Establishment of an Antimicrobial Stewardship Program to Spare the Use of Oral Fluoroquinolones for Acute Uncomplicated Cystitis in Outpatients
Abstract
1. Introduction
2. Results
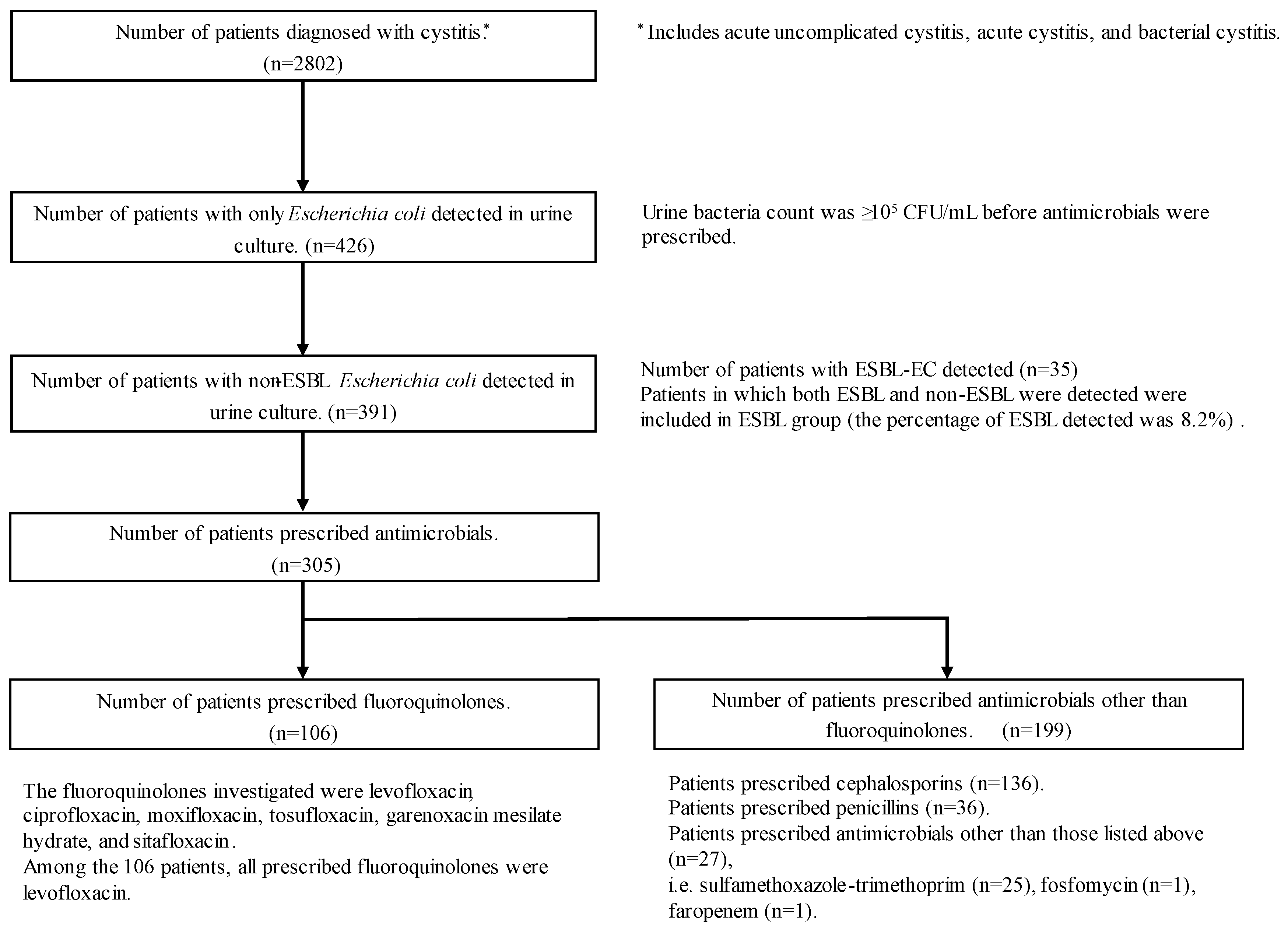
2.1. Study Patients
2.2. Antibiogram Trends for EC
2.3. Trends in Antimicrobial Usage by Each Clinical Department
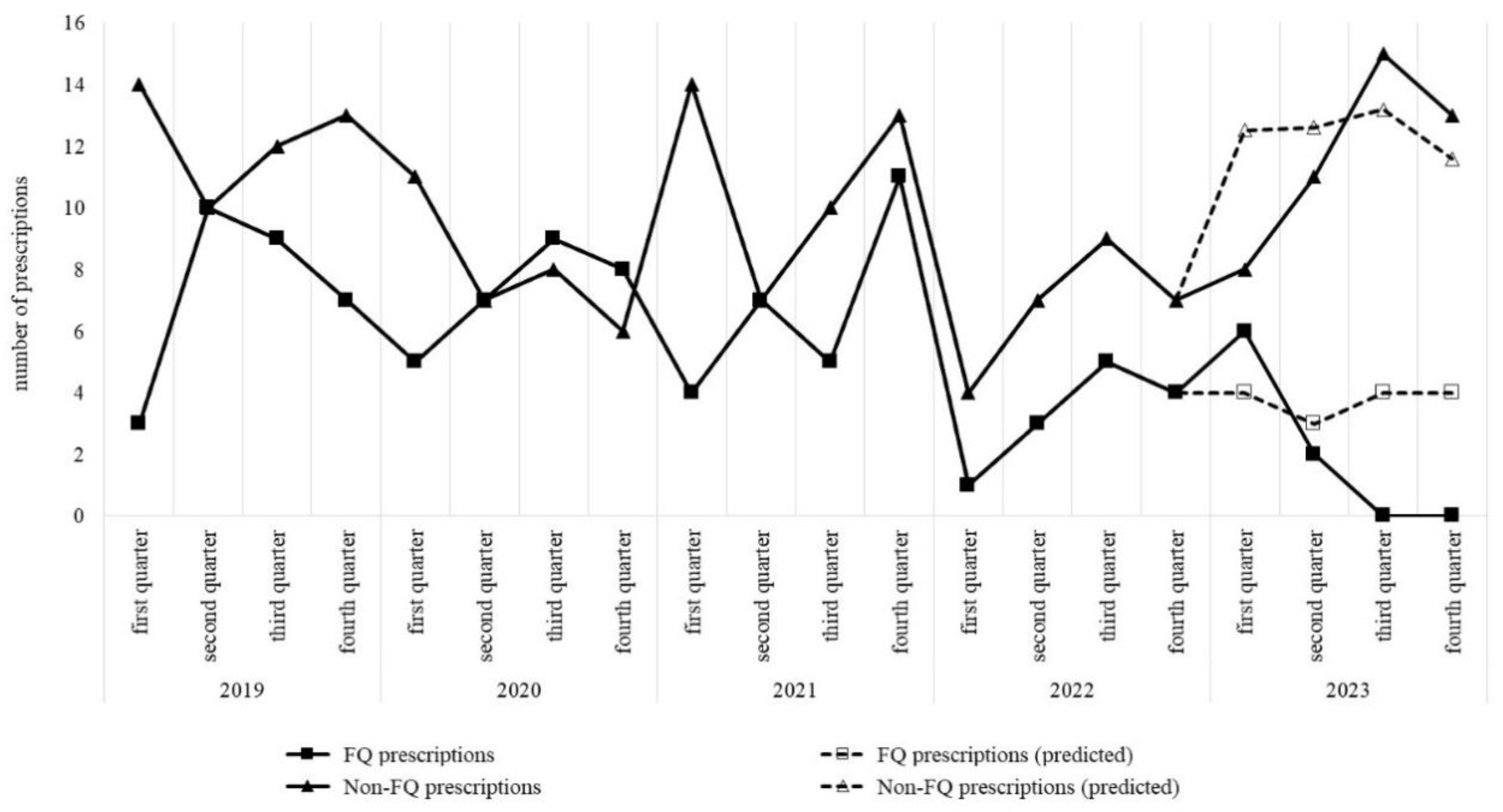
2.4. Changes in Antimicrobial Treatment for AUC
2.5. Support and Intervention for Antimicrobial Treatment for AUC through Cooperation between AST and Clinical Pharmacists
2.6. Treatment Results of Ceph and FQ for AUC
3. Discussion
4. Materials and Methods
4.1. Study Design and Setting
4.2. Performing Antimicrobial Susceptibility Tests and Creating Antibiograms
4.3. Antimicrobial Usage by Clinical Department
4.4. Antimicrobials Recommended for AUC Caused by EC
4.5. Collaborative Support System between Clinical Pharmacists and AST
4.6. Evaluation of the Therapeutic Efficacy of Ceph and FQ
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katie, J.A.; Robert, J.K.; Neil, O. Mechanism of quinolone action and resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar]
- Rodvold, K.A.; Neuhauser, M. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Pharmacotherapy 2001, 21, 233S–252S. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Matsuzaki, K.; Kishi, N.; Koyama, H.; Hasegawa, M.; Ikeda, F.; Matsumoto, T.; Yamaguchi, H.; Okutani, Y. In vitro activity of sitafloxacin against clinical isolates in 2012. Jpn. J. Antibiot. 2013, 66, 311–330. [Google Scholar] [PubMed]
- Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance Chaired by Jim O’Neill December 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 7 July 2024).
