Abstract
Objectives: Antimicrobial resistance is a major global health threat. This study aimed to assess the impact of antimicrobial-resistant bacterial pneumonia on in-hospital mortality and length of hospital stay in Spain using a large, nationally representative cohort. Methods: A retrospective cohort study that used data from Spain’s Registry of Specialized Health Care Activity (RAE-CMBD) between 2017 and 2022. Hospitalized adults with bacterial pneumonia were included. Hospitalization episodes with bacterial antimicrobial resistance, defined according to ICD-10-CM codes for antimicrobial resistance (Z16.1, Z16.2), were analyzed versus hospitalization episodes without these codes. Multivariate logistic regression models, adjusted for potential confounders (e.g., age, comorbidity, intensive care unit admission) and sensitivity analyses (Poisson regression and propensity score matching test), were performed. Results: Of the 116,901 eligible hospitalizations, 6017 (5.15%) involved antimicrobial-resistant bacteria. Patients with antimicrobial-resistant bacterial pneumonia were older (median 75 vs. 72 years), had greater comorbidity (Elixhauser–van Walraven index: 8 vs. 5), and were more frequently admitted to the intensive care unit (22% vs. 14%). Crude in-hospital mortality was higher in the antimicrobial resistance group (18.46% vs. 10.05%, p < 0.0001), with an adjusted odds ratio of 1.47 (95% confidence interval, 1.36–1.58), p < 0.0001. Length of hospital stay was prolonged in antimicrobial resistance patients (median 14 vs. 8 days; adjusted incident rate ratio of 1.46; 95% confidence interval of 1.41 to 1.50). The most prevalent antimicrobial resistant pathogens were Staphylococcus aureus and Gram-negative bacilli (Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli). Conclusions: Antimicrobial resistance is associated with longer hospital stays and an up to 50% higher risk of mortality. Despite the implementation of control policies in place over the past decade, policymakers must strengthen AMR surveillance and ensure adequate resource allocation. Clinicians, in turn, must reinforce antimicrobial stewardship and incorporate rapid diagnostic tools to minimize the impact of antimicrobial resistance on patient outcomes.
1. Introduction
Antimicrobial resistance (AMR) is a slow-moving and silent pandemic that poses a major threat to global health and economic stability [1].
There are four major categories of antimicrobial resistance exhibiting microorganisms: multidrug-resistant (MDR), extensively drug-resistant (XDR), pan drug-resistant (PDR), and difficult-to-treat resistance (DTR). MDR microorganisms acquired non-susceptibility to at least one agent in three or more antimicrobial classes. XDR microorganisms are non-susceptible to at least one agent in all but two or fewer antimicrobial classes. Microorganisms with non-susceptibility to all agents in all antimicrobial classes are referred to as PDR. Finally, difficult-to-treat resistance (DTR) is characterized by non-susceptibility to all first-line antimicrobials, including beta-lactams and fluoroquinolones, thereby restricting limiting treatment options to more toxic and/or less effective antimicrobials [2].
The World Health Organization (WHO) estimated that bacterial AMR was directly responsible for 1.27 million deaths worldwide in 2019 and contributed to an additional 4.95 million deaths [3].
The Centers for Disease Control and Prevention (CDC) reported that each year more than 2.8 million AMR infections occur in the United States (U.S.), resulting in over 35,000 deaths [4]. In the European Union (EU), this corresponds to approximately 25,000 deaths per year [1]. In Spain, 4000 people die each year, four times more than the number of deaths caused by traffic accidents in 2021 [5].
Although poorly quantified, the global burden of AMR is likely concentrated in three major areas: (i) prolonged illness and higher mortality rates in patients with resistant infections; (ii) increased treatment costs for such infections; and (iii) the inability to perform medical procedures that depend on effective antimicrobial prophylaxis [6]. Barrasa-Villar et al. concluded that hospital mortality from all-cause hospital mortality was 1.7 times higher in infections caused by multidrug-resistant organisms compared to those caused by susceptible microorganisms [7].
Various policy frameworks have been established to address the growing challenge of AMR. “The Global action plan on antimicrobial resistance” to tackle the growing problem of antimicrobial medicines, endorsed by the World Health Assembly in May 2015, provided a strategic framework for developing national action plans, outlining key measures to be implemented within 5–10 years by multiple stakeholders to combat AMR [8].
Several countries have subsequently developed their own action plans to combat this “silent pandemic” such as “A European One Health Action Plan against Antimicrobial Resistance” (EU since 2011) or the “National Action Plan for Combating Antibiotic-Resistant Bacteria” (U.S. since 2014) [1,4].
Given the transmission of bacterial pathogens, the health of humans, domestic and wild animals, plants, and the wider environment (including ecosystems) are closely interconnected. This recognition led to the development of the “One Health” concept by the WHO, later adopted globally as an integrated and unifying approach that seeks to sustainably balance and optimize the health of people, animals, and ecosystems [9].
The One Health joint plan of action (2022–2026) of the WHO, the Agriculture Organization of the United Nations (FAO), the World Organization for Animal Health (WOAH), and the (United Nations Environment Programme UNEP) was established as a quadripartite cooperation. Its purpose is to provide coordinated support for the integration of the One Health approach worldwide. Among its priority action tracks, it specifically addresses the challenge of antimicrobial resistance (AMR) as a “silent pandemic” and emphasizes the integration of environmental considerations into One Health strategies [10].
The 2024 WHO Bacterial Priority Pathogens List (WHO BPPL) is an important tool in the global response to antimicrobial resistance. The updated list identifies 24 bacterial pathogens of greatest concern, with the aim of addressing the evolving challenges of AMR and guiding priorities in research, the development of new antimicrobials, and public health interventions [11].
The WHO BPPL stratified the most worrying bacterial pathogens worldwide into three priority groups [11].
- Critical priority group—The highest threat to public health due to limited treatment options: Enterobacterales carbapenem-resistant, Enterobacterales third-generation cephalosporin-resistant, Acinetobacter baumannii carbapenem-resistant, and Mycobacterium tuberculosis rifampicin-resistant.
