Vascular Access Device Infections: Current Management Practices and the Role of Multidisciplinary Teams at a Large Hospital in Northern Italy
Abstract
:1. Introduction
2. Results
2.1. Clinical and Microbiological Characteristics of CABSI and CRBSI Episodes
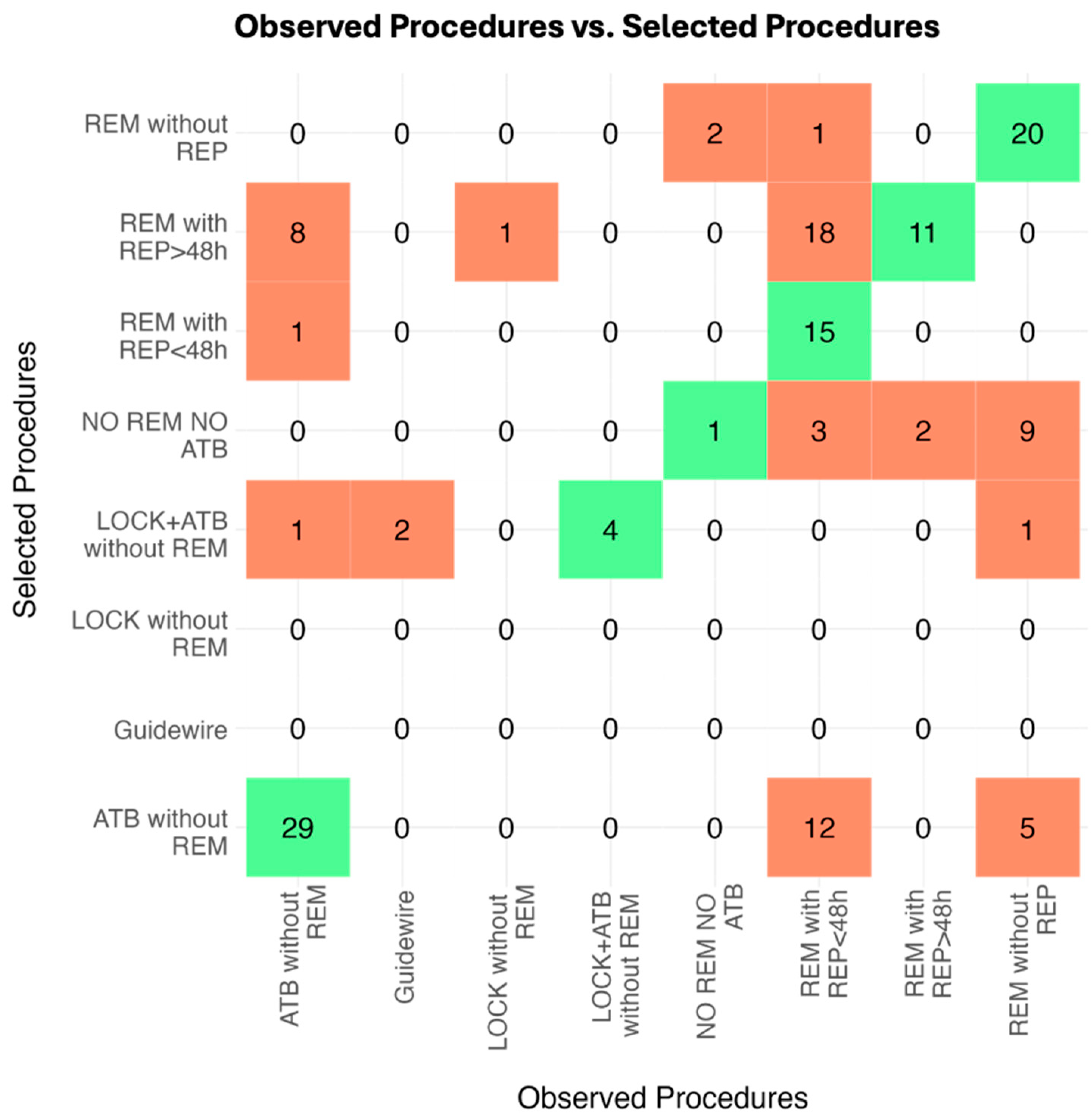
2.2. Observed Real-Life Procedures and VAT Agreement
3. Discussion
4. Materials and Methods
4.1. Study Design and Clinical Setting
4.2. Study Population
4.3. Ethics
4.4. Definitions
4.5. Available Data
4.6. Study Procedure
4.7. Potential Choices
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaves, F.; Garnacho-Montero, J.; del Pozo, J.; Bouza, E.; Capdevila, J.; de Cueto, M.; Domínguez, M.; Esteban, J.; Fernández-Hidalgo, N.; Sampedro, M.F.; et al. Diagnosis and treatment of catheter-related bloodstream infection: Clinical guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med. Intensiv. 2018, 42, 5–36. [Google Scholar] [CrossRef]
- Borgonovo, F.; Quici, M.; Gidaro, A.; Giustivi, D.; Cattaneo, D.; Gervasoni, C.; Calloni, M.; Martini, E.; La Cava, L.; Antinori, S.; et al. Physicochemical Characteristics of Antimicrobials and Practical Recommendations for Intravenous Administration: A Systematic Review. Antibiotics 2023, 12, 1338. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Osborne, S.; Rickard, C.M.; Marsh, N. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst. Rev. 2019, 2019, CD007798. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chang, Q.; Zhou, Y.; Liao, L. Risk factors of central catheter bloodstream infections in intensive care units: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0296723. [Google Scholar] [CrossRef] [PubMed]
- Catho, G.; Fortchantre, L.; Teixeira, D.; Galas-Haddad, M.; Boroli, F.; Chraïti, M.-N.; Abbas, M.; Harbarth, S.; Buetti, N.; Swissnoso Group; et al. Surveillance of catheter-associated bloodstream infections: Development and validation of a fully automated algorithm. Antimicrob. Resist. Infect. Control. 2024, 13, 38. [Google Scholar] [CrossRef]
- Leistner, R.; Hirsemann, E.; Bloch, A.; Gastmeier, P.; Geffers, C. Costs and prolonged length of stay of central venous catheter-associated bloodstream infections (CVC BSI): A matched prospective cohort study. Infection 2014, 42, 31–36. [Google Scholar] [CrossRef]
- DiGIOVINE, B.; Chenoweth, C.; Watts, C.; Higgins, M. The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am. J. Respir. Crit. Care Med. 1999, 160, 976–981. [Google Scholar] [CrossRef]
