Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics. Demographic and Clinical Aspects
2.2. Laboratory Data
2.3. Pathogens Involved in VAP Resistance to Antibiotics
- -
- Carbapenem Resistance: 51.6% was attributed to Acinetobacter spp., 25.8% to Pseudomonas spp., and 22.6% to Klebsiella spp..
- -
- Resistance to Third-Generation Cephalosporins: 66.7% was attributed to Pseudomonas spp., followed by Acinetobacter spp. and Klebsiella spp., both at 16.7%.
- -
- Fluoroquinolone Resistance: 47.8% was attributed to Acinetobacter spp., 43.5% to Pseudomonas spp., and 8.7% to Klebsiella spp..
- -
- Aminoglycoside Resistance: 48.1% was attributed to Acinetobacter spp., 33.3% to Pseudomonas spp., and 18.5% to Klebsiella spp..
2.4. Antimicrobial Treatment
2.5. Severity Scores in the ICU
2.6. Invasive Devices
2.7. VAP/MDR Relationship
2.8. Mortality
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Participants and Procedure
4.3. Diagnosis of VAP
4.4. Statistical Analysis
4.5. Definition of MDR/XDR/PDR/DTR
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APACHE | Acute Physiology and Chronic Health Evaluation |
| AUC | Areas under the curve |
| COVID-19 | Coronavirus disease 2019 |
| CPIS | Clinical Pulmonary Infection Score |
| CRP | C reactive protein |
| DTR | Difficult-to-treat resistance |
| ETA | Endotracheal aspiration |
| ETT | Endotracheal tube intubation |
| HAP | Hospital acquired pneumonia |
| ICU | Intensive care unit |
| IMV | Invasive mechanical ventilation |
| LDH | Lactate dehydrogenase |
| NIV | Noninvasive mechanical ventilation |
| MDR | Multiple drug resistance |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| MV | Mechanical MRSA |
| ROC | Receiver operating characteristic curves |
| PACU | Post-anesthesia Intermediate Intensive Care Unit |
| PCT | Procalcitonin |
| PCR | C-reactive protein |
| PDR | Pan-drug resistance |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SD | Standard Deviation |
| SOFA | Sequential Organ Failure Assessment |
| VAP | Ventilator-associated pneumonia |
| Q1 | First quartile |
| Q3 | Third quartile |
| XDR | Extensive drug resistance |
References
- Rouyer, M.; Strazzulla, A.; Youbong, T.; Tarteret, P.; Pitsch, A.; de Pontfarcy, A.; Cassard, B.; Vignier, N.; Pourcine, F.; Jochmans, S.; et al. Ventilator-Associated Pneumonia in COVID-19 Patients: A Retrospective Cohort Study. Antibiotics 2021, 10, 988. [Google Scholar] [CrossRef]
- Stoian, M.; Andone, A.; Boeriu, A.; Bândilă, S.R.; Dobru, D.; Laszlo, S.Ș.; Corău, D.; Arbănași, E.M.; Russu, E.; Stoian, A. COVID-19 and Clostridioides Difficile Coinfection Analysis in the Intensive Care Unit. Antibiotics 2024, 13, 367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoian, M.; Roman, A.; Boeriu, A.; Onișor, D.; Bandila, S.R.; Babă, D.F.; Cocuz, I.; Niculescu, R.; Costan, A.; Laszlo, S.Ș.; et al. Long-Term Radiological Pulmonary Changes in Mechanically Ventilated Patients with Respiratory Failure Due to SARS-CoV-2 Infection. Biomedicines 2023, 11, 2637. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.N.; Camporota, L.; Formenti, F. Mechanical Ventilation in COVID-19: A Physiological Perspective. Exp. Physiol. 2022, 107, 683–693. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hickey, S.M.; Sankari, A.; Giwa, A.O. Mechanical Ventilation. 30 March 2024. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Stoian, A.; Bălașa, R.; Grigorescu, B.L.; Maier, S.; Andone, S.; Cocuz, I.G.; Bajko, Z.; Filep, C.R.; Stoian, M. Guillain-Barré Syndrome Associated with COVID-19: A Close Relationship or Just a Coincidence? (Review). Exp. Ther. Med. 2021, 22, 916. [Google Scholar] [CrossRef] [PubMed]
- Stoian, A.; Șerban, G.; Bajko, Z.; Andone, S.; Mosora, O.; Bălașa, A. Therapeutic Plasma Exchange as a First-Choice Therapy for Axonal Guillain-Barré Syndrome: A Case-Based Review of the Literature (Review). Exp. Ther. Med. 2021, 21, 265. [Google Scholar] [CrossRef]
- Stoian, A.; Bajko, Z.; Maier, S.; Cioflinc, R.A.; Grigorescu, B.L.; Moțățăianu, A.; Bărcuțean, L.; Balașa, R.; Stoian, M. High-dose Intravenous Immunoglobulins as a Therapeutic Option in Critical Illness Polyneuropathy Accompanying SARS-CoV-2 Infection: A Case-based Review of the Literature (Review). Exp. Ther. Med. 2021, 22, 1182. [Google Scholar] [CrossRef] [PubMed]
- Craven, D.E. Epidemiology of Ventilator-Associated Pneumonia. Chest 2000, 117, 186S–187S. [Google Scholar] [CrossRef]
- Kohbodi, G.A.; Rajasurya, V.; Noor, A. Ventilator-Associated Pneumonia. 4 September 2023. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Miron, M.; Blaj, M.; Ristescu, A.I.; Iosep, G.; Avădanei, A.N.; Iosep, D.G.; Crișan-Dabija, R.; Ciocan, A.; Perțea, M.; Manciuc, C.D.; et al. Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: A Literature Review. Microorganisms 2024, 12, 213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. Multistate Point-Prevalence Survey of Health Care-Associated Infections. N. Engl. J. Med. 2014, 370, 1198–1208, Erratum in N. Engl. J. Med. 2022, 386, 2348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Healthcare-Associated Infections Prevalence Study Group. Prevalence of Healthcare-Associated Infections, Estimated Incidence and Composite Antimicrobial Resistance Index in Acute Care Hospitals and Long-Term Care Facilities: Results from Two European Point Prevalence Surveys, 2016 to 2017. Euro Surveill. 2018, 23, 1800516, Erratum in Euro Surveill. 