- High priority group—AMR pathogens that are significantly difficult to treat: Salmonella typhi fluoroquinolone-resistant, Shigella spp. fluoroquinolone-resistant, Enterococcus faecium vancomycin-resistant, Pseudomonas aeruginosa carbapenem-resistant, non-typhoidal Salmonella fluoroquinolone-resistant, Neisseria gonorrhoeae third-generation cephalosporin and/or fluoroquinolone-resistant, and Staphylococcus aureus methicillin-resistant (MRSA).
- Medium priority group—AMR pathogens that are associated with moderate difficulty for treatment): Group A Streptococci macrolide-resistant, Streptococcus pneumoniae macrolide-resistant, Haemophilus influenzae ampicillin-resistant, and Group B Streptococci penicillin-resistant.
Pneumonia is one of the leading causes of hospital admissions in the U.S. and remains a major cause of death [12]. In 2023, the CDC reported 41,210 deaths in the U.S. due to pneumonia, with a crude death rate of 12.3 per 100,000 population [13].
Some authors have estimated the mortality rate of community-acquired pneumonia in hospitalized patients to be approximately 15%. However, the emergence of resistant pathogens complicates management [14].
The WHO recognizes that although recent studies position AMR as one of the leading causes of death worldwide, the exact morbidity and mortality associated with AMR are very difficult to establish, and, in many settings, reliable estimates are unavailable. These knowledge gaps highlight the need to foster studies on AMR attributable mortality and morbidity [15]. The U.S. and EU action plan for combating AMR include among their goals the need for new research and evidence-based analysis and data to better understand the risks of AMR [1,4].
Since the causative pathogen is identified in fewer than 40% of pneumonia cases and is bacterial in less than 14% of isolates, most studies conducted in hospitalized patients with pneumonia include a limited sample of patients with bacterial isolates [7,16].
Large-scale epidemiological studies using real-world administrative data are a high-quality source of information on the true impact of AMR in a population. Administrative databases, such as Spain’s Registry of Specialized Health Care Activity (RAE-CMBD), provide a valuable resource for analyzing the impact of AMR on health [17].
This study, based on a large national cohort of patients with bacterial pneumonia and isolation of the responsible pathogen over six years (2017–2022), analyzed the real impact of AMR-related bacterial pneumonia on in-hospital mortality and LOHS in Spain. The primary objective was to determine the impact of AMR on in-hospital mortality in patients with bacterial pneumonia. Secondary objectives included assessing differences in hospital stay duration, identifying others risk factors associated with increased in-hospital mortality and prolonged hospitalization, and evaluating trends in the prevalence of AMR bacterial pneumonia requiring hospital admission in Spain.
2. Results
The total number of hospitalizations of patients over 18 years of age in Spain recorded in the national RAE-CMBD from 2017 to 2022, except hospitalizations for childbirth (1,857,052), was 22,331,410 [18]. Of these, 3,664,087 hospitalizations (16.40%) corresponded to bacterial infections.
We identified 186,800 (5.10%) episodes of hospitalization of patients with bacterial pneumonia diagnosis (excluding Mycobacteriales/Spirochaetales orders). After applying the inclusion and exclusion criteria, 116,901 hospitalizations were confirmed as eligible for this study.
The median age at hospital admission was 73 years (IQR: 60–83). The country of birth was Spain in 79.43% of cases (92,852). The hospitalizations with the code for AMR bacteria represented 5.15% (6017) of all bacterial pneumonia hospitalizations. Table 1 shows the prevalence of AMR across the years of study.

Table 1.
Prevalence of antimicrobial drug resistance (AMR) across the study years.
The demographic and clinical characteristics in hospitalized adults with registry of the AMR bacteria code are shown in Table 2. The AMR bacteria group were significantly older: median 75 years (IQR: 64–84) versus (vs.) 72 years (IQR: 69–83), respectively (p < 0.0001); and had a significantly higher comorbidity [median of EVCI 8 (IQR: 3–15) vs. 5 (IQR: 0–12), p < 0.0001]. ICU admission, mechanical ventilation, sepsis, ventilator-associated pneumonia (VAP), nosocomial condition, and extracorporeal membrane oxygenation (ECMO) were also significantly higher for the AMR bacteria group (Table 2).

Table 2.
Demographic and clinical characteristics, length of stay, and in-hospital mortality in hospitalized adults with and without an AMR bacteria code.
According to the APR-DRG severity classification, 43.73% of AMR patients were categorized as “extreme severity” vs. 24.32% of non-AMR patients (p < 0.0001). The risk of mortality by APR-DRG was also significantly higher in the AMR group, with 30.16% ranked as “extreme risk” vs. 19.45% in the non-AMR population (p < 0.0001). Patients with AMR bacterial infection had more fungal pneumonia co-infection (Table 2).
Crude analysis showed that patients with AMR bacterial pneumonia had a median LOHS of 14 days (IQR: 8–24) vs. 8 days (5–13) in patients without AMR bacterial infection (p < 0.0001). The median of length of ICU stay was also significantly longer: 13 days (IQR: 5–26) versus 8 days (IQR: 3–17) (p < 0.00001). Hospitalized patients with AMR bacterial pneumonia had significantly higher in-hospital mortality: 18.46% vs. 10.05% (p < 0.0001) (Table 2).
The microbial etiology of pneumonia, according to the ICD-10-CM classification, is detailed in Table 3. Streptococcus pneumoniae was the most frequent microorganism, representing 60.15% of all bacterial pneumonias, followed by Pseudomonas aeruginosa (7.10%), Legionella pneumophila (6.40%), Staphylococcus aureus (5.29%), Haemophilus influenzae (4.82%), other Gram-negative aerobic bacteria (4.15%), Klebsiella pneumoniae (3.32%), and Escherichia coli (2.58%).

Table 3.