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2016–2020. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000138942.pdf (accessed on 7 July 2024).
- The Government of Japan. National Action Plan on Antimicrobial Resistance (AMR) 2023–2027. Available online: https://www.mhlw.go.jp/content/10900000/001096228.pdf (accessed on 7 July 2024).
- Harrington, R.D.; Hooton, T.M. Urinary tract infection risk factors and gender. J. Gend. Specif. Med. 2000, 3, 27–34. [Google Scholar]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- Saperston, K.N.; Shapiro, D.J.; Hersh, A.L.; Copp, H.L. A comparison of inpatient versus outpatient resistance patterns of pediatric urinary tract infection. J. Urol. 2014, 191, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Kim, J.H.; Lee, J.Y.; Ku, N.S.; Lee, H.S.; Park, J.Y.; Kim, J.W.; Kim, K.J.; Cho, K.S. Reappraisal of the treatment duration of antibiotic regimens for acute uncomplicated cystitis in adult women: A systematic review and network meta-analysis of 61 randomised clinical trials. Lancet Infect. Dis. 2020, 20, 1080–1088. [Google Scholar] [CrossRef]
- Muraki, Y.; Yagi, T.; Tsuji, Y.; Nishimura, N.; Tanabe, M.; Niwa, T.; Watanabe, T.; Fujimoto, S.; Takayama, K.; Murakami, N.; et al. Japanese antimicrobial consumption surveillance: First report on oral and parenteral antimicrobial consumption in Japan (2009–2013). J. Glob. Antimicrob. Resist. 2016, 7, 19–23. [Google Scholar] [CrossRef]
- Eudy, J.L.; Pallotta, A.M.; Neuner, E.A.; Brummel, G.L.; Postelnick, M.J.; Schulz, L.T.; Spivak, E.S.; Wrenn, R.H. Antimicrobial Stewardship Practice in the Ambulatory Setting From a National Cohort. Open Forum Infect. Dis. 2020, 7, ofaa513. [Google Scholar] [CrossRef]
- Wada, K.; Tsuboi, I.; Takahashi, S.; Yasuda, M.; Miyazaki, J.; Kobayashi, K.; Matsumoto, M.; Hayami, H.; Yamamoto, S.; Kiyota, H.; et al. Third nationwide surveillance of bacterial pathogens in patients with acute uncomplicated cystitis conducted by the Japanese surveillance committee during 2020 and 2021: Antimicrobial susceptibility of Escherichia coli, Klebsiella pneumoniae, and Staphylococcus saprophyticus. J. Infect. Chemother. 2024, 30, 277–285. [Google Scholar] [PubMed]
- Sonia, K.M.; Lauren, B.B.; David, S.; Lynn, Z. Mechanisms accounting for fluoroquinolone resistance in Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 2009, 53, 235–241. [Google Scholar]
- Chong, Y.; Ito, Y.; Kamimura, T. Genetic evolution and clinical impact in extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect. Genet. Evol. 2011, 11, 1499–1504. [Google Scholar] [CrossRef]
- Edward, R.B.; Annie, M.J.; Peter, M.H. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar]
- Wu, H.H.; Liu, H.Y.; Lin, Y.C.; Hsueh, P.R.; Lee, Y.J. Correlation between levofloxacin consumption and the incidence of nosocomial infections due to fluoroquinolone-resistant Escherichia coli. J. Microbiol. Immunol. Infect. 2016, 49, 424–429. [Google Scholar] [CrossRef]
- Kim, J.Y.; Yum, Y.; Joo, H.J.; An, H.; Yoon, Y.K.; Kim, J.H.; Sohn, J.W. Impact of antibiotic usage on extended-spectrum β-lactamase producing Escherichia coli prevalence. Sci. Rep. 2021, 11, 13024. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; Duin, D.V.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 2022, 75, 187–212. [Google Scholar] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; Duin, D.V.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase-Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- Kato, T.; Tanaka, I.; Seyama, Y.; Sekikawa, R.; Suzuki, S.; Nagasawa, M.; Hino, S. The effectiveness of prescription support and treatment reporting system on the appropriate usage of oral third-generation cephalosporins. J. Infect. Chemother. 2021, 27, 419–423. [Google Scholar] [CrossRef]
- Tuberculosis and Infectious Diseases Control Division. Health Service Bureau, Ministry of Health, Labour and Welfare. Guidance for Appropriate Use of Antimicrobial Agents, 3rd ed. Available online: https://www.mhlw.go.jp/content/10900000/001265278.pdf (accessed on 7 July 2024).
