Towards Sustainable Antibiotic Use in Aquaculture and Antimicrobial Resistance: Participatory Experts’ Overview and Recommendations
Abstract
1. Introduction
- Preamble: Due to the lack of data and understanding of the impact of antibiotics in aquaculture at different levels, we called an expert group to fill these gaps and provide recommendations to address this challenge as concerted efforts are required by diverse actors to formulate appropriate solutions. In this context, this study presents the outcomes of a series of workshops designed to foster global collaboration and knowledge sharing on the relevant topic of antibiotic use in aquaculture. The virtual event covered various aspects of antibiotic use in aquaculture, providing valuable networking opportunities for institutions and experts worldwide.
2. Results
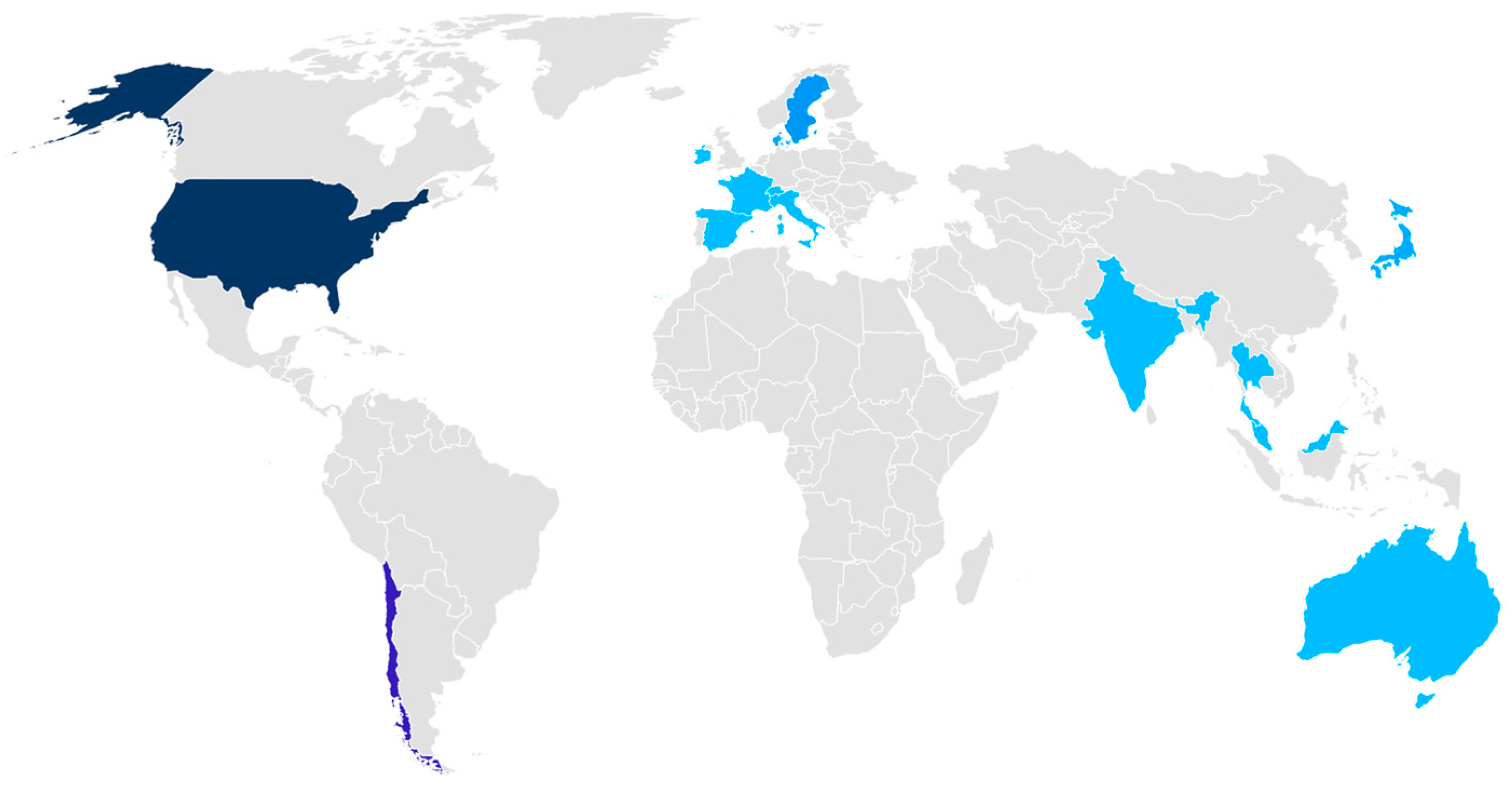
2.1. Participants’ Subsection
