Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Chickens in Hungarian Poultry Farms Between 2022 and 2023
Abstract
1. Introduction
2. Results
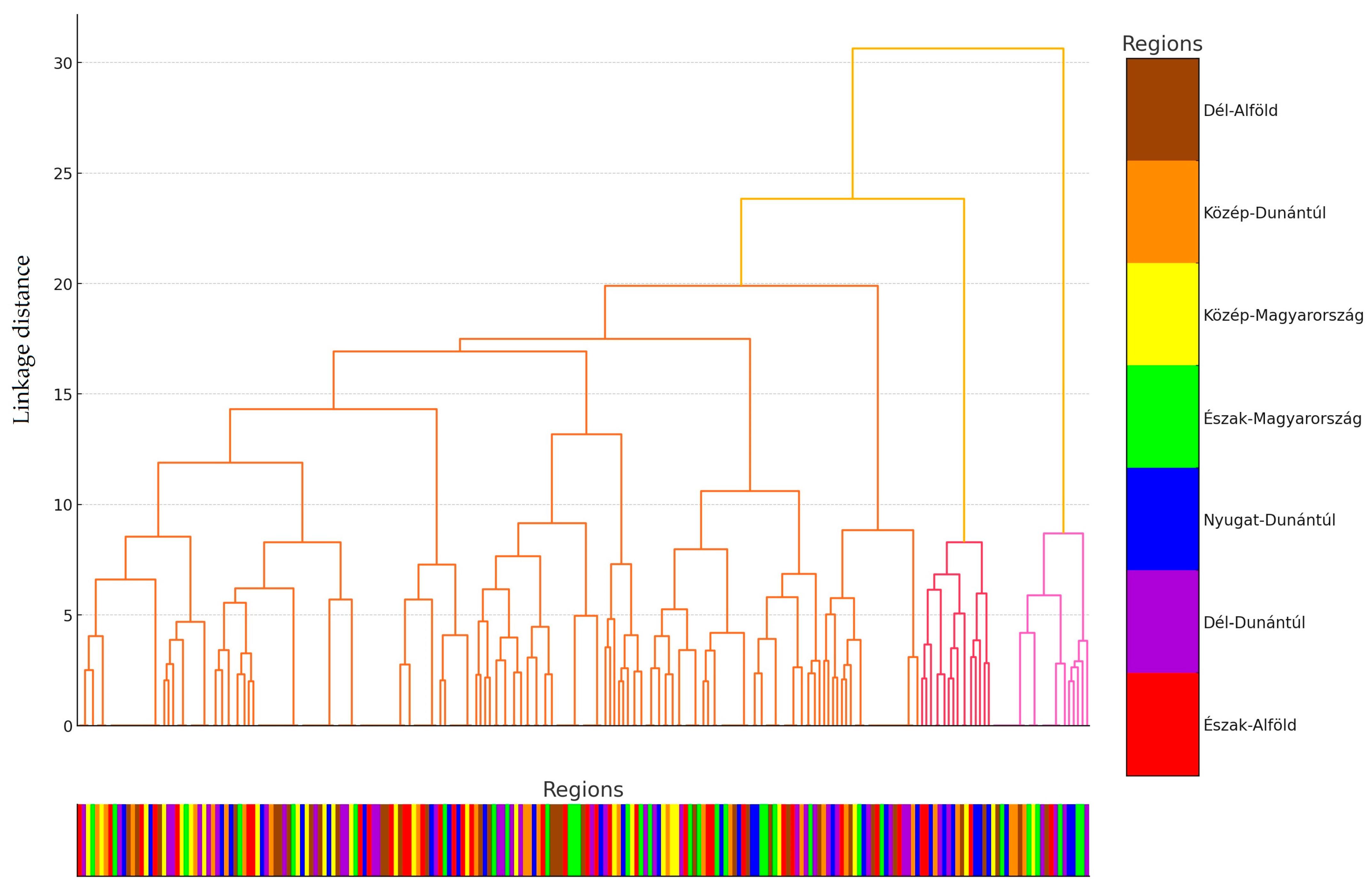
2.1. Regional Distribution and Origin of the Collected Samples
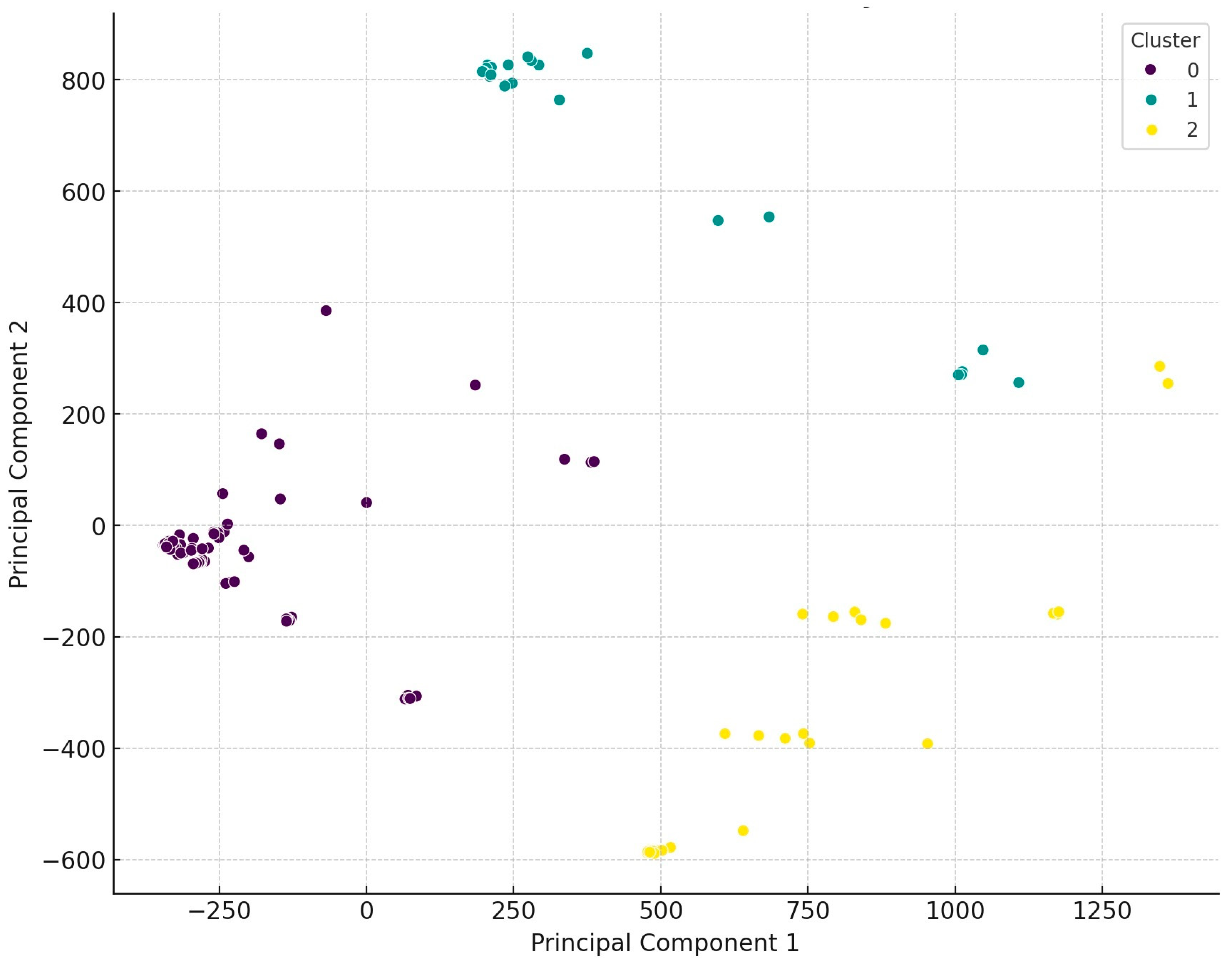
2.2. Antimicrobial Susceptibility Testing
3. Discussion
4. Materials and Methods
4.1. Origin of Strains and Human Data
4.2. Determination of Minimum Inhibitory Concentration (MIC)
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, P.; Jenner, R.; Norris, J.; Page, S.; Browning, G. Antimicrobial prescribing guidelines for poultry. Aust. Vet. J. 2021, 99, 181–235. [Google Scholar] [CrossRef]
- Ghosh, D.; Veeraraghavan, B.; Elangovan, R.; Vivekanandan, P. Antibiotic resistance and epigenetics: More to it than meets the eye. Antimicrob. Agents Chemother. 2020, 64, e02225-19. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F. Threats of antibiotic resistance: An obliged reappraisal. Int. Microbiol. 2021, 24, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef]
- Chicken Meat Production Worldwide 2012–2024. Available online: https://www.statista.com/statistics/237637/production-of-poultry-meat-worldwide-since-1990/ (accessed on 3 December 2024).