Microbial etiology of the bacterial pneumonia according to International Classification of Diseases 10th Revision Clinical Modification (ICD-10-CM).
As shown in Table 3, the Gram-positive cocci group and Gram-negative bacilli group represent most AMR bacteria (57.20% and 41.78%, respectively), while the group “other bacteria” barely included AMR bacteria (1.01%). By microbial etiology, Staphylococcus aureus had the most AMR bacteria (43.28% of the isolates), followed by Escherichia coli (15.20%), Klebsiella pneumoniae (14.39%), and Pseudomonas aeruginosa (10.35%).
To estimate the risk factors associated with higher mortality, we calculated the odds ratio (OR) from the univariate logistic regression models (Figure 1). These risk factors were treated as potential confounders (covariates) in the multivariate logistic regression model posterior.
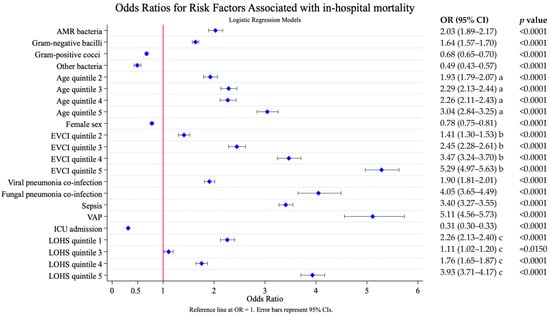
Figure 1.
Risk factors associated with in-hospital mortality. Results of the univariate logistic analysis. AMR, antimicrobial resistance; EVCI, Elixhauser–van Walraven comorbidity index; VAP, ventilator-associated pneumonia; ICU, intensive care unit. LOHS, length of hospital stay. Age (years)—quintile 1: 18–56; quintile 2: 57–68; quintile 3: 69–77; quintile 4: 78–85; and quintile 5: 86–117. EVCI—quintile 1: −18 to 0; quintile 2: 1–4; quintile 3: 5–8; quintile 4: 9–13; and quintile 5: 14–48. Length of stay (days): quintile 1: 0–4; quintile 2: 5–7; quintile 3: 8–9; quintile 4: 10–16; and quintile 5: 17–776. a. Reference age quintile; b. Reference EVCI quintile 1; c. Reference LOHS quintile 2.
Adjusted analysis showed that pneumonia due to AMR bacteria increased in-hospital mortality: OR 1.47 (95% CI 1.36–1.58), p < 0.0001 (Figure 2). Older age and higher comorbidity [OR 4.11 (95% CI 3.80–4.44) EVCI quintile 5 vs. EVCI quintile 1 and OR 3.54 (95% CI 3.31–3.79) quintile 5 vs. quintile 1, respectively], were the factors that most influenced mortality, followed by, sepsis [OR 2.71 (95% CI 2.54–2.84)], fungal pneumonia co-infection [OR 2.69 (95% CI 2.38–3.02)], viral pneumonia co-infection [OR 1.96 (95% CI 1.84–2.08)], and VAP OR 2.04 [(95% CI 1.79–2.33)], all p < 0.0001.
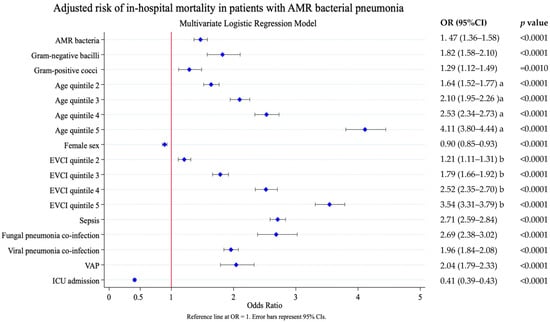
Figure 2.
Risk factors associated with in-hospital mortality. Results of the multivariate logistic analysis adjusted by the following covariates: AMR bacterial label, pneumonia due to Gram-positive cocci, pneumonia due to Gram-negative bacilli, age at admission, sex, comorbidity (EVCI), fungal pneumonia co-infection, viral pneumonia co-infection, sepsis, ventilator-associated pneumonia, and ICU admission. AMR, antimicrobial resistance; EVCI, Elixhauser–van Walraven comorbidity index; VAP, ventilator-associated pneumonia; ICU, intensive care unit. Age at admission (years)—quintile 1: 18–56; quintile 2: 57–68; quintile 3: 69–77; quintile 4: 78–85; and quintile 5: 86–117. EVCI—quintile 1: −18 to 0; quintile 2: 1–4; quintile 3: 5–8; quintile 4: 9–13; and quintile 5: 14–48. Length of stay (days)—quintile 1: 0–4; quintile 2: 5–7; quintile 3: 8–9; quintile 4: 10–16; and quintile 5: 17–776. a. Reference to age quintile 1; b. Reference EVCI quintile 1.
In the adjusted model, pneumonia due to AMR bacteria was also associated with prolonged hospital stay. The probability of a LOHS longer than 8 days (median LOHS of the population) was OR 2.87 (95% CI 2.70–3.05), p < 0.0001. Other significant predictors of prolonged stay included VAP, fungal pneumonia co-infection, comorbidity, sepsis, viral pneumonia, and age (Figure 3).
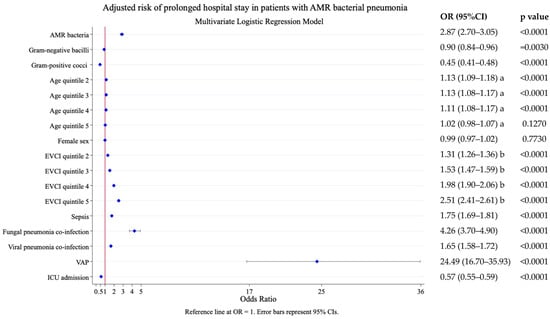
Figure 3.