- Bisanti, A.; Giammatteo, V.; Bello, G.; Giannarelli, D.; Montini, L.; Tanzarella, E.S.; Carelli, S.; Bongiovanni, F.; D’Inzeo, T.; Fiori, B.; et al. Usefulness of differential time to positivity between catheter and peripheral blood cultures for diagnosing catheter-related bloodstream infection: Data analysis from routine clinical practice in the intensive care unit. J. Crit. Care 2023, 75, 154259. [Google Scholar] [CrossRef]
- Scimò, M.; Vallecorsa, I.; Cini, A.; Cabelguenne, D.; Piriou, V. Vascular access unit: Six-years experience report in France. J. Vasc. Access 2022, 24, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Buetti, N.; Marschall, J.; Drees, M.; Fakih, M.G.; Hadaway, L.; Maragakis, L.L.; Monsees, E.; Novosad, S.; O’grady, N.P.; Rupp, M.E.; et al. Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update. Infect. Control. Hosp. Epidemiol. 2022, 43, 553–569. [Google Scholar] [CrossRef]
- Brescia, F.; Pittiruti, M.; Spencer, T.R.; Dawson, R.B. The SIP protocol update: Eight strategies, incorporating Rapid Peripheral Vein Assessment (RaPeVA), to minimize complications associated with peripherally inserted central catheter insertion. J. Vasc. Access 2024, 25, 5–13. [Google Scholar] [CrossRef]
- Garcia, R.; Septimus, E.J.; LeDonne, J.; Sturm, L.K.; Moureau, N.; DeVries, M.; DeBaun, B. Prevention of Vascular Access Device–Associated Hospital-Onset Bacteremia and Fungemia: A Review of Emerging Perspectives and Synthesis of Technical Aspects. Clin. Infect. Dis. 2024, ciae245. [Google Scholar] [CrossRef] [PubMed]
- Rosich-Soteras, A.; Bonilla-Serrano, C.; Llauradó-González, M.; Fernández-Bombín, A.; Triviño-López, J.A.; Barceló-Querol, L.; Heredia-Aguilar, L.; Frías-Martín, M.C.; Valverde-Bosch, M.; Corominas-Bosch, M.L.; et al. Implementation of a vascular access team and an intravenous therapy programme: A first-year activity analysis. J. Vasc. Access 2024, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A. Short-term Peripheral Venous Catheter–Related Bloodstream Infections: A Systematic Review. Clin. Infect. Dis. 2017, 65, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Marsh, N.; Larsen, E.N.; Ullman, A.J.; Mihala, G.; Cooke, M.; Chopra, V.; Ray-Barruel, G.; Rickard, C.M. Peripheral intravenous catheter infection and failure: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2024, 151, 104673. [Google Scholar] [CrossRef] [PubMed]
- Teja, B.; Bosch, N.A.; Diep, C.; Pereira, T.V.; Mauricio, P.; Sklar, M.C.; Sankar, A.; Wijeysundera, H.C.; Saskin, R.; Walkey, A.; et al. Complication Rates of Central Venous Catheters: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2024, 184, 474–482. [Google Scholar] [CrossRef]
- Healthcare-Associated Infections Acquired in Intensive Care Units—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-infections-acquired-intensive-care-units-annual (accessed on 12 November 2024).