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial resistance in bacterial poultry pathogens: A review. Front. Vet. Sci. 2017, 4, 28848739. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Schouler, C. Avian colibacillosis: Still many black holes. FEMS Microbiol. Lett. 2015, 362, fnv118. [Google Scholar] [CrossRef]
- Qadir, M.F.; Saleemi, M.K.; Gul, S.T. Epidemiological and pathological status of mycoplasma gallisepticum in layer chicks at faisalabad, pakistan. Pak. J. Agric. Sci. 2021, 58, 213–218. [Google Scholar]
- Pintér, K.; Ádám, K.; Tibor, M. Antibiotic susceptibility of Pasteurella multocida strains, genetic background of antimicrobial resistance Literature review. Magy. Állatorvosok Lapja 2023, 147, 239–256. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di Ilio, C. Escherichia coli in Europe: An Overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef]
- Threlfall, E.J. Antimicrobial drug resistance in Salmonella: Problems and perspectives in food- and water-borne infections. FEMS Microbiol. Rev. 2002, 26, 141–148. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Z.; Li, Z.; Zhang, X.; Jia, C.; Li, T.; Dai, M.; Tan, C.; Xu, Z.; Wu, B.; et al. Antimicrobial resistance and population genomics of multidrug-resistant Escherichia coli in pig farms in mainland China. Nat. Commun. 2022, 13, 1116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Sannes, M.R.; Croy, C.; Johnston, B.; Clabots, C.; Kuskowski, M.A.; Bender, J.; Smith, K.E.; Winokur, P.L.; Belongia, E.A. Antimicrobial drug–resistant Escherichia coli from humans and poultry products, Minnesota and Wisconsin, 2002–2004. Emerg. Infect. Dis. 2007, 13, 838–846. [Google Scholar] [CrossRef]
- Kovács, D.; Palkovicsné Pézsa, N.; Farkas, O.; Jerzsele, Á. Usage of antibiotic alternatives in pig farming: Literature review. Magy. Állatorvosok Lapja 2021, 143, 281–282. [Google Scholar]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The possibilities of antibiotic-free broiler-hen fattening, with special reference to the use of pre- and probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Huang, L.; Luo, S.; Liu, S.; Jin, M.; Wang, Y.; Zong, X. Comparative multiomics analyses reveal the breed effect on the colonic host–microbe interactions in pig. iMetaOmics 2024, 1, e8. [Google Scholar] [CrossRef]
- Zeng, M.; Zou, Y.; Shi, Z.; Wang, J.; Yang, Y.; Bai, Y.; Ping, A.; Zhang, P.; Chen, Y.; Tao, H.; et al. A broad-spectrum broth rapidly and completely repairing the sublethal injuries of Escherichia coli caused by freezing and lactic acid alone or in combination for accurate enumeration. LWT 2024, 201, 116219. [Google Scholar] [CrossRef]
- Adorján, A.; Makrai, L.; Könyves, L.; Tóth, I. Enteropatogén Escherichia coli (EPEC): Rövid irodalmi összefoglaló. Magy. Állatorvosok Lapja 2021, 143, 429–438. [Google Scholar]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial properties of essential oils–animal health aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Wang, K.; Chen, J.; Jin, K.; Peng, K.; Chen, X.; Liu, Z.; Ouyang, J.; Wang, Y.; et al. Effects of Litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front. Pharmacol. 2023, 14, 1166022. [Google Scholar] [CrossRef]
- Kerek, Á.; Szabó, Á.; Dobra, P.F.; Bárdos, K.; Ózsvári, L.; Fehérvári, P.; Bata, Z.; Molnár-Nagy, V.; Jerzsele, Á. Determining the in vivo efficacy of plant-based and probiotic-based antibiotic alternatives against mixed infection with Salmonella enterica and Escherichia coli in domestic chickens. Vet. Sci. 2023, 10, 706. [Google Scholar] [CrossRef]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of fermented wheat germ extract on artificial Salmonella Typhimurium infection in broiler chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Zeng, J.; Li, Y.; Zou, Y.; Yang, Y.; Yang, T.; Zhou, Y. Intestinal toxicity alleviation and efficacy potentiation through therapeutic administration of Lactobacillus paracasei GY-1 in the treatment of gout flares with colchicine. Food Funct. 2024, 15, 1671–1688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, Y.; Zhang, S.; Zhao, Y.; Gao, D.; Xing, J.; Cao, Y.; Xu, G. Purslane (Portulaca oleracea L.) polysaccharide attenuates carbon tetrachloride-induced acute liver injury by modulating the gut microbiota in mice. Genomics 2025, 117, 110983. [Google Scholar] [CrossRef] [PubMed]