Risk factors associated with prolonged length of hospital stay (i.e., stays longer than 8 days, the median stay of the population). Results of the multivariate logistic analysis adjusted by the following covariates: AMR bacterial label, age, sex, comorbidity (EVCI), sepsis, fungal pneumonia co-infection, viral pneumonia co-infection, VAP, and ICU admission. AMR, antimicrobial resistance; EVCI, Elixhauser–van Walraven comorbidity index; VAP, ventilator-associated pneumonia; ICU, intensive care unit. Age at admission (years)—quintile 1: 18–56; quintile 2: 57–68; quintile 3: 69–77; quintile 4: 78–85; and quintile 5: 86–117. EVCI—quintile 1: −18 to 0; quintile 2: 1–4; quintile 3: 5–8; quintile 4: 9–13; and quintile 5: 14–48. a. Reference to age quintile 1; b. Reference EVCI quintile 1.
In the subgroup analysis by adjusted model (Table 4), patients with pneumonia due to AMR Gram-positive cocci and AMR Gram-negative bacilli showed increased mortality, with OR of 1.62 (95% CI 1.48–1.78), p < 0.000, and OR 1.41 (95% CI 1.26–1.58), p < 0.0001, respectively.

Table 4.
Results by subgroup (type of bacteria) of the multivariate logistic analysis for hospital mortality adjusted by the following covariates: age at admission, sex, comorbidity (EVCI), fungal pneumonia co-infection, viral pneumonia co-infection, sepsis, ventilator-associated pneumonia, and ICU admission.
Sensitivity Analysis
Multivariate Poisson regression analysis showed an increased incidence rate ratio (IRR) of both mortality and LOHS in hospitalizations with pneumonia due to AMR bacteria compared to the non-AMR group: IRR 1.34 (95% CI 1.26–1.41), p < 0.0001, and IRR 1.46 (95% CI 1.41–1.50), p < 0.0001, respectively (Table 5).

Table 5.
Sensitivity analysis: Poisson regression: analysis adjusted for in-hospital mortality and LOHS by the following covariates: AMR bacterial label, pneumonia due to Gram-positive cocci, pneumonia due to Gram-negative bacilli, age at admission, sex, comorbidity (EVCI), fungal pneumonia co-infection, viral pneumonia co-infection, sepsis, ventilator-associated pneumonia, ICU admission and in-hospital mortality.
Propensity score matching using logistic regression showed an increase in both in-hospital mortality and LOHS associated with AMR bacterial pneumonia compared to hospitalizations without the AMR bacterial pneumonia group (average treatment effect on the treated, ATET) and in the overall population (average treatment effect, ATE) (Table 6). The standardized mean differences before and after matching for ATET in relation to in-hospital mortality are shown in Supplementary Materials File S1.

Table 6.
Sensitivity analysis: Propensity score matching test of AMR bacterial by the following covariates: pneumonia due to Gram-positive cocci group, pneumonia due to Gram-negative bacilli group, age (quintile), sex, Elixhauser–van Walraven comorbidity index (quintile), viral pneumonia co-infection, fungal pneumonia co-infection, sepsis, ventilator-associated pneumonia, and ICU admission.
3. Discussion
Pneumonia due to AMR bacteria increased in-hospital mortality: OR 1.47 (95% CI 1.36–1.58), p < 0.0001. To our knowledge, this study includes one of the largest cohorts to evaluate the association between AMR bacterial infection and in-hospital mortality in cases of bacterial pneumonia requiring hospitalization.
The 5.15% prevalence of AMR bacterial pneumonia in our cohort is consistent with that reported in Spain by Aliberti et al. (7.6%) [19]. Crude mortality was nearly twice as high in AMR cases (18.46% vs. 10.05%, p < 0.0001), in line with previous reports of bacterial pneumonia requiring hospitalization, which mortality rates of 10–15% [14,20,21,22].
Based on these data, adjusted analyses estimated that AMR infection increases the risk of mortality by 37% to 40% [23], which represents a mortality rate between 3.7% to 6.0% higher that of the reference group (without AMR), supported by a propensity score matching test (ATE 3.82% and ATET 4.01%) and a Poisson adjusted regression [IRR 1.34, (95% CI 1.26–1.41)]. Similar effects were observed internationally: Lakbar et al. reported OR 1.39 for ICU- mortality adjusted [24]; while Lambert et al. reported that the risk of death associated with antimicrobial resistance (additional risk of death to that of the infection) in patients with pneumonia admitted to the ICU was 1.2 (95% CI 1.1–1.4), with no p-value reported [25].
Wanda et al. also found an increase in ICU mortality due to AMR bacterial infection: OR 1.65 (95% CI 1.18–2.43), without making a distinction between the type of infection [26]; Hixon et al. calculated a median excess mortality difference of 5.8% (IQR: 2.6–4.0%) in cases of AMR Gram-negative bacterial pneumonia [27]; and Nelson et al. reported mortality attributable to health care-associated infections due AMR organisms of 4.9% for AMR Gram-negative bacteria and 5.9% for MRSA [28].
Patients with AMR-associated pneumonia experienced significantly prolonged LOHS (median 14 vs. 8 days; the adjusted rate of hospitalization days was 46% higher [IRR 1.46 (95% CI 1.41–1.50)], and the adjusted odds of a stay longer than the population median was or 2.87 (95% CI 2.70–3.05), with a mean increase of six days [ATE 6.06 (95% CI 5.44–6.68)]. These findings align with prior studies: Mauldin et al. observed a significant adjusted increase in LOHS in patients with health care-associated infections (including pneumonia) due to resistant pathogens, with a 23.8% increase (p = 0.0003) [29] and Lat et al. also reported that patients with pneumonia caused by AMR bacteria experienced a longer LOHS (23.9 ± 23.8 days vs. 17.9 ± 22.7 days, p = 0.020) [30].
The effect of AMR on patient outcomes can be explained by three main determinants. First, patients infected by AMR bacteria are more likely to receive inadequate empirical antimicrobial therapy. As there is an association between delayed initiation of effective antimicrobial treatment and survival [3,6,14], this hypothesis may explain the increased mortality observed in our study. Second, some resistant bacteria are suspected to have increased virulence. The third determinant relates to host factors and comorbidities. Frail patients are at higher risk of recurrent hospitalizations and antimicrobial exposure, which, in turn, increases their risk of colonization and infection by AMR bacteria [24].