2018, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Eldridge, N.; Metersky, M.L.; Verzier, N.R.; Meehan, T.P.; Pandolfi, M.M.; Foody, J.M.; Ho, S.Y.; Galusha, D.; Kliman, R.E.; et al. National Trends in Patient Safety for Four Common Conditions, 2005–2011. N. Engl. J. Med. 2014, 370, 341–351. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charles, M.P.; Kali, A.; Easow, J.M.; Joseph, N.M.; Ravishankar, M.; Srinivasan, S.; Kumar, S.; Umadevi, S. Ventilator-Associated Pneumonia. Australas Med. J. 2014, 7, 334–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papazian, L.; Klompas, M.; Luyt, C.E. Ventilator-Associated Pneumonia in Adults: A Narrative Review. Intensive Care Med. 2020, 46, 888–906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalil, A.C.; Metersky, M.L.; Klompas, M.; Muscedere, J.; Sweeney, D.A.; Palmer, L.B.; Napolitano, L.M.; O’Grady, N.P.; Bartlett, J.G.; Carratalà, J.; et al. Management of Adults with Hospital-Acquired and Ventilator-Associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016, 63, E61–E111, Erratum in Clin. Infect. Dis. 2017, 64, 1298; Erratum in Clin. Infect. Dis. 2017, 65, 1435; Erratum in Clin. Infect. Dis. 2017, 65, 2161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loyola-Cruz, M.Á.; Durán-Manuel, E.M.; Cruz-Cruz, C.; Márquez-Valdelamar, L.M.; Bravata-Alcántara, J.C.; Cortés-Ortíz, I.A.; Cureño-Díaz, M.A.; Ibáñez-Cervantes, G.; Fernández-Sánchez, V.; Castro-Escarpulli, G.; et al. ESKAPE Bacteria Characterization Reveals the Presence of Acinetobacter baumannii and Pseudomonas aeruginosa Outbreaks in COVID-19/VAP Patients. Am. J. Infect. Control. 2023, 51, 729–737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bassetti, M.; Mularoni, A.; Giacobbe, D.R.; Castaldo, N.; Vena, A. New Antibiotics for Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia. Semin. Respir. Crit. Care Med. 2022, 43, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Alnimr, A. Antimicrobial Resistance in Ventilator-Associated Pneumonia: Predictive Microbiology and Evidence-Based Therapy. Infect. Dis. Ther. 2023, 12, 1527–1552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plata-Menchaca, E.P.; Ferrer, R. Current Treatment of Nosocomial Pneumonia and Ventilator-Associated Pneumonia. Rev. Esp. Quimioter. 2022, 35 (Suppl. S3), 25–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deng, J.; Li, F.; Zhang, N.; Zhong, Y. Prevention and Treatment of Ventilator-Associated Pneumonia in COVID-19. Front. Pharmacol. 2022, 13, 945892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wicky, P.-H.; Dupuis, C.; Cerf, C.; Siami, S.; Cohen, Y.; Laurent, V.; Mourvillier, B.; Reignier, J.; Goldgran-Toledano, D.; Schwebel, C.; et al. Ventilator-Associated Pneumonia in COVID-19 Patients Admitted in Intensive Care Units: Relapse, Therapeutic Failure and Attributable Mortality—A Multicentric Observational Study from the OutcomeRea Network. J. Clin. Med. 2023, 12, 1298. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, H.; Saqib, M.; Khan, W.; Ismail, S.M.; Sohail, H.; Muneeb, M.; Sheikh, S.S. Ventilator Associated Pneumonia in Intensive Care Unit Patients: A Systematic Review. Ann. Med. Surg. 2023, 85, 2932–2939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mergulhão, P.; Pereira, J.G.; Fernandes, A.V.; Krystopchuk, A.; Ribeiro, J.M.; Miranda, D.; Castro, H.; Eira, C.; Morais, J.; Lameirão, C.; et al. Epidemiology and Burden of Ventilator-Associated Pneumonia among Adult Intensive Care Unit Patients: A Portuguese, Multicenter, Retrospective Study (eVAP-PT Study). Antibiotics 2024, 13, 290. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.; Nseir, S.; Rodriguez, A.; Martin-Loeches, I. Ventilator-Associated Pneumonia in Critically Ill Patients with COVID-19 Infection: A Narrative Review. ERJ Open Res. 2022, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Teoli, J.; Amour, S.; Dananché, C.; Dauwalder, O.; Gerbier-Colomban, S.; Mauranne, C.C.; Zorio, V.; Monard, C.; Arnal, S.; Friggeri, A.; et al. Trends in the Proportion of Resistant Bacteria Involved in Ventilator-Associated Pneumonia as the First Hospital-Acquired Infection in Intensive Care Units between 2003 and 2016 in Lyon, France. Am. J. Infect. Control. 2021, 49, 1454–1456. [Google Scholar] [CrossRef] [PubMed]
- Howroyd, F.; Chacko, C.; MacDuff, A.; Gautam, N.; Pouchet, B.; Tunnicliffe, B.; Weblin, J.; Gao-Smith, F.; Ahmed, Z.; Duggal, N.A.; et al. Ventilator-Associated Pneumonia: Pathobiological Heterogeneity and Diagnostic Challenges. Nat. Commun. 2024, 15, 6447. [Google Scholar] [CrossRef]
- Carter, E.L.; Duguid, A.; Ercole, A.; Matta, B.; Burnstein, R.M.; Veenith, T. Strategies to Prevent Ventilation-Associated Pneumonia: The Effect of Cuff Pressure Monitoring Techniques and Tracheal Tube Type on Aspiration of Subglottic Secretions: An in-Vitro Study. Eur. J. Anaesthesiol. 2014, 31, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, C.; Zhang, S.; Zhong, Y. Risk Factors of Ventilator-Associated Pneumonia in Critically III Patients. Front. Pharmacol. 2019, 10, 482. [Google Scholar] [CrossRef]
- ECDC. European Centre for Disease Prevention and Control. European Surveillance of Healthcare-Associated Infections in Intensive Care Units—HAI-Net ICU Protocol Stockholm: ECDC. 2015. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/publications/healthcare-associated-infections-hai-icu-protocol.pdf (accessed on 30 September 2024).