- Pomothy, J.M.; Barna, R.F.; Gere, E. The Effects of the rosmarinic acid in livestock animals: Literature review. Magy. Állatorvosok Lapja 2020, 142, 567–576. [Google Scholar]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial peptides as new tools to combat infectious diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In vivo efficacy of different extracts of propolis in broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial efficiency of propolis–Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Tuska-Szalay, B.; Kovács, L.; Jerzsele, Á. In vitro efficacy of hungarian propolis against bacteria, yeast, and trichomonas gallinae isolated from pigeons—A possible antibiotic alternative? Resources 2023, 12, 101. [Google Scholar] [CrossRef]
- Mag, P.; Németh, K.; Somogyi, Z.; Jerzsele, Á. Antibacterial therapy based on pharmacokinetic/pharmacodynamic models in small animal medicine-1. Literature review. Magy. Állatorvosok Lapja 2023, 145, 419–438. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Liao, L.; Tan, H.; Li, Y.; Li, Z.; Zhou, B.; Bao, M.; He, B. Pharmacokinetics effects of chuanxiong rhizoma on warfarin in pseudo germ-free rats. Front. Pharmacol. 2022, 13, 1022567. [Google Scholar] [CrossRef]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity status of large-scale poultry farms in Hungary based on the data of the national center for disease control of Nébih and the poultry product council biosecurity audit system during the 2021–2022 period. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Kovács, L.; Hejel, P.; Farkas, M.; László, L. Könyves László Study Report on the Effect of a litter treatment product containing bacillus licheniformis and zeolite in male fattening turkey flock. Magy. Állatorvosok Lapja 2024, 146, 291–305. [Google Scholar] [CrossRef]
- van der Mee-Marquet, N.L.; Corvaglia, A.; Haenni, M.; Bertrand, X.; Franck, J.-B.; Kluytmans, J.; Girard, M.; Quentin, R.; François, P. Emergence of a novel subpopulation of CC398 Staphylococcus aureus infecting animals is a serious hazard for humans. Front. Microbiol. 2014, 5, 652. [Google Scholar] [CrossRef] [PubMed]
- Cuny, C.; Wieler, L.H.; Witte, W. Livestock-associated MRSA: The impact on humans. Antibiotics 2015, 4, 521–543. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Ballhausen, B.; Kahl, B.C.; Köck, R. The clinical impact of livestock-associated methicillin-resistant Staphylococcus aureus of the clonal complex 398 for humans. Vet. Microbiol. 2017, 200, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Spoor, L.E.; McAdam, P.R.; Weinert, L.A.; Rambaut, A.; Hasman, H.; Aarestrup, F.M.; Kearns, A.M.; Larsen, A.R.; Skov, R.L.; Fitzgerald, J.R. Livestock origin for a human pandemic clone of community-associated methicillin-resistant Staphylococcus aureus. mBio 2013, 4, e00356-13. [Google Scholar] [CrossRef]
- Geenen, P.L.; Graat, E.A.M.; Haenen, A.; Hengeveld, P.D.; Van Hoek, A.H.A.M.; Huijsdens, X.W.; Kappert, C.C.; Lammers, G.A.C.; Van Duijkeren, E.; Van De Giessen, A.W. Prevalence of livestock-associated MRSA on Dutch broiler farms and in people living and/or working on these farms. Epidemiol. Infect. 2013, 141, 1099–1108. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Stefani, L.M.; Lucheis, S.B.; Okano, W.; Cruz, J.C.M.; Souza, G.V.; Casagrande, T.A.C.; Bastos, P.A.S.; Pinheiro, R.R.; Arruda, M.M.; et al. Methicillin-resistant staphylococcus aureus in poultry and poultry meat: A meta-analysis. J. Food Prot. 2018, 81, 1055–1062. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Chambers, H.F. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Invest 2009, 119, 2464–2474. [Google Scholar] [CrossRef]
- van Belkum, A. Staphylococcal colonization and infection: Homeostasis versus disbalance of human (innate) immunity and bacterial virulence. Curr. Opin. Infect. Dis. 2006, 19, 339–344. [Google Scholar] [CrossRef]
- Weems, J.J. The many faces of Staphylococcus aureus infection. Recognizing and managing its life-threatening manifestations. Postgrad. Med. 2001, 110, 24–26, 29–31, 35–36. [Google Scholar] [CrossRef]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Nagase, N.; Sasaki, A.; Yamashita, K.; Shimizu, A.; Wakita, Y.; Kitai, S.; Kawano, J. Isolation and species distribution of staphylococci from animal and human skin. J. Vet. Med. Sci. 2002, 64, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaeghen, W.; Hermans, K.; Haesebrouck, F.; Butaye, P. Methicillin-resistant Staphylococcus aureus (MRSA) in food production animals. Epidemiol. Infect. 2010, 138, 606–625. [Google Scholar] [CrossRef]
- van Cleef, B.a.G.L.; van Benthem, B.H.B.; Verkade, E.J.M.; van Rijen, M.; Kluytmans-van den Bergh, M.F.Q.; Schouls, L.M.; Duim, B.; Wagenaar, J.A.; Graveland, H.; Bos, M.E.H.; et al. Dynamics of methicillin-resistant Staphylococcus aureus and methicillin-susceptible Staphylococcus aureus carriage in pig farmers: A prospective cohort study. Clin. Microbiol. Infect. 2014, 20, O764–O771. [Google Scholar] [CrossRef]