Notably, our data highlight Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli as the predominant AMR pathogens, in line with surveillance data from the WHO and CDC [3,11].
3.1. Strengths
The strengths of this study include its large, nationally representative cohort of patients with bacterial pneumonia and etiological confirmation of the causative agent. Given the low identification rates in pneumonia cases (less than 40%, with bacterial isolates accounting for less than 14%) [16], most published studies include smaller sample sizes.
Furthermore, the use of the largest administrative database in Spain, containing standardized clinical data on hospitalized patients, ensured a comprehensive capture of clinical outcomes and risk factors, allowing for rigorous adjustment for confounders (up to eleven, such as comorbidities, VAP, age, ICU admission) using multivariate models (logistic and Poisson regression) and propensity score analyses.
We applied rigorous exclusion criteria (Figure 4) to the study sample—including the elimination of pneumonia cases without microbiological isolation, the exclusion of polymicrobial infections, and the exclusion of patients with another concomitant bacterial infection in addition to pneumonia—in order to minimize bias when comparing populations with similar clinical characteristics. Although this approach resulted in a substantial reduction in the sample size, it ensured a more homogeneous and comparable population.
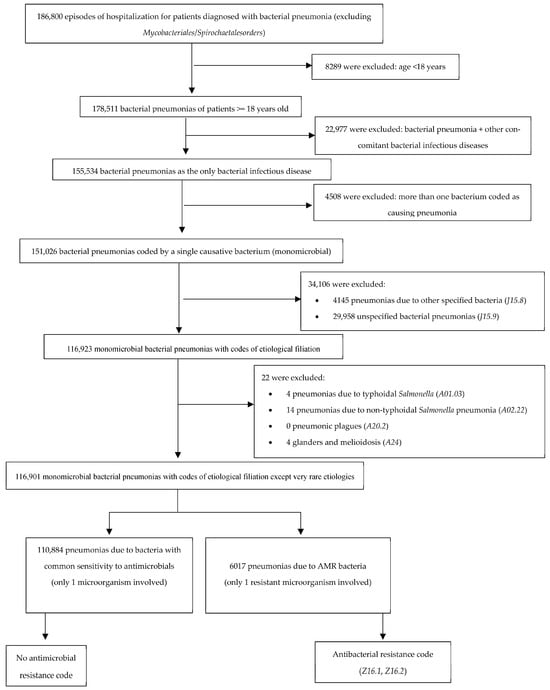
Figure 4.
Selection of study population. Number of potentially eligible participants, algorithm for determining their eligibility, and those confirmed as eligible for the study. AMR, antimicrobial resistance.
To further strengthen the accuracy of our findings and avoid underestimating the prevalence of antimicrobial resistance (AMR), we restricted our analysis to data from 2017 onwards. The transition from ICD-9-CM to ICD-10-CM in 2016 introduced substantial changes in diagnostic coding that could have compromised the consistency and reliability of AMR classification. Given that 2016 represented a transitional period, with a higher likelihood of coding inconsistencies and errors, we excluded this year from the analysis to improve data quality and comparability over time.
Finally, the observed effect, despite an unbalanced population (1 AMR case vs. 18 non-AMR cases), reinforces the magnitude of the impact of AMR bacteria.
3.2. Limitations
Coding biases and under-reporting (ICD-10-CM): There is a potential risk of misclassification that may be due to ICD-10-CM coding errors or under-reporting. Nevertheless, the Spanish RAE-CMBD has been validated for research in previous studies, and such errors are partially mitigated by the large dataset size [31,32,33]. Furthermore, the volume of data analyzed contributes to minimizing the impact of occasional misclassification or under-reporting.
Unmeasured confounders: Key variables such as antibiotic timing or severity scores (e.g., CURB-65) were not available, which could inflate the apparent impact of AMR. However, sensitivity analyses (e.g., Poisson regression) confirmed robust effect sizes, reducing the likelihood of significant bias.
Selection bias: The exclusion of polymicrobial infections may have led to an underestimation of the true burden of AMR. Nonetheless, this criterion ensured a more homogeneous and clinically comparable cohort.
Definition of AMR and appropriateness of therapy: The definition of AMR relied on each attending physician, with variability in sampling techniques. Moreover, the database did not provide information on the adequacy or timeliness of empirical treatment, which is a major determinant of mortality. Future studies should integrate microbiological data with administrative records to refine resistance patterns and assess therapeutic adequacy.
Competing risk bias in length of stay analyses: The analysis of length of hospital stay (LOHS) using a Poisson regression model may be influenced by in-hospital mortality since death shortens the observation period and can bias estimates. However, it should be noted that several influential and widely cited studies have adopted similar modeling strategies, given that Poisson regression offers a simple and interpretable framework for estimating incidence rate ratios (IRR) [34].
Finally, selection bias is inherent in the retrospective design. To minimize this, our group previously validated the EVCI as the best predictor of in-hospital mortality in our population, to eliminate the confounding effect caused by the patients’ different levels of comorbidity [35]. In addition, we applied strict exclusion criteria (e.g., polymicrobial bacterial pneumonia, other bacterial infections) to obtain a more reproducible and comparable cohort.
3.3. Conclusions
Consistent with previous evidence, our findings demonstrate that antimicrobial-resistant (AMR) is associated with longer hospital stays and an up to 50% higher risk of mortality, emphasizing the urgent need for action.
Despite the implementation of control policies in place over the past decade, these alarming data highlight the persistence of the problem and compel policymakers to strengthen AMR surveillance and ensure adequate resource allocation in order to mitigate its clinical and economic impact. Clinicians, in turn, must reinforce antimicrobial stewardship to limit the emergence of resistant infections and incorporate rapid diagnostic tools to minimize the impact of AMR on patient outcomes.