- Koenig, S.M.; Truwit, J.D. Ventilator-Associated Pneumonia: Diagnosis, Treatment, and Prevention. Clin. Microbiol. Rev. 2006, 19, 637–657. [Google Scholar] [CrossRef]
- Fàbregas, N.; Ewig, S.; Torres, A.; El-Ebiary, M.; Ramirez, J.; de La Bellacasa, J.P.; Bauer, T.; Cabello, H. Clinical Diagnosis of Ventilator Associated Pneumonia Revisited: Comparative Validation Using Immediate Post-Mortem Lung Biopsies. Thorax 1999, 54, 867–873. [Google Scholar] [CrossRef]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Li Bassi, G.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT Guidelines for the Management of Hospital-Acquired Pneumonia and Ventilator-Associated Pneumonia: Guidelines for the Management of Hospital-Acquired Pneumonia (HAP)/Ventilator-Associated Pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociación Latinoamericana Del Tórax (ALAT). Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [PubMed]
- Adukauskiene, D.; Ciginskiene, A.; Adukauskaite, A.; Koulenti, D.; Rello, J. Clinical Features and Outcomes of VAP Due to Multidrug-Resistant Klebsiella Spp.: A Retrospective Study Comparing Monobacterial and Polybacterial Episodes. Antibiotics 2023, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Saliba, R.; Zahar, J.-R.; Dabar, G.; Riachy, M.; Karam-Sarkis, D.; Husni, R. Limiting the Spread of Multidrug-Resistant Bacteria in Low-to-Middle-Income Countries: One Size Does Not Fit All. Pathogens 2023, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Ravi, K.; Singh, B. ESKAPE: Navigating the Global Battlefield for Antimicrobial Resistance and Defense in Hospitals. Bacteria 2024, 3, 76–98. [Google Scholar] [CrossRef]
- Badger-Emeka, L.; Al Rashed, A.S.; Aljindan, R.Y.; Emeka, P.M.; Quadri, S.A.; Almutairi, H.H. Incidence of Drug-Resistant Hospital-Associated Gram-Negative Bacterial Infections, the Accompanying Risk Factors, and Clinical Outcomes with Treatment. Antibiotics 2023, 12, 1425. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Klompas, M.; Kyeremanteng, K.; Mehta, S.; English, S.W.; Muscedere, J.; Cook, D.J.; Torres, A.; et al. Diagnosis of Ventilator-Associated Pneumonia in Critically Ill Adult Patients-a Systematic Review and Meta-Analysis. Intensive Care Med. 2020, 46, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Ali, A.; Patel, A.A.; Abbagoni, V.; Goswami, R.; Kumar, A.; Velasquez Botero, F.; Otite, E.; Tomar, H.; Desai, M.; et al. Trends and Factors Associated with Ventilator-Associated Pneumonia: A National Perspective. Cureus 2022, 14, E23634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Froes, F.; Paiva, J.A.; Amaro, P.; Baptista, J.P.; Brum, G.; Bento, H.; Duarte, P.; Dias, C.S.; Gloria, C.; Estrada, H.; et al. Consensus Document on Nosocomial Pneumonia. Rev. Port. Pneumol. 2007, 13, 419–486. [Google Scholar] [CrossRef] [PubMed]
- Melsen, W.G.; Rovers, M.M.; Koeman, M.; Bonten, M.J. Estimating the Attributable Mortality of Ventilator-Associated Pneumonia from Randomized Prevention Studies. Crit. Care Med. 2011, 39, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, P.S.; Wyncoll, D.L. The Tracheal Tube: Gateway to Ventilator-Associated Pneumonia. Crit. Care 2011, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Ventilator Associated Pneumonia. Postgrad. Med. J. 2006, 82, 172–178. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Luo, K.; He, J.; Ma, Y.; Li, Z.; Zhao, N.; Xu, Q.; Li, Y.; Yu, X. Noninvasive versus Invasive Mechanical Ventilation for Immunocompromised Patients with Acute Respiratory Failure: A Systematic Review and Meta-Analysis. BMC Pulm. Med. 2016, 16, 129. [Google Scholar] [CrossRef]
- Cook, D.J.; Walter, S.D.; Cook, R.J.; Griffith, L.E.; Guyatt, G.H.; Leasa, D.; Jaeschke, R.Z.; Brun-Buisson, C. Incidence of and Risk Factors for Ventilator-Associated Pneumonia in Critically Ill Patients. Ann. Intern. Med. 1998, 129, 433–440. [Google Scholar] [CrossRef]
