel, C.; Barbar, S.; Aho, S.; Prin, S.; Doise, J.M.; Olsson, N.O.; Blettery, B.; Quenot, J.P. Procalcitonin kinetics within the first days of sepsis: Relationship with the appropriateness of antibiotic therapy and the outcome. Crit. Care 2009, 13, R38. [Google Scholar] [CrossRef]
- Gibot, S.; Béné, M.C.; Noel, R.; Massin, F.; Guy, J.; Cravoisy, A.; Barraud, D.; Bittencourt, M.D.C.; Quenot, J.-P.; Bollaert, P.-E.; et al. Combination Biomarkers to Diagnose Sepsis in the Critically Ill Patient. Am. J. Respir. Crit. Care Med. 2012, 186, 65–71. [Google Scholar] [CrossRef]
- Nobre, V.; Harbarth, S.; Graf, J.D.; Rohner, P.; Pugin, J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: A randomized trial. Am. J. Respir. Crit. Care Med. 2008, 177, 498–505. [Google Scholar] [CrossRef]
- Bouadma, L.; Luyt, C.-E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef]
- de Jong, E.; van Oers, J.A.; Beishuizen, A.; Vos, P.; Vermeijden, W.J.; Haas, L.E.; Loef, B.G.; Dormans, T.; van Melsen, G.C.; Kluiters, Y.C.; et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: A randomised, controlled, open-label trial. Lancet Infect. Dis. 2016, 16, 819–827. [Google Scholar] [CrossRef]
- Christ-Crain, M.; Stolz, D.; Bingisser, R.; Muller, C.; Miedinger, D.; Huber, P.R.; Zimmerli, W.; Harbarth, S.; Tamm, M.; Muller, B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: A randomized trial. Am. J. Respir. Crit. Care Med. 2006, 174, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Albrich, W.C.; Dusemund, F.; Bucher, B.; Meyer, S.; Thomann, R.; Kuhn, F.; Bassetti, S.; Sprenger, M.; Bachli, E.; Sigrist, T.; et al. Effectiveness and safety of procalcitonin-guided antibiotic therapy in lower respiratory tract infections in “real life”: An international, multicenter poststudy survey (ProREAL). Arch. Intern. Med. 2012, 172, 715–722. [Google Scholar] [PubMed]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect. Dis. 2018, 18, 95–107. [Google Scholar] [CrossRef]
- Stolz, D.; Smyrnios, N.; Eggimann, P.; Pargger, H.; Thakkar, N.; Siegemund, M.; Marsch, S.; Azzola, A.; Rakic, J.; Mueller, B.; et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: A randomised study. Eur. Respir. J. 2009, 34, 1364–1375. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Hochreiter, M.; Koehler, T.; Schweiger, A.-M.; Bein, B.; Keck, F.S.; von Spiegel, T. Procalcitonin (PCT)-guided algorithm reduces length of antibiotic treatment in surgical intensive care patients with severe sepsis: Results of a prospective randomized study. Langenbeck’s. Arch. Surg. 2008, 394, 221–226. [Google Scholar] [CrossRef]
- Hochreiter, M.; Köhler, T.; Schweiger, A.M.; Keck, F.S.; Bein, B.; von Spiegel, T.; Schroeder, S. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: A randomized prospective controlled trial. Crit. Care 2009, 13, R83. [Google Scholar] [CrossRef]
- Shehabi, Y.; Sterba, M.; Garrett, P.M.; Rachakonda, K.S.; Stephens, D.; Harrigan, P.; Walker, A.; Bailey, M.J.; Johnson, B.; Millis, D.; et al. Procalcitonin Algorithm in Critically Ill Adults with Undifferentiated Infection or Suspected Sepsis. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2014, 190, 1102–1110. [Google Scholar] [CrossRef]
- Kopterides, P.; Siempos, I.I.; Tsangaris, I.; Tsantes, A.; Armaganidis, A. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: A systematic review and meta-analysis of randomized controlled trials. Crit. Care Med. 2010, 38, 2229–2241. [Google Scholar] [CrossRef]
- Wirz, Y.; Meier, M.A.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; Tubach, F.; Schroeder, S.; Nobre, V.; Annane, D.; et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit. Care 2018, 22, 191. [Google Scholar] [CrossRef]