- Hayden, D.A.; White, B.P.; Neely, S.; Bennett, K.K. Impact of Fluoroquinolone Susceptibility Suppression on Discharge Prescribing for Acute Uncomplicated Cystitis. Open Forum Infect. Dis. 2023, 10, ofad459. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gupta, A.; Petty, L.A.; Malani, A.N.; Osterholzer, D.; Patel, P.K.; Younas, M.; Bernstein, S.J.; Burdick, S.; Ratz, D.; et al. A Statewide Quality Initiative to Reduce Unnecessary Antibiotic Treatment of Asymptomatic Bacteriuria. JAMA Intern. Med. 2023, 183, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Hooton, T.M.; Vecchio, M.; Iroz, A.; Tack, I.; Dornic, Q.; Seksek, I.; Lotan, Y. Effect of Increased Daily Water Intake in Premenopausal Women With Recurrent Urinary Tract Infections: A Randomized Clinical Trial. JAMA Intern. Med. 2018, 178, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Ishikawa, K.; Hayami, H.; Nakamura, T.; Miyairi, I.; Hoshino, T.; Hasui, M.; Tanaka, K.; Kiyota, H.; Arakawa, S. JAID/JSC Guidelines for Clinical Management of Infectious Disease 2015—Urinary tract infection/male genital infection. J. Infect. Chemother. 2017, 23, 733–751. [Google Scholar] [CrossRef] [PubMed]
- WHO. Antibiotic Categorization. Available online: https://aware.essentialmeds.org/groups (accessed on 7 July 2024).
- Mary, F.W.; Piotr, K.; Brandon, M.Z.; William, E.T.; Michael, S.; Robert, A.W. Development of a clinical data Warehouse for hospital infection control. J. Am. Med. Inform. Assoc. 2003, 10, 454–462. [Google Scholar]
- Yasuda, M.; Muratani, T.; Ishikawa, K.; Kiyota, H.; Sakata, H.; Shigemura, K.; Takahashi, S.; Hamasuna, R.; Hayami, H.; Mikamo, H.; et al. Japanese guideline for clinical research of antimicrobial agents on urogenital infections: Second edition. J. Infect. Chemother. 2016, 22, 651–661. [Google Scholar] [CrossRef]
- CLSI Document M39-A4; Analysis and Presentation of Cumulative Antimicrobial Susceptibility Test Data; Approved Guideline–Fourth Edition. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2014.
- AMR Clinical Reference Center. Guidelines for Create Antibiogram. Available online: https://amr.ncgm.go.jp/pdf/201904_antibaiogram_guideline.pdf (accessed on 7 July 2024).
| Fluoroquinolones (n = 106) | Other Antimicrobials a (n = 199) | p-Value | |
|---|---|---|---|
| Female (n, %) | 53 (50%) | 152 (76.4%) | <0.01 b |
| Year (median, IQR) | 66 (49–79) | 67 (47–77) | 0.424 |
| ≤49 years | 14 | 47 | 0.245 |
| ≥50 years | 39 | 105 | |
| Antimicrobials duration (median, IQR) | 3 (3–5) | 5 (3–7) | <0.01 b |
| 2019 | 2020 | 2021 | 2022 | 2023 | p-Value | |
|---|---|---|---|---|---|---|
| Number of detected strains | 867 | 800 | 786 | 923 | 994 | 0.35 |
| Antimicrobials | ||||||
| LVFX | 83.5 | 81.9 | 81.9 | 80.2 | 79.4 | 0.162 |
| CCL | 91.2 | 92.1 | 92.6 | 94.1 | 93.3 | 0.072 |
| AMPC/CVA | 73.6 | 73.8 | 73.7 | 76.2 | 76.9 | 0.072 |
| 2019 | 2020 | 2021 | 2022 | 2023 | Correlation Coefficient a | p-Value | |
|---|---|---|---|---|---|---|---|
| Urology | |||||||
| FQ group | 1.690 | 1.860 | 1.841 | 0.948 | 0.541 | −0.363 | <0.01 b |
| Non-FQ group | 1.623 | 1.085 | 1.761 | 0.862 | 1.805 | 0.064 | 0.626 |
| Departments other than urology | |||||||