- Sivanathan, L.; Wunsch, H.; Vigod, S.; Hill, A.; Pinto, R.; Scales, D.C. Mental Illness after Admission to an Intensive Care Unit. Intensive Care Med. 2019, 45, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Hatch, R.; Young, D.; Barber, V.; Griffiths, J.; Harrison, D.A.; Watkinson, P. Anxiety, Depression and Post Traumatic Stress Disorder after Critical Illness: A UK-Wide Prospective Cohort Study. Crit. Care 2018, 22, 310. [Google Scholar] [CrossRef]
- Mossberg, R.; Ahlström, B.; Lipcsey, M. A Nationwide Cohort Study on the Association between Intensive Care Treatments and Mental Distress Linked Psychiatric Disorders. Sci. Rep. 2024, 14, 4519. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Kress, J.; Arabi, Y.M. High-Flow Nasal Oxygen and Noninvasive Ventilation for COVID-19. Crit. Care Clin. 2022, 38, 601–621. [Google Scholar] [CrossRef] [PubMed]
- Shallik, N.; Bashir, K.; Elmoheen, A.; Iftikhar, H.; Zaki, H.A. High Flow Nasal Oxygen (HFNO) in the Treatment of COVID-19 Infection of Adult Patients from—An Emergency Perspective: A Systematic Review and Meta-Analysis. Trends Anaesth. Crit. Care 2023, 50, 101238. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, A.P.; Martins, G.; Martins, C.M.; Marques, V.; Christovam, S.; Battaglini, D.; Robba, C.; Pelosi, P.; Rocco, P.R.M.; Cruz, F.F.; et al. Comparison between High-Flow Nasal Oxygen (HFNO) Alternated with Non-Invasive Ventilation (NIV) and HFNO and NIV Alone in Patients with COVID-19: A Retrospective Cohort Study. Eur. J. Med. Res. 2024, 29, 248. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Carvelli, J.; Lesaux, A.; Boucekine, M.; Tonon, D.; Bichon, A.; Gainnier, M.; Bourenne, J. Ventilator Acquired Pneumonia in COVID-19 ICU Patients: A Retrospective Cohort Study during Pandemia in France. J. Clin. Med. 2023, 12, 421. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Younan, D.; Delozier, S.J.; Adamski, J.; Loudon, A.; Violette, A.; Ustin, J.; Tinkoff, G.; Moorman, M.L.; McQuay, N.; UHRISES Research Consortium. Factors Predictive of Ventilator-Associated Pneumonia in Critically Ill Trauma Patients. World J. Surg. 2020, 44, 1121–1125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubánková, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical Phenotype of Blood Cells Is Altered in COVID-19. Biophys. J. 2021, 120, 2838–2847. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seyit, M.; Avci, E.; Nar, R.; Senol, H.; Yilmaz, A.; Ozen, M.; Oskay, A.; Aybek, H. Neutrophil to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio and Platelet to Lymphocyte Ratio to Predict the Severity of COVID-19. Am. J. Emerg. Med. 2021, 40, 110–114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Semet, C. The Ongoing Challenge of Ventilator-Associated Pneumonia: Epidemiology, Prevention, and Risk Factors for Mortality in a Secondary Care Hospital Intensive Care Unit. Infect. Prev. Pract. 2023, 5, 100320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, N.R.; Balraj, T.A.; Kempegowda, S.N.; Prashant, A. Multidrug-Resistant Sepsis: A Critical Healthcare Challenge. Antibiotics 2024, 13, 46. [Google Scholar] [CrossRef]
- Djordjevic, D.; Rondovic, G.; Surbatovic, M.; Stanojevic, I.; Udovicic, I.; Andjelic, T.; Zeba, S.; Milosavljevic, S.; Stankovic, N.; Abazovic, D.; et al. Neutrophil-to-Lymphocyte Ratio, Monocyte-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Mean Platelet Volume-to-Platelet Count Ratio as Biomarkers in Critically Ill and Injured Patients: Which Ratio to Choose to Predict Outcome and Nature of Bacteremia? Mediat. Inflamm. 2018, 2018, 3758068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taşcı, H.