- Huang, D.T.; Yealy, D.M.; Filbin, M.R.; Brown, A.M.; Chang, C.-C.H.; Doi, Y.; Donnino, M.W.; Fine, J.; Fine, M.J.; Fischer, M.A.; et al. Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N. Engl. J. Med. 2018, 379, 236–249. [Google Scholar] [CrossRef]
- Chaitidis, N.; Kokkinidis, D.G.; Papadopoulou, Z.; Kyriazopoulou, M.; Schizas, D.; Bakoyiannis, C. Treatment of chronic venous disorder: A comprehensive review. Dermatol. Ther. 2021, 35, e15238. [Google Scholar] [CrossRef]
- Dark, P.; Hossain, A.; McAuley, D.F.; Brealey, D.; Carlson, G.; Clayton, J.C.; Felton, T.W.; Ghuman, B.K.; Gordon, A.C.; Hellyer, T.P.; et al. Biomarker-Guided Antibiotic Duration for Hospitalized Patients with Suspected Sepsis: The ADAPT-Sepsis Randomized Clinical Trial. JAMA 2025, 333, 682–693. [Google Scholar] [CrossRef]
- Beye, F.; Vigneron, C.; Dargent, A.; Prin, S.; Andreu, P.; Large, A.; Quenot, J.-P.; Bador, J.; Bruyere, R.; Charles, P.-E. Adhering to the procalcitonin algorithm allows antibiotic therapy to be shortened in patients with ventilator-associated pneumonia. J. Crit. Care 2019, 53, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Daneman, N.; Gruneir, A.; Bronskill, S.E.; Newman, A.; Fischer, H.D.; Rochon, P.A.; Anderson, G.M.; Bell, C.M. Prolonged antibiotic treatment in long-term care: Role of the prescriber. JAMA Intern. Med. 2013, 173, 673–682. [Google Scholar] [CrossRef]
- Wilke, M.; Grube, R.; Bodmann, K. The use of a standardized PCT-algorithm reduces costs in intensive care in septic patients—A DRG-based simulation model. Eur. J. Med. Res. 2011, 16, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Kip, M.M.A.; van Oers, J.A.; Shajiei, A.; Beishuizen, A.; Berghuis, A.M.S.; Girbes, A.R.; de Jong, E.; de Lange, D.W.; Nijsten, M.W.N.; IJzerman, M.J.; et al. Cost-effectiveness of procalcitonin testing to guide antibiotic treatment duration in critically ill patients: Results from a randomised controlled multicentre trial in the Netherlands. Crit. Care 2018, 22, 293. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; de Mendonca, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, J.-R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef] [PubMed]

| Procalcitonin Group (n = 60) | Standard of Care Group (n = 52) | p | |
|---|---|---|---|
| Number of PCT measurements | 4 (3–5) | 4 (2–5) | 0.76 |
| PCT value at initiation of ABT (ug/L) | 1.3 (0.2–4.8) | 1.1 (0.5–4.1) | 0.52 |
| Peak value of PCT (ug/L) | 4.0 (0.9–10.4) | 3.3 (1.1–15.7) | 0.23 |
| PCT value at discontinuation of ABT (ug/L) | 0.6 (0.2–1.2) | 1.1 (0.5–2.7) | 0.70 |
| Duration of antibiotic therapy (days) | 5 (4–7) | 7 (5–10) | <0.001 |
| Duration of antibiotic therapy as compared to guidelines (days) | −2 (−3; 0) | 0 (−1; 3) | <0.001 |
| Resumption antibiotic therapy within 48 h | 3 (5) | 4 (8) | 0.70 |
| Duration of mechanical ventilation (days) | 4 (2–10) | 2 (0–7) | 0.67 |
| Length of stay (days) | 9 (7–15) | 5 (3–15) | 0.28 |
| ICU mortality | 10 (17) | 7 (13) | 0.63 |
| 28-day mortality | 9 (15) | 11 (21) | 0.40 |
| Procalcitonin Group (n = 60) | Standard of Care Group (n = 52) | p | |
|---|---|---|---|
| Cause of ICU admission | |||
| Acute respiratory failure | 37 (62) | 17 (33) | 0.002 |
| Septic shock | 8 (13) | 12 (23) | 0.18 |
| Hemorrhagic shock | 2 (3) | 4 (8) | 0.41 |
| Cardiogenic shock | 0 (0) | 1 (2) | 0.46 |
| Obstructive shock | 0 (0) | 1 (2) | 0.46 |
| Cardiac arrest | 4 (7) | 9 (17) | 0.14 |
| Coma | 4 (7) | 3 (6) | 1.00 |
| Framing invasive procedure | 0 (0) | 1 (2) | 0.46 |
| Voluntary medical intoxication | 3 (5) | 0 (0) | 0.25 |
| Others | 2 (3) | 5 (10) | 0.25 |