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.A.; Sherertz, R.J.; Warren, D.K. Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Weiner, L.M.; Webb, A.K.; Limbago, B.; Dudeck, M.A.; Patel, J.; Kallen, A.J.; Edwards, J.R.; Sievert, D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef] [PubMed]
- Reigadas, E.; Rodríguez-Creixems, M.; Guembe, M.; Sanchez-Carrillo, C.; Martín-Rabadán, P.; Bouza, E. Catheter-related bloodstream infection caused by Enterococcus spp. Clin. Microbiol. Infect. 2013, 19, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, A.S.; Edwards, J.R.; Ricks, P.M.; Sievert, D.M.; Fridkin, S.K.; Gould, C.V. Device-associated infection rates, device utilization, and antimicrobial resistance in long-term acute care hospitals reporting to the National Healthcare Safety Network, 2010. Infect. Control. Hosp. Epidemiol. 2012, 33, 993–1000. [Google Scholar] [CrossRef]
- Phua, A.I.-H.; Hon, K.Y.; Holt, A.; O’Callaghan, M.; Bihari, S. Candida catheter-related bloodstream infection in patients on home parenteral nutrition—Rates, risk factors, outcomes, and management. Clin. Nutr. ESPEN 2019, 31, 1–9. [Google Scholar] [CrossRef]
- Oladapo-Shittu, O.; Cosgrove, S.E.; Rock, C.; Hsu, Y.-J.; Klein, E.; Harris, A.D.; Mejia-Chew, C.; Saunders, H.; Ching, P.R.; Gadala, A.; et al. Characterizing Patients Presenting on Hospital Admission with Central Line–Associated Bloodstream Infections: A Multicenter Study. Clin. Infect. Dis. 2024, 78, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Delgado, V.; Delgado, V.; Marsan, N.A.; Marsan, N.A.; de Waha, S.; de Waha, S.; Bonaros, N.; Bonaros, N.; Brida, M.; Brida, M.; et al. 2023 ESC Guidelines for the management of endocarditis. Eur. Heart J. 2023, 44, 3948–4042. [Google Scholar] [CrossRef]
- Chen, X.-X.; Lo, Y.-C.; Su, L.-H.; Chang, C.-L. Investigation of the case numbers of catheter-related bloodstream infection overestimated by the central line-associated bloodstream infection surveillance definition. J. Microbiol. Immunol. Infect. 2015, 48, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Catton, J.A.; Dobbins, B.M.; Kite, P.; Wood, J.M.; Eastwood, K.; Sugden, S.; Sandoe, J.A.T.; Burke, D.; McMahon, M.J.; Wilcox, M.H. In situ diagnosis of intravascular catheter-related bloodstream infection: A comparison of quantitative culture, differential time to positivity, and endoluminal brushing. Crit. Care Med. 2005, 33, 787–791. [Google Scholar] [CrossRef]
- Muñoz, P.; Guembe, M.; Pérez-Granda, M.J.; del Pozo, J.L.; López-Cortés, L.E.; Pittiruti, M.; Martín-Delgado, M.C.; Bouza, E. Vascular catheter-related infections: An endemic disease in healthcare institutions. An opinion paper of the Spanish Society of Cardiovascular Infections (SEICAV). Rev. Espanola De Quimioter. 2024, 37, 387–400. [Google Scholar] [CrossRef]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the prevention of intravascular catheter-related infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- Bouzidi, H.; Emirian, A.; Marty, A.; Chachaty, E.; Laplanche, A.; Gachot, B.; Blot, F. Differential time to positivity of central and peripheral blood cultures is inaccurate for the diagnosis of Staphylococcus aureus long-term catheter-related sepsis. J. Hosp. Infect. 