İ. The Role of Neutrophil-to-Lymphocyte Ratio in Predicting Disease Progression and Emergency Surgery Indication in Benign Intestinal Obstructions. Ulus. Travma Acil Cerrahi Derg. 2022, 28, 1238–1247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bahçe, Y.G.; Acer, Ö.; Özüdoğru, O. Evaluation of Bacterial Agents Isolated from Endotracheal Aspirate Cultures of COVID-19 General Intensive Care Patients and Their Antibiotic Resistance Profiles Compared to Pre-Pandemic Conditions. Microb. Pathog. 2022, 164, 105409. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Chávez, L.A.; Esteban-Dionicio, M.L.; Rodriguez-Mendoza, C.R.E. Microbiological Profile of Bacteria Causing Ventilator-Associated Pneumonia in the Intensive Care Unit of a High-Complexity Hospital. Rev. Peru. Med. Exp. Salud Publica 2023, 40, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Aguirregabiria, M.; Lázaro-Perona, F.; Cacho-Calvo, J.B.; Arellano-Serrano, M.S.; Ramos-Ramos, J.C.; Rubio-Mora, E.; Díaz-Almirón, M.; Asensio-Martín, M.J. Challenges Facing Two Outbreaks of Carbapenem-Resistant Acinetobacter baumannii: From Cefiderocol Susceptibility Testing to the Emergence of Cefiderocol-Resistant Mutants. Antibiotics 2024, 13, 784. [Google Scholar] [CrossRef]
- Kollef, M.; Dupont, H.; Greenberg, D.E.; Viale, P.; Echols, R.; Yamano, Y.; Nicolau, D.P. Prospective Role of Cefiderocol in the Management of Carbapenem-Resistant Acinetobacter baumannii Infections: Review of the Evidence. Int. J. Antimicrob. Agents 2023, 62, 106882. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.; Putra, V.; Lodise, T.P. Treatment of Patients with Serious Infections Due to Carbapenem-Resistant Acinetobacter baumannii: How Viable Are the Current Options? Pharmacotherapy 2021, 41, 762–780. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the Critically Ill: Complex Infections Get Complicated. Front. Microbiol. 2023, 22, 1196774. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Attili, A.-R.; De Martino, L. Acinetobacter baumannii: Its Clinical Significance in Human and Veterinary Medicine. Pathogens 2021, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Eggimann, P.; Bille, J.; Marchetti, O. Diagnosis of Invasive Candidiasis in the ICU. Ann. Intensive Care 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Foucrier, A.; Dessalle, T.; Tuffet, S.; Federici, L.; Dahyot-Fizelier, C.; Barbier, F.; Pottecher, J.; Monsel, A.; Hissem, T.; Lefrant, J.Y.; et al. Association between Combination Antibiotic Therapy as Opposed as Monotherapy and Outcomes of ICU Patients with Pseudomonas Aeruginosa Ventilator-Associated Pneumonia: An Ancillary Study of the iDIAPASON Trial. Crit. Care 2023, 27, 211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramadan, R.A.; Bedawy, A.M.; Negm, E.M.; Hassan, T.H.; Ibrahim, D.A.; ElSheikh, S.M.; Amer, R.M. Carbapenem-Resistant Klebsiella pneumoniae Among Patients with Ventilator-Associated Pneumonia: Evaluation of Antibiotic Combinations and Susceptibility to New Antibiotics. Infect. Drug Resist. 2022, 15, 3537–3548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arthur, L.E.; Kizor, R.S.; Selim, A.G.; van Driel, M.L.; Seoane, L. Antibiotics for Ventilator-Associated Pneumonia. Cochrane Database Syst. Rev. 2016, 10, CD004267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Souza, G.H.A.; Rossato, L.; Brito, G.T.; Bet, G.M.D.S.; Simionatto, S. Carbapenem-Resistant Pseudomonas Aeruginosa Strains: A Worrying Health Problem in Intensive Care Units. Rev. Inst. Med. Trop. Sao Paulo. 