| Patients characteristics | |||
| Male gender | 42 (70) | 37 (71) | 0.89 |
| Age (years) | 64 (55–78) | 70 (61–79) | 0.19 |
| Chronic renal failure | 4 (7) | 14 (27) | 0.004 |
| Chronic heart failure | 6 (10) | 6 (12) | 0.79 |
| Chronic respiratory failure | 4 (7) | 7 (13) | 0.34 |
| Cirrhosis | 0 (0) | 10 (19) | <0.001 |
| Immunosuppression | 9 (15) | 15 (29) | 0.07 |
| ICU admission SOFA score (points) | 7 (4–9) | 7 (5–10) | 0.55 |
| ICU discharge SOFA score (points) | 2 (1–3) | 2 (0–3) | 0.70 |
| ICU admission SAPS 2 (points) | 46 (36–61) | 50 (36–62) | 0.56 |
| Sepsis main characteristics | |||
| Septic shock | 24 (40) | 22 (42) | 0.80 |
| Infection type | |||
| Community-acquired | 22 (37) | 29 (56) | 0.04 |
| Hospital-acquired | 21 (35) | 18 (35) | 0.97 |
| Healthcare-associated | 3 (5) | 5 (10) | 0.47 |
| Infection site | |||
| Lung | 52 (87) | 35 (67) | 0.01 |
| Digestive tract | 2 (3) | 7 (13) | 0.08 |
| Urinary tract | 3 (5) | 2 (4) | 1.00 |
| Skin and soft tissue | 2 (3) | 5 (10) | 0.25 |
| Bone | 0 (0) | 0 (0) | / |
| Others | 0 (0) | 3 (6) | 0.10 |
| Unknown | 1 (2) | 0 (0) | 1.00 |
| Bacteremia | 9 (15) | 11 (2) | 0.39 |
| Documentation | 36 (60) | 26 (50) | 0.06 |
| Gram-positive bacteria | 12 (20) | 7 (15) | 0.52 |
| Gram-negative bacteria | 24 (40) | 19 (42) | 0.80 |
| Multidrug-resistant bacteria | 8 (13) | 8 (15) | 0.76 |
| Initial antibiotic therapy | |||
| Empirical | 59 (98) | 50 (96) | 0.48 |
| Amoxicillin, Amoxicillin-clavulanic acid | 13 (22) | 12 (23) | 0.86 |
| Cephalosporins | 21 (35) | 15 (29) | 0.49 |
| Piperacillin-Tazobactam | 21 (35) | 17 (33) | 0.80 |
| Carbapenems | 4 (7) | 7 (13) | 0.34 |
| Aminoglycosides | 4 (7) | 3 (6) | 1.00 |
| Appropriateness | 22 (61) | 19 (86) | 0.98 |
| Odd Ratio | 95% CI | p | |
|---|---|---|---|
| Age (years) | 0.99 | 0.06–1.02 | 0.37 |
| Cirrhosis | 1.95 × 10−9 | 0.00–0.00 | 0.99 |
| Immunosuppression | 0.38 | 0.13–1.17 | 0.92 |
| SOFA score on admission (points) | 1.07 | 0.92–1.24 | 0.37 |
| Source of infection | |||
| Lung | 4264.8 | 0.00–0.00 | 0.99 |
| Digestive tract | 1.23 | 0.05–30.00 | 0.89 |
| Urinary tract | 16.20 | 0.52–507.74 | 0.11 |
| Skin and soft tissue | 3.43 | 0.13–94.51 | 0.47 |
| Duration of ABT (days) | 0.74 | 0.62–0.88 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Péju, E.; Dargent, A.; Roudaut, J.-B.; Prin, S.; Andreu, P.; Large, A.; Quenot, J.-P.; Charles, P.-E. Impact of Complying with a Procalcitonin-Guided Stopping Rule on the Duration of Antibiotic Therapy in Critically Ill Patients: A Real-Life Study. Antibiotics 2025, 14, 1012. https://doi.org/10.3390/antibiotics14101012
Péju E, Dargent A, Roudaut J-B, Prin S, Andreu P, Large A, Quenot J-P, Charles P-E. Impact of Complying with a Procalcitonin-Guided Stopping Rule on the Duration of Antibiotic Therapy in Critically Ill Patients: A Real-Life Study. Antibiotics. 2025; 14(10):1012. https://doi.org/10.3390/antibiotics14101012
Chicago/Turabian StylePéju, Edwige, Auguste Dargent, Jean-Baptiste Roudaut, Sébastien Prin, Pascal Andreu, Audrey Large, Jean-Pierre Quenot, and Pierre-Emmanuel Charles. 2025. "Impact of Complying with a Procalcitonin-Guided Stopping Rule on the Duration of Antibiotic Therapy in Critically Ill Patients: A Real-Life Study" Antibiotics 14, no. 10: 1012. https://doi.org/10.3390/antibiotics14101012
APA StylePéju, E., Dargent, A., Roudaut, J.-B., Prin, S., Andreu, P., Large, A., Quenot, J.-P., & Charles, P.-E. (2025). Impact of Complying with a Procalcitonin-Guided Stopping Rule on the Duration of Antibiotic Therapy in Critically Ill Patients: A Real-Life Study. Antibiotics, 14(10), 1012. https://doi.org/10.3390/antibiotics14101012