3.4. Key Recommendations and Future Directions
Future research should investigate region-specific AMR trends to guide local antimicrobial stewardship programs, explore cost-effectiveness of rapid diagnostics for reducing AMR-related mortality, and assess long-term outcomes (e.g., post-discharge survival).
The presence of AMR bacteria complicates the clinical management of infections [11]. Our findings advocate for multifaceted interventions:
Optimization of antimicrobial use: Personalized empirical therapy guided by local resistance patterns.
Improved diagnosis: Rapid molecular testing to enable the early identification of AMR pathogens and the timely initiation of targeted therapy.
Strengthening antimicrobial stewardship programs: This will mitigate the natural progression of resistance.
Investment in nosocomial infection control measures: This is particularly important for high-risk pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Klebsiella pneumoniae.
In Spain, the implementation of the “Zero Resistance” project recommendations has been associated with a significant reduction in infections caused by multidrug-resistant bacteria acquired during ICU stays [IRR 0.67 (95% CI 0.57–0.80)] [34].
Future research should investigate region-specific AMR trends to inform local antimicrobial stewardship programs, evaluate the cost-effectiveness of rapid diagnostics in reducing AMR-related mortality, and assess long-term patient outcomes, including post-discharge survival.
4. Materials and Methods
4.1. Study Design
A retrospective cohort study was conducted on adult patients hospitalized with bacterial pneumonia and discharged from hospitals within the Spanish National Health System between 2017 and 2022, using data from the RAE-CMBD administrative database. Hospitalization episodes with antimicrobial resistance bacteria—identified through ICD-10-CM resistance codes (Z16.1, Z16.2)—were compared with hospitalization episodes without these codes.
4.2. Patients
Adult patients admitted to public or private hospitals with a diagnosis of bacterial pneumonia were eligible. Patients were followed until discharge.
Key exclusion criteria were as follows:
- All patients under 18 years of age.
- All cases of pneumonia due to bacteria from the Mycobacteriales and Spirochaetales orders.
- All patients with bacterial pneumonia and any other concurrent bacterial infectious disease.
- All cases coded with bacterial pneumonia attributed to more than one causative agent.
- All cases coded with a diagnosis of bacterial pneumonia in which the causative agent was not specified or not identified.
- Patients codified with diagnosis codes for very unusual bacterial pneumonias, e.g., pneumonia due to typhoidal salmonella, pneumonia due to non-typhoidal Salmonella pneumonia, pneumonic plague, glanders, and melioidosis.
Supplementary Materials Files S2 and S3 detail the ICD-10-CM exclusion codes applied to the study cohort to select eligible patients.
4.3. Procedures
The RAE-CMBD is the largest administrative database maintained by the Spanish ministry of health, containing the standardized clinical data of hospitalized patients. This registry contains, for each hospitalization, the coding of the main diagnosis (cause of the hospital admission) and up to 19 secondary diagnoses (other conditions that influence the patient’s clinical course), coded according to the ICD-10-CM, Spanish version [14].
The following variables were collected from the RAE-CMBD:
- Administrative variables: Year of the registry, center, and anonymized patient ID number.
- Sociodemographic variables: Date of birth, sex (male/female/not specified), and country of birth. Sex data are collected in RAE-CMBD as recorded in the patient’s medical history during admission according to the patient’s self-reported information.
- Clinical variables: Date of hospital admission, date of discharge, type of discharge (death/home/transfer to another hospital/voluntary discharge/transfer to social-health center), intensive care unit (ICU) admission (yes/no), length of ICU stay (in days), main diagnosis (ICD-10-CM), secondary diagnoses (ICD-10-CM), procedures 1 to 20 (ICD-10-CM), all patient-refined diagnosis-related groups (APR-DRG) severity level (null/minor/moderate/major/extreme), and APR-DRG mortality risk level (low/medium/high/extreme).
4.4. Outcomes
Mortality was defined as the number of hospitalization episodes in which death was the reason for hospital discharge. LOHS was defined as the number of days between the discharge date and the hospital admission date.
The outcomes were evaluated in both the AMR group and the non-AMR based on the final cohort of eligible hospitalization episodes, as detailed in the following flow chart (Figure 4).
4.5. Statistical Analysis
First, a univariate regression model studied the risk factors for in-hospital mortality in patients hospitalized with bacterial pneumonia. Comorbidity at each hospitalization episode was assessed using the Elixhauser–van Walraven Comorbidity Index (EVCI). Supplementary Materials File S4 shows the calculation of the EVCI. Supplementary Materials File S5 details the ICD-10-CM codes used to study the risk factors. Subsequently, multivariate logistic regression models were built, as primary analyses, including the AMR bacteria label, with appropriate covariates based on the risk factors.
We used a phylogenetic structural classification (Gram-negative and Gram-positive) rather than an anatomical one (respiratory vs. enteric microorganisms), which would have been more appropriate for pneumonia etiology since the primary goal was to assess the impact of microbial resistance on health outcomes, regardless of the infection source.
To apply logistic regression, the EVCI score, age at admission (years), and LOHS (days) were categorized into quintiles based on their distributions (stratum 1: quintile 1; strata 2: quintile 2; strata 3: quintile 3; strata 4: quintile 4; and strata 5: quintile 5). This approach enables a clearer comparison of the influence of each stratum (quintile) on the outcome variable (in-hospital mortality) as this relationship is non-linear (see Supplementary Materials File S6). Similar methods have been reported by other authors to yield more reliable results [31,35]. The stratum associated with lower mortality was chosen as the reference category: quintile 1 in age and EVCI and quintile 2 in LOHS. In logistic regression, the dependent effect of LOHS was measured as the probability of a prolonged hospitalization, using the population median (8 days) as the cut-off point.
4.6. Sensitivity Analysis
As a secondary method, we tested the hypotheses using the same covariates as in the original analysis using a modified Poisson multivariate regression model with robust standard errors, which provided the incidence rate ratio (IRR).