- Abdalrahman, L.S.; Stanley, A.; Wells, H.; Fakhr, M.K. Isolation, virulence, and antimicrobial resistance of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) Strains from Oklahoma retail poultry meats. Int. J. Environ. Res. Public Health 2015, 12, 6148–6161. [Google Scholar] [CrossRef]
- Kraushaar, B.; Ballhausen, B.; Leeser, D.; Tenhagen, B.-A.; Käsbohrer, A.; Fetsch, A. Antimicrobial resistances and virulence markers in Methicillin-resistant Staphylococcus aureus from broiler and turkey: A molecular view from farm to fork. Vet. Microbiol. 2017, 200, 25–32. [Google Scholar] [CrossRef]
- Salgado, C.D.; Farr, B.M.; Calfee, D.P. Community-acquired methicillin-resistant Staphylococcus aureus: A meta-analysis of prevalence and risk factors. Clin. Infect. Dis. 2003, 36, 131–139. [Google Scholar] [CrossRef]
- Ashok, S.; Ejaz, M.; Muhammad, F.Q.; Manzoor, A.; Qadir, M.F.; Nazir, S. Antibacterial activity of trifluoperazine; in vitro susceptibility of MRSA Staphylococcus aureus, Pseudomonas aeruginosa, and E. coli, and in vivo evaluation against methicillin-resistant Staphylococcus aureus in a surgical wound infection model. J. Anim. Plant Sci. 2021, 31, 1287–1292. [Google Scholar] [CrossRef]
- Abolghait, S.K.; Fathi, A.G.; Youssef, F.M.; Algammal, A.M. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from chicken meat and giblets often produces staphylococcal enterotoxin B (SEB) in non-refrigerated raw chicken livers. Int. J. Food Microbiol. 2020, 328, 108669. [Google Scholar] [CrossRef]
- Sergelidis, D.; Angelidis, A.S. Methicillin-resistant Staphylococcus aureus: A controversial food-borne pathogen. Lett. Appl. Microbiol. 2017, 64, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.T.; Dalsgaard, A.; Andersen, P.S.; Nguyen, H.M.; Ta, Y.T.; Nguyen, T.T. Large-Scale Staphylococcus aureus foodborne disease poisoning outbreak among primary school children. Microbiol. Res. 2021, 12, 43–52. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Beshiru, A.; Igbinosa, I.H.; Ogofure, A.G.; Uwhuba, K.E. Prevalence and characterization of food-borne vibrio parahaemolyticus from african salad in southern Nigeria. Front. Microbiol. 2021, 12, 632266. [Google Scholar] [CrossRef] [PubMed]
- Beshiru, A.; Okoh, A.I.; Igbinosa, E.O. Processed ready-to-eat (RTE) foods sold in Yenagoa Nigeria were colonized by diarrheagenic Escherichia coli which constitute a probable hazard to human health. PLoS ONE 2022, 17, e0266059. [Google Scholar] [CrossRef]
- Zehra, A.; Gulzar, M.; Singh, R.; Kaur, S.; Gill, J.P.S. Prevalence, multidrug resistance and molecular typing of methicillin-resistant Staphylococcus aureus (MRSA) in retail meat from Punjab, India. J. Glob. Antimicrob. Resist. 2019, 16, 152–158. [Google Scholar] [CrossRef]
- Tegegne, H.A.; Koláčková, I.; Florianová, M.; Gelbíčová, T.; Madec, J.-Y.; Haenni, M.; Karpíšková, R. Detection and molecular characterisation of methicillin-resistant Staphylococcus aureus isolated from raw meat in the retail market. J. Glob. Antimicrob. Resist. 2021, 26, 233–238. [Google Scholar] [CrossRef]
- Fisher, E.L.; Otto, M.; Cheung, G.Y.C. Basis of Virulence in Enterotoxin-Mediated Staphylococcal Food Poisoning. Front. Microbiol. 2018, 9, 436. [Google Scholar] [CrossRef]
- Igbinosa, E.O.; Beshiru, A.; Odjadjare, E.E.O. Diversity, antimicrobial characterization and biofilm formation of Enterococci isolated from aquaculture and slaughterhouse sources in Benin City, Nigeria. Ife J. Sci. 2020, 22, 51–63. [Google Scholar] [CrossRef]
- Abbasi, K.; Tajbakhsh, E.; Momtaz, H. Antimicrobial resistance, virulence genes, and biofilm formation in Staphylococcus aureus strains isolated from meat and meat products. J. Food Saf. 2021, 41, e12933. [Google Scholar] [CrossRef]
- Beshiru, A.; Igbinosa, I.H.; Igbinosa, E.O. Characterization of enterotoxigenic Staphylococcus aureus from ready-to-eat seafood (RTES). LWT 2021, 135, 110042. [Google Scholar] [CrossRef]
- Nemati, M.; Hermans, K.; Devriese, L.A.; Maes, D.; Haesebrouck, F. Screening of genes encoding adhesion factors and biofilm formation in Staphylococcus aureus isolates from poultry. Avian Pathol. 2009, 38, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Lachance, J.; Arsenault, J.; Usongo, V.; Parent, É.; Labrie, J.; Jacques, M.; Malouin, F.; Archambault, M. Prevalence and characteristics of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) isolated from chicken meat in the province of Quebec, Canada. PLoS ONE 2020, 15, e0227183. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance Surveillance in Europe 2014. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2014 (accessed on 10 July 2023).