2018, 99, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Kaasch, A.J.; Rieg, S.; Hellmich, M.; Kern, W.V.; Seifert, H. Differential time to positivity is not predictive for central line-related Staphylococcus aureus bloodstream infection in routine clinical care. J. Infect. 2014, 68, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Orihuela-Martín, J.; Rodríguez-Núñez, O.; Morata, L.; Cardozo, C.; Puerta-Alcalde, P.; Hernández-Meneses, M.; Ambrosioni, J.; Linares, L.; Bodro, M.; Guerrero-León, M.d.L.A.; et al. Performance of differential time to positivity as a routine diagnostic test for catheter-related bloodstream infections: A single-centre experience. Clin. Microbiol. Infect. 2020, 26, 383.e1–383.e7. [Google Scholar] [CrossRef]
- Nickel, B.A.-C.; Gorski, L.M.; Kleidon, T.P.; Kyes, A.M.; DeVries, M.M.; Keogh, S.P.; Meyer, B.P.; Sarver, M.J.M.; Crickman, R.D.; Ong, J.; et al. Infusion Therapy Standards of Practice, 9th Edition. J. Infus. Nurs. 2024, 47, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
| Total (N = 135) | CABSI (N = 74) | CRBSI (N = 61) | p Value * | |
|---|---|---|---|---|
| Age (IQR) | 60 (49, 67) | 62 (53, 67) | 57 (46, 66) | 0.129 |
| Female (%) | 75 (55.6%) | 41 (55.4%) | 34 (55.7%) | 1.000 |
| CCI (IQR) | 6 (5, 8) | 6 (5, 8) | 7 (5, 8) | 0.214 |
| Comorbidity count (IQR) | 4 (3, 5) | 4 (3, 5) | 4 (3, 5) | 0.641 |
| Cancer (%) | 27 (20.0%) | 8 (10.8%) | 19 (31.1%) | 0.005 |
| Hematologic malignacies (%) | 10 (7.4%) | 8 (10.8%) | 2 (3.3%) | 0.113 |
| Diabetes (%) | 34 (25.2%) | 16 (21.6%) | 18 (29.5%) | 0.324 |
| CVD (%) | 54 (40.0%) | 27 (36.5%) | 27 (44.3%) | 0.382 |
| COPD (%) | 29 (21.5%) | 15 (20.3%) | 14 (23.0%) | 0.834 |
| Neurological disease (%) | 57 (42.2%) | 29 (39.2%) | 28 (45.9%) | 0.486 |
| Mental disorder (%) | 15 (11.1%) | 6 (8.1%) | 9 (14.8%) | 0.275 |
| Cirrhosis (%) | 7 (5.2%) | 4 (5.4%) | 3 (4.9%) | 1.000 |
| CKD (%) | 13 (9.6%) | 8 (10.8%) | 5 (8.2%) | 0.772 |
| Hypertension (%) | 91 (67.4%) | 54 (73.0%) | 37 (60.7%) | 0.143 |
| Obesity (%) | 15 (11.1%) | 13 (17.6%) | 2 (3.3%) | 0.011 |
| Immunosuppression (%) | 121 (89.6%) | 72 (97.3%) | 49 (80.3%) | 0.001 |
| Previous CABSI/CRBSI (%) | 8 (5.9%) | 5 (6.8%) | 3 (4.9%) | 0.729 |
| Albumin g/L (IQR) | 16 (13, 21) | 16 (13, 20) | 17 (13, 22) | 0.442 |
| Missing | 8 | 5 | 3 | |
| LOS (day) (IQR) | 18 (12, 29) | 16 (12, 28) | 21 (12, 29) | 0.183 |
| Missing | 25 | 14 | 11 | |
| Transferred from ICU (%) | 8 (5.9%) | 3 (4.1%) | 5 (8.2%) | 0.467 |
| Death (%) | 34 (25.2%) | 17 (23.0%) | 17 (27.9%) | 0.554 |
| Transferred to ICU (%) | 7 (5.2%) | 4 (5.4%) | 3 (4.9%) | 1.000 |
| Total (N = 146) | CABSI (N = 84) | CRBSI (N = 62) | p Value * | |
|---|---|---|---|---|
| Isolated pathogens | ||||
| Staphylococcus aureus | 6 (4.1%) | 1 (1.2%) | 5 (8.1%) | 0.083 |
| CoNS | 76 (52.1%) | 47 (56.0%) | 29 (46.8%) | 0.316 |
| Streptococcus spp. | 4 (2.7%) | 3 (3.6%) | 1 (1.6%) | 0.637 |
| Enterococcus spp. | 30 (20.5%) | 16 (19.0%) | 14 (22.6%) | 0.680 |
| Enterobacteriaceae spp. | 17 (11.6%) | 13 (15.5%) | 4 (6.5%) | 0.120 |
| Pseudomonas aeruginosa | 2 (1.4%) | 0 (0.0%) | 2 (3.2%) | 0.179 |
| Bacillus spp. | 3 (2.1%) | 2 (2.4%) | 1 (1.6%) | 1.000 |