Also, propensity score matching tests without replacement (matching ratio: 1:1 exact; caliper of 0.1) were performed for both the entire population (average treatment effect (ATE)) and for the AMR bacterial group (average treatment effect on the treated (ATET)).
Statistical analysis was performed using Stata/MP v17.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14101006/s1, Supplementary Materials File S1. Balance Diagnostics Before and After 1:1 Propensity Score Matching (ATET in-hospital mortality. Supplementary Materials File S2. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM-CM) codes for definition of bacterial pneumonia. Supplementary Materials File S3. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM-CM) codes for definition other bacterial infectious diseases. Supplementary Materials File S4. Calculation of the Elixhauser–van Walraven comorbidity index according to Elixhauser comorbidities using the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) codes. Supplementary Materials File S5. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM-CM) codes for risk factors definition. Supplementary Materials File S6. Marginal plots of predicted probability of in-hospital mortality, by strata (quintile) based on univariate logistic regression analysis.
Author Contributions
I.O.-M., conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, and visualization; M.P.-E., conceptualization, methodology, writing—review and editing, supervision, and project Administration; F.J.C.-G., conceptualization, writing—review and editing, and supervision; S.L.-M., conceptualization, resources, writing—review and editing, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Institutional Review Board Statement
The Alcorcon Foundation University Hospital Research Ethics Committee (REC) approved the study protocol (23/10, 12 September 2023).
Informed Consent Statement
The Alcorcon Foundation University Hospital Research Ethics Committee (REC) granted an exemption for informed consent due to the use of anonymized data.
Data Availability Statement
The database used (RAE-CMBD) was provided by the Spanish Ministry of Health. Access to these data is public, and any author may request access to the anonymized data following the instructions of the Spanish government through the following website: https://www.sanidad.gob.es/estadEstudios/estadisticas/estadisticas/estMinisterio/SolicitudCMBD.htm.
Acknowledgments
The authors thank the Spanish Ministry of Health for providing the records of the RAE-CMBD. During the preparation of this work, the authors used DeepSeek-V3 to summarize certain individual texts previously provided by the authors, as well as to revise them to improve the final manuscript. On no occasion was the AI provided with the full draft or asked to create content. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Directorate-General for Health and Food Safety. A European One Health Action Plan Against Antimicrobial Resistance (AMR). 2017. Available online: https://health.ec.europa.eu/publications/european-one-health-action-plan-against-antimicrobial-resistance-amr_en (accessed on 1 August 2025).
- Alkofide, H.; Alhammad, A.M.; Alruwaili, A.; Aldemerdash, A.; Almangour, T.A.; Alsuwayegh, A.; Almoqbel, D.; Albati, A.; Alsaud, A.; Enani, M. Multidrug-Resistant and Extensively Drug-Resistant Enterobacteriaceae: Prevalence, Treatments, and Outcomes—A Retrospective Cohort Study. Infect. Drug Resist. 2020, 13, 4653–4662. [Google Scholar]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet Lond. Engl. 2022, 399, 629–655. [Google Scholar]
- Federal Task Force on Combating Antibiotic-Resistant Bacteria. National Action Plan for Combating Antibiotic-Resistant Bacteria, 2020–2025 [Internet]. Office of the Assistant Secretary for Planning & Evaluation: U.S. Department of Health & Human Services, 2020. Available online: http://aspe.hhs.gov/reports/national-action-plan-combating-antibiotic-resistant-bacteria-2020-2025 (accessed on 1 August 2025).
- Spain Remains on the Front Line Against the Silent Pandemic: Antibiotic Resistance. Spanish Agency for Medicines and Health Products: Ministry of Health. 2021. Available online: https://www.aemps.gob.es/informa/notasinformativas/laaemps/espana-mantiene-el-pulso-frente-a-la-pandemia-silenciosa-la-resistencia-a-los-antibioticos/ (accessed on 1 August 2025).
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic resistance—The need for global solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Barrasa-Villar, J.I.; Aibar-Remón, C.; Prieto-Andrés, P.; Mareca-Doñate, R.; Moliner-Lahoz, J. Impact on Morbidity, Mortality, and Length of Stay of Hospital-Acquired Infections by Resistant Microorganisms. Clin. Infect. Dis. 2017, 65, 644–652. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan on Antimicrobial Resistance. 2015. Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 1 August 2025).
- World Health Organization. One Health. 2015. Available online: https://www.who.int/health-topics/one-health (accessed on 1 August 2025).
- One Health Joint Plan of Action (2022–2026): Working Together for the Health of Humans, Animals, Plants and the Environment. Available online: https://www.who.int/publications/i/item/9789240059139 (accessed on 1 August 2025).
- World Health Organization. WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 1 August 2025).
- Bondarchuk, C.P.; Grobman, B.; Mansur, A.; Lu, C.Y. National trends in pneumonia-related mortality in the United States, 1999–2019. Infect. Dis. 2024, 57, 56–65. [Google Scholar]
- Underlying Cause of Death, 2018–2023, Single Race Results Form. Available online: https://wonder.cdc.gov/controller/datarequest/D158;jsessionid=3A8160D25E64B52113AD31741CFD#Options (accessed on 1 August 2025).
- Lanks, C.W.; Musani, A.I.; Hsia, D.W. Community-acquired Pneumonia and Hospital-acquired Pneumonia. Med. Clin. N. Am. 2019, 103, 487–501. [Google Scholar]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2022. Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 1 August 2025).
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Royal Decree 69/2015, of February 6, Regulating the Registry of Specialized Healthcare Activities (RAE-CMBD). Ministry of Health, Social Services and Equality. Spain Official State Bulletin. 2015. Available online: https://www.boe.es/eli/es/rd/2015/02/06/69 (accessed on 1 August 2025).
- Statistical Portal. Interactive Consultation of the National Health System. Management Intelligence Area: Spain Ministry of Health. Available online: https://pestadistico.inteligenciadegestion.sanidad.gob.es/publicoSNS/N/rae-cmbd/rae-cmbd/informacion-general (accessed on 1 August 2025).