| FQ group | 0.013 | 0.018 | 0.014 | 0.007 | 0.007 | −0.087 | 0.507 |
| Non-FQ group | 0.081 | 0.064 | 0.075 | 0.061 | 0.100 | 0.077 | 0.56 |
| 2019 (n = 78) | 2020 (n = 61) | 2021 (n = 71) | 2022 (n = 40) | 2023 (n = 55) | Correlation Coefficient c | p-Value | |
|---|---|---|---|---|---|---|---|
| Fluoroquinolones | |||||||
| Number of prescriptions | 29 | 29 | 27 | 13 | 8 | −0.157 | <0.01 d |
| Days of administration (median, IQR) | 3 (3–7) | 4 (3–7) | 5 (3–7) | 3 (3–7) | 3 (3–5) | −0.039 | 0.690 |
| Cephalosporins | |||||||
| Number of prescriptions | 33 | 21 | 28 | 14 | 40 | 0.156 | <0.01 d |
| Days of administration (median, IQR) | 7 (5–7) | 7 (3–7) | 7 (5–7) | 7 (5–7) | 5 (3–7) | −0.149 | 0.084 |
| Penicillin | |||||||
| Number of prescriptions | 10 | 6 | 11 | 6 | 3 | −0.037 | 0.515 |
| Days of administration (median, IQR) | 7 (5–7) | 3 (2–5) | 6 (3–7) | 4 (3–5) | 5 (5–7) | −0.131 | 0.446 |
| Other antimicrobials | |||||||
| Number of prescriptions | 6 | 5 | 5 | 7 | 4 | 0.034 | 0.557 |
| Days of administration (median, IQR) | 3 (3–5) | 3 (3–14) | 5 (3–5) | 5 (3–6) | 6 (4–7) | 0.186 | 0.352 |
| Evaluation of Treatment | |||
|---|---|---|---|
| Antimicrobials | Ineffective | Effective | p-Value |
| Cephs | 14 | 100 | <0.01 e |
| FQs | 27 | 59 | |
| Variables | Odds ratio (95% CI) | p-Value | |
| Antimicrobials | |||
| FQs | Reference | ||
| CCL CEX | 0.28 (0.088–0.882) 0.33 (0.104–1.070) | 0.030 e 0.066 | |
| Resistant to CCL | 5.01 (1.600–15.70) | <0.01 e | |
| Resistant to LVFX | 1.64 (0.660–4.090) | 0.286 | |
| Duration (day) | 0.95 (0.815–1.110) | 0.488 | |
| Age | 1.01 (0.985–1.030) | 0.625 | |
| Sex | |||
| Male | Reference | ||
| Female | 0.51 (0.215–1.190) | 0.120 | |
| Clinical department | |||
| other than urology | Reference | ||
| Urology | 1.03 (0.384–2.760) | 0.953 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Nagasawa, M.; Tanaka, I.; Seyama, Y.; Sekikawa, R.; Yamada, S.; Ishikawa, E.; Kitajima, K. Establishment of an Antimicrobial Stewardship Program to Spare the Use of Oral Fluoroquinolones for Acute Uncomplicated Cystitis in Outpatients. Antibiotics 2024, 13, 886. https://doi.org/10.3390/antibiotics13090886
Kato T, Nagasawa M, Tanaka I, Seyama Y, Sekikawa R, Yamada S, Ishikawa E, Kitajima K. Establishment of an Antimicrobial Stewardship Program to Spare the Use of Oral Fluoroquinolones for Acute Uncomplicated Cystitis in Outpatients. Antibiotics. 2024; 13(9):886. https://doi.org/10.3390/antibiotics13090886
Chicago/Turabian StyleKato, Tomoyuki, Masayuki Nagasawa, Ippei Tanaka, Yuka Seyama, Reiko Sekikawa, Shiori Yamada, Eriko Ishikawa, and Kento Kitajima. 2024. "Establishment of an Antimicrobial Stewardship Program to Spare the Use of Oral Fluoroquinolones for Acute Uncomplicated Cystitis in Outpatients" Antibiotics 13, no. 9: 886. https://doi.org/10.3390/antibiotics13090886
APA StyleKato, T., Nagasawa, M., Tanaka, I., Seyama, Y., Sekikawa, R., Yamada, S., Ishikawa, E., & Kitajima, K. (2024). Establishment of an Antimicrobial Stewardship Program to Spare the Use of Oral Fluoroquinolones for Acute Uncomplicated Cystitis in Outpatients. Antibiotics, 13(9), 886. https://doi.org/10.3390/antibiotics13090886