2021, 63, E71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gondal, A.J.; Choudhry, N.; Niaz, A.; Yasmin, N. Molecular Analysis of Carbapenem and Aminoglycoside Resistance Genes in Carbapenem-Resistant Pseudomonas Aeruginosa Clinical Strains: A Challenge for Tertiary Care Hospitals. Antibiotics 2024, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Gursel, G.; Demirtas, S. Value of APACHE II, SOFA and CPIS Scores in Predicting Prognosis in Patients with Ventilator-Associated Pneumonia. Respiration 2006, 73, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Huang, C.Y.; Li, L.F. Prolonged Mechanical Ventilation: Outcomes and Management. J. Clin. Med. 2022, 11, 2451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodrigues, A.R.; Oliveira, A.; Vieira, T.; Assis, R.; Lume, C.; Gonçalves-Pereira, J.; Fernandes, S.M. A Prolonged Intensive Care Unit Stay Defines a Worse Long-Term Prognosis—Insights from the Critically Ill Mortality by Age (Cimba) Study. Aust. Crit. Care 2024, 37, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Bouadma, L.; Bouhemad, B.; Brissaud, O.; Dauger, S.; Gibot, S.; Hraiech, S.; Jung, B.; Kipnis, E.; Launey, Y.; et al. Brief Summary of French Guidelines for the Prevention, Diagnosis and Treatment of Hospital-Acquired Pneumonia in ICU. Ann. Intensive Care 2018, 8, 104. [Google Scholar] [CrossRef]
- Cosentino, F.; Viale, P.; Giannella, M. MDR/XDR/PDR or DTR? Which Definition Best. Fits the Resistance Profile of Pseudomonas Aeruginosa? Curr. Opin. Infect. Dis. 2023, 36, 564–571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]



| Attrition Criteria | N |
|---|---|
| Initial population: patients admitted to Mures County Clinical Hospital from 1 April 2021 to 1 April 2023. | 11,648 |
| Patients hospitalized in departments other than the ICU at Mures County Clinical Hospital. | 7111 |
| Patients who were hospitalized in post-anesthesia care units. | 1196 |
| Patients admitted to the ICU during this interval. | 3341 |
| Patients who required MV in the ICU. | 912 |
| Final eligible population. | 122 |
| Sample Characteristics | All Patients N = 122, n (%) | COVID Positive Patients N = 53, n (%) | COVID Negative Patients N = 69, n (%) | p Value |
|---|---|---|---|---|
| Sex | 0.553 | |||
| Male | 75 (61.5) | 31 (58.5) | 44 (63.8) | |
| Female | 47 (38.5) | 22 (41.5) | 25 (36.2) | |
| Living environment | 0.422 | |||
| Rural | 74 (60.7) | 30 (56.6) | 44 (63.8) | |
| Urban | 48 (39.3) | 23 (43.4) | 25 (36.2) | |
| Carmeli score | 0.005 | |||
| 1 | 3 (2.5) | 2 (3.8) | 1 (1.4) | |
| 2 | 55 (45) | 32 (60.4) | 25 (33.3) | |
| 3 | 64 (52.5) | 19 (35.8) | 45 (65.2) | |
| Comorbidities | 0.155 | |||
| Cardiovascular | ||||
| Yes | 97 (79.5) | 39 (73.5) | 58 (84) | |
| No | 25 (20.5) | 14 (26.5) | 11 (16) | |
| Renal | 0.323 | |||
| Yes | 38 (31.1) | 14 (26.4) | 24 (34.7) | |
| No | 84 (68.9) | 39 (73.6) | 45 (65.3) | |
| Pulmonary | 0.089 | |||
| Yes | 40 (32.8) | 13 (24.5) | 27 (39.1) | |
| No | 82 (67.2) | 40 (75.5) | 42 (60.9) | |
| Oncologic | 0.512 | |||
| Yes | 24 (19.7) | 9 (16.98) | 15 (21.7) | |
| No | 98 (80.3) | 44 (82.02) | 54 (78.3) | |
| Digestive | 0.08 | |||
| Yes | 50 (40.9) | 17 (32) | 33 (47.8) | |
| No | 72 (59.1) | 36 (68) | 36 (52.2) | |
| Psychiatric | 0.952 * | |||
| Yes | 17 (13.9) | 8 (15) | 9 (13) | |
| No | 105 (86.1) | 45 (85) | 60 (87) | |
| Diabetes | 0.063 * | |||
| Yes | 27 (22.1) | 7 (13.2) | 20 (28.9) | |
| No | 95 (77.9) | 46 (86.8) | 49 (71.1) | |
| Obesity | 0.376 * | |||
| Yes | 24 (19.7) | 8 (15) | 16 (23.1) | |
| No | 98 (80.3) | 45 (85) | 53 (76,9) | |
| Number of comorbidities | ||||
| 0 | 2 (1.6) | 2 (3.8) | 0 (0) | <0.001 † |
| 1 | 23 (18.9) | 15 (28.3) | 8 (11.6) | |
| 2 | 29 (23.8) | 17 (32.1) | 12 (17.4) | |
| 3 | 43 (35.2) | 13 (24.5) | 30 (43.5) | |
| 4 | 19 (15.6) | 4 (7.5) | 15 (21.7) | |
| 5 | 5 (4.1) | 1 (1.9) | 4 (5.8) | |
| 6 | 1 (0.8) | 1 (1.9) | 0 (0) | |
| Median (IQR) | 3 (1) | 2 (2) | 3 (2) | |
| Invasive devices | ||||
| Central venous catheter | ||||
| Yes | 110 (90.2) | 52 (98.1) | 58 (84) | 0.012 # |
| No | 12 (9.8) | 1 (1.9) | 11 (16) | |
| Urinary catheterization | ||||
| Yes | 73 (59.8) | 16 (30.2) | 57 (82.6) | <0.001 |
| No | 49 (40.2) | 37 (69.8) | 12 (17.4) | |
| Surgical intervention during the last 30 days | ||||