- Kourtis, A.P.; Hatfield, K.; Baggs, J.; Mu, Y.; See, I.; Epson, E.; Nadle, J.; Kainer, M.A.; Dumyati, G.; Petit, S.; et al. Vital signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-susceptible staphylococcus aureus bloodstream infections—United States. MMWR Morb. Mortal Wkly. Rep. 2019, 68, 214–219. [Google Scholar] [CrossRef]
- Kim, Y.B.; Seo, K.W.; Jeon, H.Y.; Lim, S.-K.; Lee, Y.J. Characteristics of the antimicrobial resistance of Staphylococcus aureus isolated from chicken meat produced by different integrated broiler operations in Korea. Poult. Sci. 2018, 97, 962–969. [Google Scholar] [CrossRef]
- Abunna, F.; Adugna, B.; Tufa, T.B.; Ayana, D.; Gutema, F.D.; Waktole, H.; Regassa, F.; Abdi, R.D. Detection and Antimicrobial Resistance of Staphylococcus Species from chicken, chicken litter, and humans in Addis Ababa, Ethiopia. Vet. Med. Int. 2022, 2022, 9084334. [Google Scholar] [CrossRef]
- Rafiq, K.; Islam, M.R.; Siddiky, N.A.; Samad, M.A.; Chowdhury, S.; Hossain, K.M.M.; Rume, F.I.; Hossain, M.K.; Mahbub-E-Elahi, A.; Ali, M.Z.; et al. Antimicrobial Resistance profile of common foodborne pathogens recovered from livestock and poultry in Bangladesh. Antibiotics 2022, 11, 1551. [Google Scholar] [CrossRef]
- Miranda, J.M.; Vázquez, B.I.; Fente, C.A.; Calo-Mata, P.; Cepeda, A.; Franco, C.M. Comparison of antimicrobial resistance in Escherichia coli, Staphylococcus aureus, and Listeria monocytogenes strains isolated from organic and conventional poultry meat. J. Food Prot. 2008, 71, 2537–2542. [Google Scholar] [CrossRef]
- Boamah, V.E.; Agyare, C.; Odoi, H.; Adu, F.; Gbedema, S.Y.; Dalsgaard, A. Prevalence and antibiotic resistance of coagulase-negative Staphylococci isolated from poultry farms in three regions of Ghana. Infect. Drug. Resist. 2017, 10, 175–183. [Google Scholar] [CrossRef]
- Sonola, V.S.; Misinzo, G.; Matee, M.I. Occurrence of Multidrug-Resistant Staphylococcus aureus among Humans, Rodents, Chickens, and Household Soils in Karatu, Northern Tanzania. Int. J. Environ. Res. Public Health 2021, 18, 8496. [Google Scholar] [CrossRef]
- Mkize, N.; Zishiri, O.T.; Mukaratirwa, S. Genetic characterisation of antimicrobial resistance and virulence genes in Staphylococcus aureus isolated from commercial broiler chickens in the Durban metropolitan area, South Africa. J. S. Afr. Vet. Assoc. 2017, 88, e1–e7. [Google Scholar] [CrossRef]
- Benrabia, I.; Hamdi, T.M.; Shehata, A.A.; Neubauer, H.; Wareth, G. Methicillin-resistant staphylococcus Aureus (MRSA) in poultry species in Algeria: Long-term study on prevalence and antimicrobial resistance. Vet. Sci. 2020, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yeh, K.-S.; Liu, H.-T.; Lin, J.-H. Staphylococcus aureus isolated from pork and chicken carcasses in Taiwan: Prevalence and antimicrobial susceptibility. J. Food Prot. 2009, 72, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Moawad, A.A.; Hotzel, H.; Awad, O.; Roesler, U.; Hafez, H.M.; Tomaso, H.; Neubauer, H.; El-Adawy, H. Evolution of antibiotic resistance of coagulase-negative staphylococci isolated from healthy turkeys in Egypt: First report of linezolid resistance. Microorganisms 2019, 7, 476. [Google Scholar] [CrossRef]
- Nemeghaire, S.; Argudín, M.A.; Haesebrouck, F.; Butaye, P. Molecular epidemiology of methicillin-resistant Staphylococcus sciuri in healthy chickens. Vet. Microbiol. 2014, 171, 357–363. [Google Scholar] [CrossRef]
- Barnácz, F.; Kerek, Á.; Csirmaz, B.; Román, I.L.; Gál, C.; Horváth, Á.; Hajduk, E.; Szabó, Á.; Jerzsele, Á.; Kovács, L. The status of antimicrobial resistance in domestic poultry with different breeding purposes in Hungary between 2022–2023. Magy. Állatorvosok Lapja 2024, 146, 339–356. [Google Scholar] [CrossRef]
- Nazarchuk, O.A.; Nahaichuk, V.I.; Osadchuk, N.I.; Dmytriiev, D.V.; Dmytriiev, K.D.; Turzhanska, O.S. Prognostic parameters of the susceptibility of Staphylococcus spp. to aminoglycosides and doxycycline. Wiad. Lek. 2020, 73, 1615–1619. [Google Scholar] [CrossRef]
- Benmazouz, I.; Kövér, L.; Kardos, G. The rise of Antimicrobial resistance in wild birds: Potential AMR sources and wild birds as AMR reservoirs and disseminators: Literature review. Magy. Állatorvosok Lapja 2024, 146, 91–105. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- R Core Team R. A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org (accessed on 15 January 2025).