- Aliberti, S.; Cilloniz, C.; Chalmers, J.D.; Zanaboni, A.M.; Cosentini, R.; Tarsia, P.; Pesci, A.; Blasi, F.; Torres, A. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: A European perspective. Thorax 2013, 68, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Sato, S.; Maruyama, E.; Ohashi, T.; Ogawa, M.; Hashimoto, N.; Imaizumi, K.; Sato, T.; Hasegawa, Y. Health-Care-Associated Pneumonia Among Hospitalized Patients in a Japanese Community Hospital. Chest 2009, 135, 633–640. [Google Scholar]
- Carratalà, J.; Mykietiuk, A.; Fernández-Sabé, N.; Suárez, C.; Dorca, J.; Verdaguer, R.; Manresa, F.; Gudiol, F. Health Care–Associated Pneumonia Requiring Hospital Admission: Epidemiology, Antibiotic Therapy, and Clinical Outcomes. Arch. Intern. Med. 2007, 167, 1393–1399. [Google Scholar] [PubMed]
- Muthumbi, E.; Lowe, B.S.; Muyodi, C.; Getambu, E.; Gleeson, F.; Scott, J.A.G. Risk factors for community-acquired pneumonia among adults in Kenya: A case—Control study. Pneumonia 2017, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.L. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 2014, 348, f7450. [Google Scholar] [CrossRef] [PubMed]
- Lakbar, I.; Medam, S.; Ronflé, R.; Cassir, N.; Delamarre, L.; Hammad, E.; Lopez, A.; Lepape, A.; Machut, A.; Boucekine, M.; et al. Association between mortality and highly antimicrobial-resistant bacteria in intensive care unit-acquired pneumonia. Sci. Rep. 2021, 11, 16497. [Google Scholar] [CrossRef]
- Lambert, M.-L.; Suetens, C.; Savey, A.; Palomar, M.; Hiesmayr, M.; Morales, I.; Agodi, A.; Frank, U.; Mertens, K.; Schumacher, M.; et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: A cohort study. Lancet Infect. Dis. 2011, 11, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Cornistein, W.; Balasini, C.; Nuccetelli, Y.; Rodriguez, V.M.; Cudmani, N.; Roca, M.V.; Sadino, G.; Brizuela, M.; Fernández, A.; González, S.; et al. Prevalence and mortality associated with multidrug-resistant infections in adult intensive care units in Argentina (PREV-AR). Antimicrob. Agents Chemother. 2025, 69, e0142624. [Google Scholar] [CrossRef]
- Hixon, A.M.; Micek, S.; Fraser, V.J.; Kollef, M.; Guillamet, M.C.V. Impact of Gram-Negative Bacilli Resistance Rates on Risk of Death in Septic Shock and Pneumonia. Open Forum Infect. Dis. 2024, 11, ofae219. [Google Scholar] [CrossRef]
- Nelson, R.E.; Slayton, R.B.; Stevens, V.W.; Jones, M.M.; Khader, K.; Rubin, M.A.; Jernigan, J.A.; Samore, M.H. Attributable Mortality of Healthcare-Associated Infections Due to Multidrug-Resistant Gram-Negative Bacteria and Methicillin-Resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 2017, 38, 848–856. [Google Scholar] [CrossRef]
- Mauldin, P.D.; Salgado, C.D.; Hansen, I.S.; Durup, D.T.; Bosso, J.A. Attributable Hospital Cost and Length of Stay Associated with Health Care-Associated Infections Caused by Antibiotic-Resistant Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2010, 54, 109–115. [Google Scholar] [CrossRef]
- Lat, I.; Daley, M.J.; Shewale, A.; Pangrazzi, M.H.; Hammond, D.; Olsen, K.M.; the DEFINE Study Group and the Discovery Research Network. A Multicenter, Prospective, Observational Study to Determine Predictive Factors for Multidrug-Resistant Pneumonia in Critically Ill Adults: The DEFINE Study. Pharmacotherapy 2019, 39, 253–260. [Google Scholar] [CrossRef]
- Pérez-Encinas, M.; Lorenzo-Martínez, S.; Losa-García, J.E.; Walter, S.; Tejedor-Alonso, M.A. Impact of Penicillin Allergy Label on Length of Stay and Mortality in Hospitalized Patients through a Clinical Administrative National Dataset. Int. Arch. Allergy Immunol. 2021, 183, 498–506. [Google Scholar] [CrossRef]
- Oterino-Moreira, I.; Sanz-Márquez, S.; Zhan-Zhou, E.; Martínez-Simón, J.J.; Morales-Catalán, M.C.; Lorenzo-Martínez, S.; Pérez-Encinas, M. Incidencia de reacciones adversas a medicamentos en pacientes hospitalizados por COVID-19 a través del conjunto mínimo básico de datos. Rev. OFIL 2023, 33, 161–168. [Google Scholar]
- Oterino-Moreira, I.; Lorenzo-Martínez, S.; Pérez-Encinas, M. Use big data as a source of data for health care quality indicators construction. Med. Clin. 2023, 160, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Lerma, F.; Catalán-González, M.; Álvarez, J.; Sánchez-García, M.; Palomar-Martínez, M.; Fernández-Moreno, I.; Garnacho-Montero, J.; Barcenilla-Gaite, F.; García, R.; Aranaz-Andrés, J.; et al. Impact of the “Zero Resistance” program on acquisition of multidrug-resistant bacteria in patients admitted to Intensive Care Units in Spain. A prospective, intervention, multimodal, multicenter study. Med. Intensiv. Engl. Ed. 2023, 47, 193–202. [Google Scholar] [CrossRef]
- Oterino-Moreira, I.; Lorenzo-Martínez, S.; López-Delgado, Á.; Pérez-Encinas, M. Comparison of Three Comorbidity Measures for Predicting In-Hospital Death through a Clinical Administrative Nacional Database. Int. J. Environ. Res. Public Health 2022, 19, 11262. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).