| No | 101 (82.8) | 48 (90.6) | 53 (76.8) | 0.055 # |
| Yes | 21 (17.2) | 5 (9.4) | 16 (23.2) | |
| APACHE score | ||||
| Median (IQR) | 22 (10) | 24 (10) | 17 (11) | 0.065 † |
| SOFA score | ||||
| Median (IQR) | 10 (5) | 10 (4.25) | 8 (6.5) | 0.360 † |
| Conventional radiology result | ||||
| Persistent infiltrates | 52 (49) | 27 (40) | 34 (55.7) | 0.109 |
| Evolutive radiological changes | 54 (51) | 18 (60) | 27 (44.3) | |
| Computed tomography result | ||||
| Persistent infiltrates | 18 (48.6) | 5 (29.4) | 13 (65) | 0.049 * |
| Evolutive radiological changes | 19 (51.4) | 12 (70.6) | 7 (35) | |
| VAP bacteriologic diagnosis | ||||
| No | 40 (32.8) | 20 (37.8) | 20 (29) | 0.307 |
| Yes | 82 (67.2) | 33 (62.2) | 49 (71) |
| COVID-19 Positive Patients Mean ± SD/Median (Q1–Q3) | COVID-19 Negative Patients Mean ± SD/Median (Q1–Q3) | p-Value | |
|---|---|---|---|
| Age (years) | 70.04 ± 11.48 | 66.58 ± 11.79 | 0.107 † |
| Intensive care stay (days) | 12 (8–18.5) | 10 (7–15) | 0.242 |
| Duration of ventilation (hours) | 245 (134–404.5) | 152 (86–240) | 0.001 * |
| Neutrophil (×103/µL) at admission | 9.09 (5.89–14.04) | 8.74 (6.43–13.79) | 0.938 |
| Neutrophil (×103/µL) at 48 h | 11.74 (7.28–16.99) | 9.2 (6.15–13.94) | 0.139 |
| Neutrophil (×103/µL) at 72 h | 12.60 (8.54–19.15) | 9.28 (6.61–13.75) | 0.006 * |
| Lymphocytes (×103/µL) at admission | 0.55 (0.44–0.93) | 0.8 (0.47–1.32) | 0.073 |
| Lymphocytes (×103/µL) at 48 h | 0.59 (0.38–0.89) | 0.88 (0.41–1.16) | 0.083 |
| Lymphocytes (×103/µL) at 72 h | 0.46 (0.37–0.74) | 0.88 (0.53–1.39) | <0.001 * |
| Monocytes (×103/µL) at admission | 0.41 (0.26–0.72) | 0.59 (0.38–0.9) | 0.039 * |
| Monocytes (×103/µL) at 48 h | 0.45 (0.28–0.69) | 0.56 (0.29–0.84) | 0.292 |
| Monocytes (×103/µL) at 72 h | 0.41 (0.26–0.81) | 0.57 (0.37–0.97) | 0.006 * |
| Eosinophils (×103/µL) at admission | 0.02 (0–0.09) | 0.02 (0–0.08) | 0.935 |
| Eosinophils (×103/µL) at 48 h | 0.01 (0–0.04) | 0.04 (0.01–0.15) | 0.016 * |
| Eosinophils (×103/µL) at 72 h | 0.01 (0–0.08) | 0.08 (0.01–0.26) | 0.001 * |
| LDH (U/L) | 456 (274.5–709) | 298 (224.5–387.75) | 0.007 * |
| Neutrophil/lymphocyte ratio at admission | 15.11 (7.72–25.66) | 11.80 (7.18–23.70) | 0.311 |
| Neutrophil/lymphocyte ratio at 48 h | 17.80 (10.11–35.37) | 12.02 (6.26–20.78) | 0.016 * |
| Neutrophil/lymphocyte ratio at 72 h | 24.31 (11.74–44.23) | 10.13 (5.63–19.11) | <0.001 * |
| Pathogen Agent | COVID-19 Positive Patients’ Relative Frequency | COVID-19 Negative Patients’ Relative Frequency |
|---|---|---|
| Acinetobacter spp. | 39.29% | 32.34% |
| Baumannii | 10.71% | 2.94% |
| Baumannii MDR | 17.86% | 29.41% |
| Jejuni MDR | 10.71% | 0.00% |
| Pseudomonas spp. | 21.43% | 11.76% |
| Aeruginosa | 7.14% | 5.88% |
| Aeruginosa MDR | 14.29% | 11.76% |
| Klebsiella spp. | 14.29% | 11.76% |
| Pneumoniae | 3.57% | 2.94% |
| Pneumoniae MDR | 10.71% | 8.82% |
| Stafilococus aureus spp. | 7.14% | 8.82% |
| MSSA | 3.57% | 5.88% |
| MRSA | 3.57% | 2.94% |
| Stenotrophomonasmaltophilia | 3.57% | 5.88% |
| Escherichia spp. | 0.00% | 0.00% |
| Coli | 0.00% | 2.94% |
| Coli MDR | 0.00% | 2.94% |
| Citrobacter Freundii | 3.57% | 0.00% |
| Corymenacterium Striatum | 0.00% | 2.94% |
| Morganella morganii | 0.00% | 2.94% |
| Serratia Marcescens | 0.00% | 2.94% |
| Candida Albicans | 3.57% | 2.94% |
| Candida Non-Albicans | 3.57% | 2.94% |
| Candida Glabrata | 3.57% | 0.00% |
| Candida Krusei | 0.00% | 2.94% |
| Antibiotics | Relative Frequency |
|---|---|
| Penicillins | 4.6% |
| Second-generation cephalosporins | 0.6% |
| Third-generation cephalosporins | 24.9% |
| Carbapenems | 26% |
| Aminoglycosides | 6.9% |
| Antifungals | 2.9% |
| Oxazolidinones | 9.8% |
| Fluoroquinolones | 13.9% |
| Polymyxins | 10.4% |
| Type of therapy | |
| Monotherapy | 13.1% |
| Polytherapy | 86.9% |
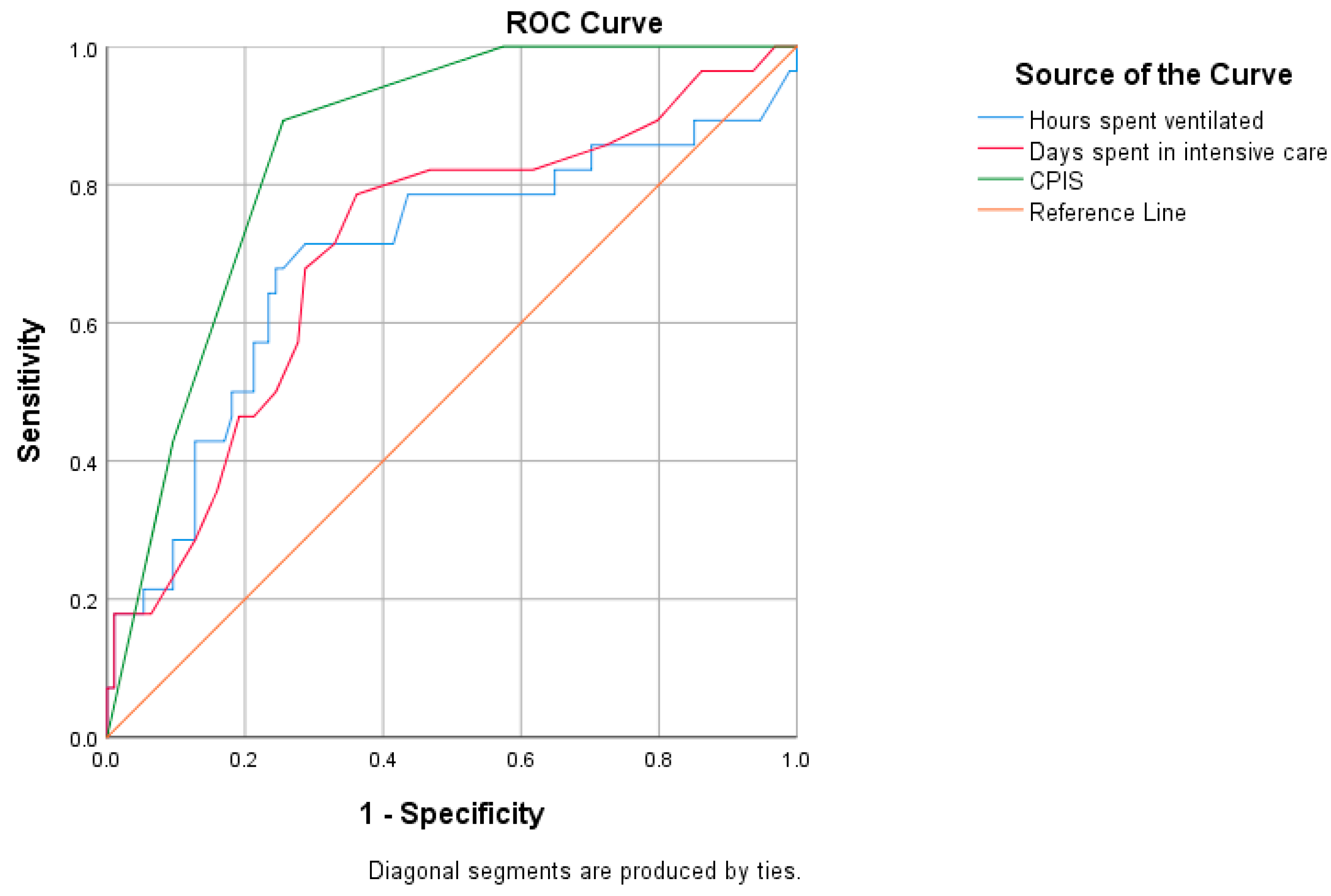