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rules. Stat. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple comparisons among means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Sibson, R. SLINK: An optimally efficient algorithm for the single-link cluster method. Comput. J. 1973, 16, 30–34. [Google Scholar] [CrossRef]



| Antibiotics | Respiratory–Cloaca Comparation |
|---|---|
| p-Values | |
| Doxycycline | >0.0001 * |
| Enrofloxacin | 0.4387 |
| 1 Potentiated sulfonamide | 0.5184 |
| Vancomycin | 0.8005 |
| Amoxicillin | 0.6334 |
| 2 Amoxicillin–clavulanic acid | 0.6437 |
| Imipenem | 0.6401 |
| Tylosin | 0.5466 |
| Tiamulin | 0.1318 |
| Antibiotics | Laying–Broiler | Laying–Breeding | Broiler–Breeding |
|---|---|---|---|
| p-Values | |||
| Doxycycline | 0.0336 * | 0.8927 | 0.0730 |
| Enrofloxacin | 0.2883 | 0.0402 * | 0.6045 |
| 1 Potentiated sulfonamide | 0.0074 * | 0.0109 * | 0.9978 |
| Vancomycin | 0.5427 | 0.7159 | 0.2272 |
| Amoxicillin | 0.8889 | 0.1360 | 0.1438 |
| 2 Amoxicillin–clavulanic acid | 0.3540 | 0.6012 | 0.7879 |
| Imipenem | 0.0607 | 0.6122 | 0.0233 * |
| Tylosin | 0.0077 * | 0.7487 | 0.0291 * |
| Tiamulin | 0.8055 | 0.2606 | 0.1805 |
| Antibiotics | 3 Young–4 Adult Comparation |
|---|---|
| p-Values | |
| Doxycycline | 0.0058 * |
| Enrofloxacin | 0.6738 |
| 1 Potentiated sulfonamide | 0.0860 |
| Vancomycin | 0.3538 |
| Amoxicillin | 0.4141 |
| 2 Amoxicillin–clavulanic acid | 0.4741 |
| Imipenem | 0.0124 * |
| Tylosin | 0.0026 * |
| Tiamulin | 0.4009 |
| Antibiotics | Small–Medium | Small–Large | Medium–Large |
|---|---|---|---|
| p-Values | |||
| Doxycycline | 0.1310 | 0.5647 | 0.0196 * |
| Enrofloxacin | 0.3589 | >0.0001 * | 0.0597 |
| 1 Potentiated sulfonamide | 0.6906 | 0.0019 | 0.0006 * |
| Vancomycin | 0.0012 * | >0.0001 * | 0.0635 |
| Amoxicillin | 0.0392 * | >0.0001 * | 0.4693 |
| 2 Amoxicillin–clavulanic acid | 0.0552 | >0.0001 * | 0.1816 |
| Imipenem | 0.2127 | 0.3035 | 0.6089 |
| Tylosin | 0.1331 | 0.4267 | 0.3763 |
| Tiamulin | >0.0001 * | >0.0001 * | 0.2507 |
| Antibiotic | 1 BP * | 0.001 | 0.002 | 0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1024 | MIC50 | MIC90 | 2 ECOFF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µg/mL | µg/mL | ||||||||||||||||||||||||
| Amoxicillin | 0.5 | 1 | 1 | 1 | 5 | 2 | 9 | 18 | 17 | 40 | 51 | 26 | 10 | 18 | 10 | 3 | 1 | 1 | 1 | 3 | 6 | 3 | 0.5 | 8 | 0.5 |
| 0.4% | 0.4% | 0.4% | 2.2% | 0.9% | 4.0% | 7.9% | 7.5% | 17.6% | 22.5% | 11.5% | 4.4% | 7.9% | 4.4% | 1.3% | 0.4% | 0.4% | 0.4% | 1.3% | 2.6% | 1.3% | |||||
| Doxycycline | 0.5 | 2 | 1 | 4 | 12 | 22 | 0 | 7 | 2 | 8 | 18 | 48 | 19 | 16 | 14 | 10 | 14 | 20 | 8 | 0 | 0 | 2 | 1 | 64 | 0.5 |
| 0.9% | 0.4% | 1.8% | 5.3% | 9.7% | 0.0% | 3.1% | 0.9% | 3.5% | 7.9% | 21.1% | 8.4% | 7.0% | 6.2% | 4.4% | 6.2% | 8.8% | 3.5% | 0.0% | 0.0% | 0.9% | |||||