| Candida spp. | 18 (12.3%) | 1 (1.2%) | 17 (27.4%) | <0.001 |
| Polymicrobial | 19 (13.0%) | 8 (9.5%) | 11 (17.7%) | 0.213 |
| VAD type | <0.001 | |||
| Mid-thigh | 45 (30.8%) | 34 (40.5%) | 11 (17.7%) | |
| Midline | 36 (24.7%) | 25 (29.8%) | 11 (17.7%) | |
| Other CVADs | 15 (10.3%) | 8 (9.5%) | 7 (11.3%) | |
| PICC | 50 (34.2%) | 17 (20.2%) | 33 (53.2%) |
| Total (N = 146) | CABSI (N = 84) | CRBSI (N = 62) | p Value * | |
|---|---|---|---|---|
| REM without REP | 35 (24.0%) | 22 (26.2%) | 13 (21.0%) | 0.558 |
| REM with REP > 48 h | 13 (8.9%) | 5 (6.0%) | 8 (12.9%) | 0.156 |
| REM with REP < 48 h | 49 (33.6%) | 25 (29.8%) | 24 (38.7%) | 0.290 |
| Guidewire | 2 (1.4%) | 2 (2.4%) | 0 (0.0%) | 0.508 |
| LOCK without REM | 1 (0.7%) | 0 (0.0%) | 1 (1.6%) | 0.425 |
| LOCK + ATB without REM | 4 (2.7%) | 0 (0.0%) | 4 (6.5%) | 0.031 |
| ATB without REM | 39 (26.7%) | 29 (34.5%) | 10 (16.1%) | 0.014 |
| NO REM and NO ATB | 3 (2.1%) | 1 (1.2%) | 2 (3.2%) | 0.575 |
| Total (N = 146) | CABSI (N = 84) | CRBSI (N = 62) | p Value * | |
|---|---|---|---|---|
| REM without REP | 23 (15.8%) | 7 (8.3%) | 16 (25.8%) | 0.006 |
| REM with REP > 48 h | 38 (26.0%) | 6 (7.1%) | 32 (51.6%) | <0.001 |
| REM with REP < 48 h | 16 (11.0%) | 8 (9.5%) | 8 (12.9%) | 0.596 |
| Guidewire | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| LOCK without REM | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| LOCK + ATB without REM | 8 (5.5%) | 4 (4.8%) | 4 (6.5%) | 0.723 |
| ATB without REM | 46 (31.5%) | 44 (52.4%) | 2 (3.2%) | <0.001 |
| NO REM and NO ATB | 15 (10.3%) | 15 (17.9%) | 0 (0.0%) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colaneri, M.; Galli, L.; Offer, M.; Borgonovo, F.; Scaglione, G.; Genovese, C.; Fattore, R.; Schiavini, M.; De Capitani, G.; Calloni, M.; et al. Vascular Access Device Infections: Current Management Practices and the Role of Multidisciplinary Teams at a Large Hospital in Northern Italy. Antibiotics 2025, 14, 27. https://doi.org/10.3390/antibiotics14010027
Colaneri M, Galli L, Offer M, Borgonovo F, Scaglione G, Genovese C, Fattore R, Schiavini M, De Capitani G, Calloni M, et al. Vascular Access Device Infections: Current Management Practices and the Role of Multidisciplinary Teams at a Large Hospital in Northern Italy. Antibiotics. 2025; 14(1):27. https://doi.org/10.3390/antibiotics14010027
Chicago/Turabian StyleColaneri, Marta, Lucia Galli, Martina Offer, Fabio Borgonovo, Giovanni Scaglione, Camilla Genovese, Rebecca Fattore, Monica Schiavini, Giovanni De Capitani, Maria Calloni, and et al. 2025. "Vascular Access Device Infections: Current Management Practices and the Role of Multidisciplinary Teams at a Large Hospital in Northern Italy" Antibiotics 14, no. 1: 27. https://doi.org/10.3390/antibiotics14010027
APA StyleColaneri, M., Galli, L., Offer, M., Borgonovo, F., Scaglione, G., Genovese, C., Fattore, R., Schiavini, M., De Capitani, G., Calloni, M., Bartoli, A., Gidaro, A., Cogliati, C., Antinori, S., Gori, A., & Foschi, A. (2025). Vascular Access Device Infections: Current Management Practices and the Role of Multidisciplinary Teams at a Large Hospital in Northern Italy. Antibiotics, 14(1), 27. https://doi.org/10.3390/antibiotics14010027