| Variable | Cut-Off | AUC | 95% CI | Sensitivity | Specificity | p-Value |
|---|---|---|---|---|---|---|
| Multi-drug resistance in all patients | ||||||
| Days spent in intensive care | 11.5 | 0.708 | 0.595–0.820 | 78.6% | 63.8% | <0.001 |
| Hours spent ventilated | 196 | 0.695 | 0.570–0.820 | 78.6% | 56.4% | 0.002 |
| CPIS | 5 | 0.854 | 0.786–0.921 | 89.3% | 74.5% | <0.001 |
| Multi-drug resistance in COVID-positive patients | ||||||
| Days spent in intensive care | 11.5 | 0.618 | 0.453–0.783 | 78.6% | 56.4% | 0.193 |
| Hours spent ventilated | 261.5 | 0.604 | 0.433–0.776 | 71.4% | 61.5% | 0.250 |
| CPIS | 5 | 0.905 | 0.825–0.984 | 100% | 79.5% | <0.001 |
| Multi-drug resistance in COVID-19-negative patients | ||||||
| Hours spent in intensive care | 11.5 | 0.773 | 0.621–0.925 | 78.6% | 69.1% | 0.002 |
| Days spent ventilated | 196 | 0.731 | 0.533–0.929 | 78.6% | 72.7% | 0.008 |
| CPIS | 5 | 0.800 | 0.692–0.908 | 78.6% | 70.9% | 0.001 |
| Variable | Discharged Dead Median (Q1–Q3) | Discharged Alive Median (Q1–Q3) | p-Value |
|---|---|---|---|
| Intensive care stay (days) | 12.00 (8.00–18.00) | 9.00 (6.75–15.00) | 0.017 * |
| Duration of ventilation (hours) | 222.50 (138.50–396.00) | 122.50 (90.00–232.50) | 0.001 * |
| Leucocytes (×103/µL) at admission | 10.85 (7.73–16.41) | 9.44 (6.85–13.44) | 0.122 |
| Leucocytes (×103/µL) at 48 h | 13.37 (9.84–20.81) | 9.07 (6.49–11.05) | <0.001 * |
| Leucocytes (×103/µL) at 72 h | 14.15 (10.42–20.20) | 10.65 (7.04–12.81) | <0.001 * |
| Neutrophil (×103/µL) at 48 h | 12 (8.64–18.83) | 7.24 (5.32–9.75) | <0.001 * |
| Neutrophil (×103/µL) at 72 h | 12.61 (8.6–18.44) | 8.02 (5.23–10.4) | <0.001 * |
| Lymphocytes (×103/µL) at 72 h | 0.56 (0.37–0.99) | 0.94 (0.51–1.49) | 0.004 * |
| Eosinophils (×103/µL) at 48 h | 0.02 (0.00–0.07) | 0.04 (0.01–0.16) | 0.100 |
| Eosinophils (×103/µL) at 72 h | 0.02 (0.01–0.11) | 0.10 (0.02–0.31) | 0.001 * |
| Basophils (×103/µL) at 72 h | 0.00 (0.00–0.01) | 0.01 (0.00–0.02) | 0.004 * |
| CRP (mg/dL) at 48 h | 9.74 (5.39–15.87) | 4.82 (2.42–10.84) | 0.011 * |
| LDH (U/L) | 387.50 (240.25–720.25) | 313.00 (230.00–383.00) | 0.045 * |
| COVID-19 | |||
| Yes | 43 (35.3%) | 10 (8.2%) | 0.002 * |
| No | 37 (30.3%) | 32 (26.2%) | |
| Type of ventilation | |||
| NIV | 15 (12.3%) | 29 (23.8%) | <0.001 * |
| IMV | 46 (37.7%) | 8 (6.6%) | |
| Mixed | 19 (15.5%) | 5 (4.1%) | |
| Chest radiography | |||
| Evolutive radiological changes | 42 (39.7%) | 12 (11.3%) | 0.003 * |
| Persistent infiltrates | 26 (24.5%) | 26 (24.5%) | |
| Chest CT | |||
| Evolutive radiological changes | 16 (43.2%) | 3 (8.2%) | 0.007 *,† |
| Persistent infiltrates | 7 (18.9%) | 11 (29.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoian, M.; Andone, A.; Bândilă, S.R.; Onișor, D.; Laszlo, S.Ș.; Lupu, G.; Danielescu, A.; Baba, D.-F.; Văsieșiu, A.M.; Manea, A.; et al. Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics 2025, 14, 28. https://doi.org/10.3390/antibiotics14010028
Stoian M, Andone A, Bândilă SR, Onișor D, Laszlo SȘ, Lupu G, Danielescu A, Baba D-F, Văsieșiu AM, Manea A, et al. Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics. 2025; 14(1):28. https://doi.org/10.3390/antibiotics14010028
Chicago/Turabian StyleStoian, Mircea, Adina Andone, Sergiu Rareș Bândilă, Danusia Onișor, Sergiu Ștefan Laszlo, Gabriela Lupu, Alina Danielescu, Dragoș-Florin Baba, Anca Meda Văsieșiu, Andrei Manea, and et al. 2025. "Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units" Antibiotics 14, no. 1: 28. https://doi.org/10.3390/antibiotics14010028
APA StyleStoian, M., Andone, A., Bândilă, S. R., Onișor, D., Laszlo, S. Ș., Lupu, G., Danielescu, A., Baba, D.-F., Văsieșiu, A. M., Manea, A., & Stoian, A. (2025). Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics, 14(1), 28. https://doi.org/10.3390/antibiotics14010028