| 3 Amoxicillin–clavulanic acid | 1 | 1 | 3 | 8 | 13 | 13 | 19 | 53 | 36 | 31 | 7 | 16 | 16 | 2 | 7 | 2 | 0.5 | 4 | 0.5 | ||||||
| 0.4% | 1.3% | 3.5% | 5.7% | 5.7% | 8.4% | 23.3% | 15.9% | 13.7% | 3.1% | 7.0% | 7.0% | 0.9% | 3.1% | 0.9% | |||||||||||
| Tiamulin | 4 | 2 | 3 | 1 | 27 | 6 | 14 | 10 | 10 | 44 | 68 | 16 | 9 | 11 | 6 | 32 | 256 | 2 | |||||||
| 0.9% | 1.3% | 0.4% | 11.9% | 2.6% | 6.2% | 4.4% | 4.4% | 19.4% | 30.0% | 7.0% | 4.0% | 4.8% | 2.6% | ||||||||||||
| Enrofloxacin | 4 | 4 | 5 | 19 | 9 | 18 | 12 | 35 | 24 | 14 | 25 | 21 | 26 | 11 | 3 | 1 | 4 | 64 | 0.5 | ||||||
| 1.8% | 2.2% | 8.4% | 4.0% | 7.9% | 5.3% | 15.4% | 10.6% | 6.2% | 11.0% | 9.3% | 11.5% | 4.8% | 1.3% | 0.4% | |||||||||||
| 4 Potentiated sulfonamide | 4 | 1 | 18 | 20 | 31 | 39 | 20 | 24 | 6 | 9 | 14 | 45 | 2 | 64 | 0.25 | ||||||||||
| 0.4% | 7.9% | 8.8% | 13.7% | 17.2% | 8.8% | 10.6% | 2.6% | 4.0% | 6.2% | 19.8% | |||||||||||||||
| Imipenem | 8 | 4 | 11 | 20 | 33 | 45 | 34 | 27 | 16 | 7 | 22 | 8 | 0.25 | 4 | 0.125 | ||||||||||
| 1.8% | 4.8% | 8.8% | 14.5% | 19.8% | 15.0% | 11.9% | 7.0% | 3.1% | 9.7% | 3.5% | |||||||||||||||
| Vancomycin | 32 | 1 | 0 | 1 | 0 | 2 | 3 | 7 | 10 | 49 | 41 | 59 | 25 | 6 | 1 | 1 | 0 | 1 | 0 | 12 | 6 | 2 | 0.5 | 4 | 2 |
| 0.4% | 0.0% | 0.4% | 0.0% | 0.9% | 1.3% | 3.1% | 4.4% | 21.6% | 18.1% | 26.0% | 11.0% | 2.6% | 0.4% | 0.4% | 0.0% | 0.4% | 0.0% | 5.3% | 2.6% | 0.9% | |||||
| Tylosin | 64 | 1 | 0 | 1 | 1 | 0 | 6 | 6 | 2 | 5 | 16 | 88 | 11 | 10 | 13 | 0 | 4 | 2 | 2 | 9 | 14 | 36 | 1 | 1024 | 2 |
| 0.4% | 0.0% | 0.4% | 0.4% | 0.0% | 2.6% | 2.6% | 0.9% | 2.2% | 7.0% | 38.8% | 4.8% | 4.4% | 5.7% | 0.0% | 1.8% | 0.9% | 0.9% | 4.0% | 6.2% | 15.9% | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, Á.; Jerzsele, Á.; Kovács, L.; Kerek, Á. Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Chickens in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics 2025, 14, 103. https://doi.org/10.3390/antibiotics14010103
Szabó Á, Jerzsele Á, Kovács L, Kerek Á. Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Chickens in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics. 2025; 14(1):103. https://doi.org/10.3390/antibiotics14010103
Chicago/Turabian StyleSzabó, Ábel, Ákos Jerzsele, László Kovács, and Ádám Kerek. 2025. "Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Chickens in Hungarian Poultry Farms Between 2022 and 2023" Antibiotics 14, no. 1: 103. https://doi.org/10.3390/antibiotics14010103
APA StyleSzabó, Á., Jerzsele, Á., Kovács, L., & Kerek, Á. (2025). Antimicrobial Susceptibility Profiles of Commensal Staphylococcus spp. Isolates from Chickens in Hungarian Poultry Farms Between 2022 and 2023. Antibiotics, 14(1), 103. https://doi.org/10.3390/antibiotics14010103