2.2. Workshop 1: General AMU and Ecological Impacts in Aquatic Ecosystem
2.3. Workshop 2: Socioeconomic Perspective
2.4. Workshop 3: Antibiotics in Aquaculture
2.5. Workshop 4: Methods for Determining Impact
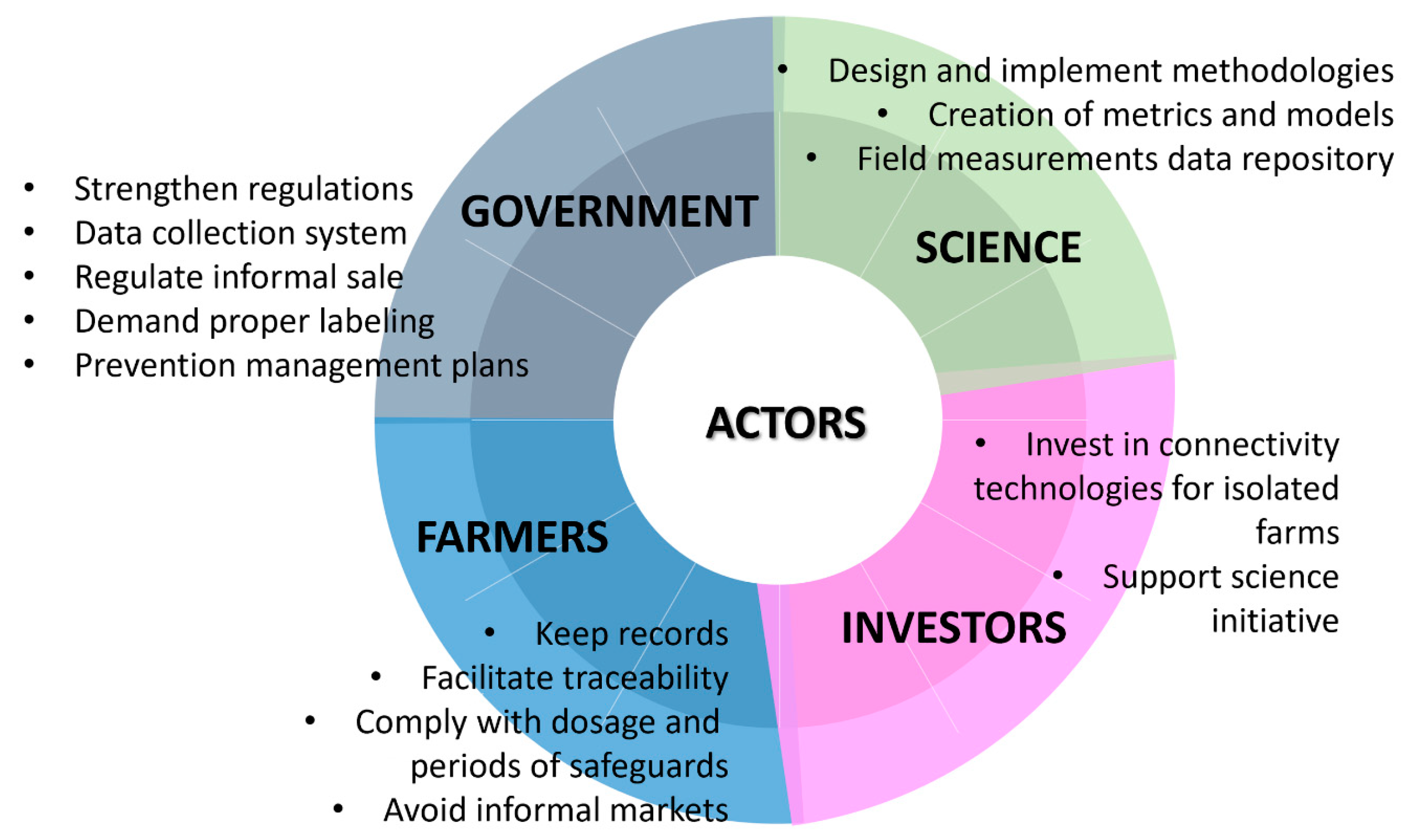
2.6. Challenges and Recommendations
3. Discussion
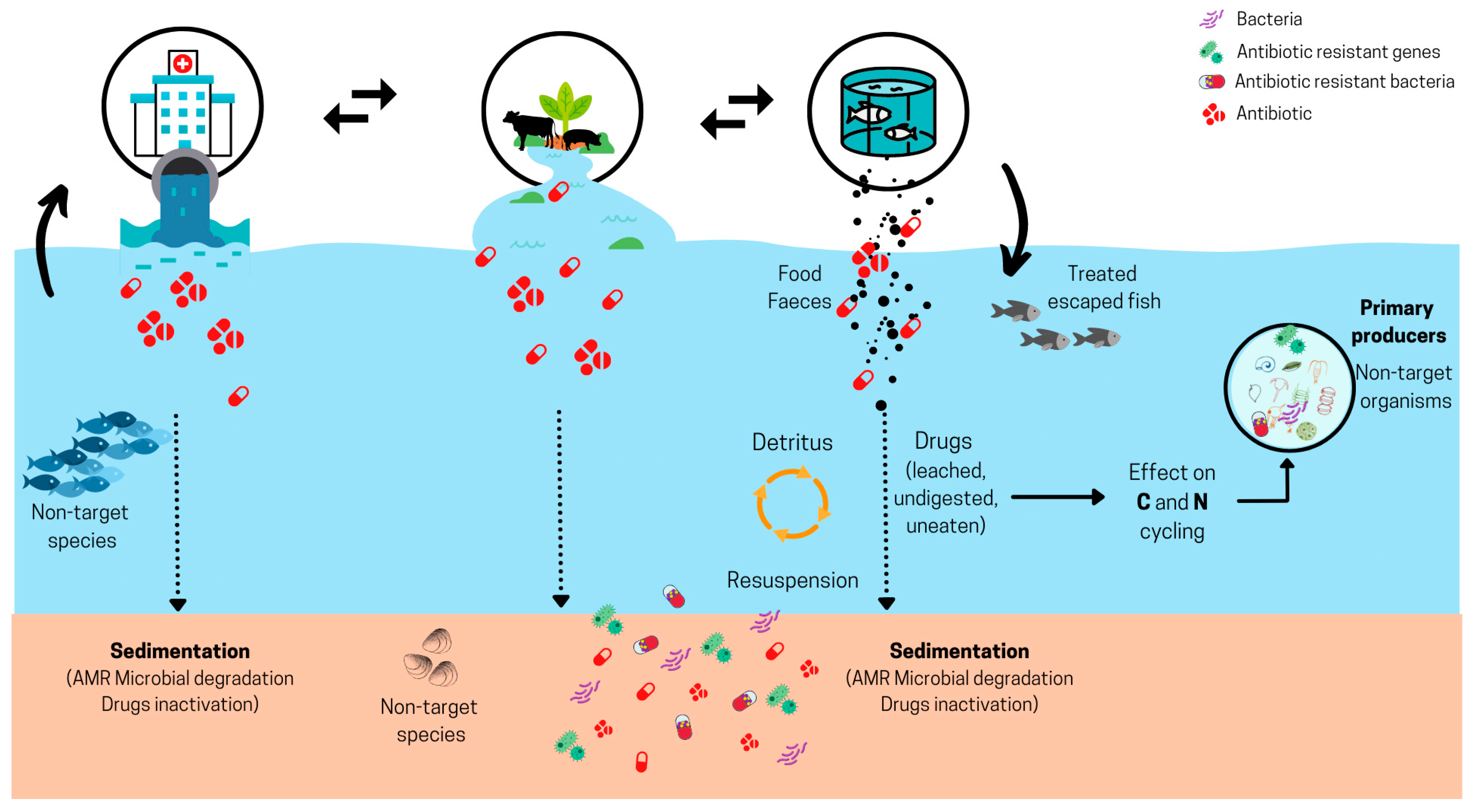
3.1. Antibiotics in Aquatic Ecosystems
3.2. General Recommendations and Actions Taken
3.3. Participatory Experts’ Initiative
4. Materials and Methods
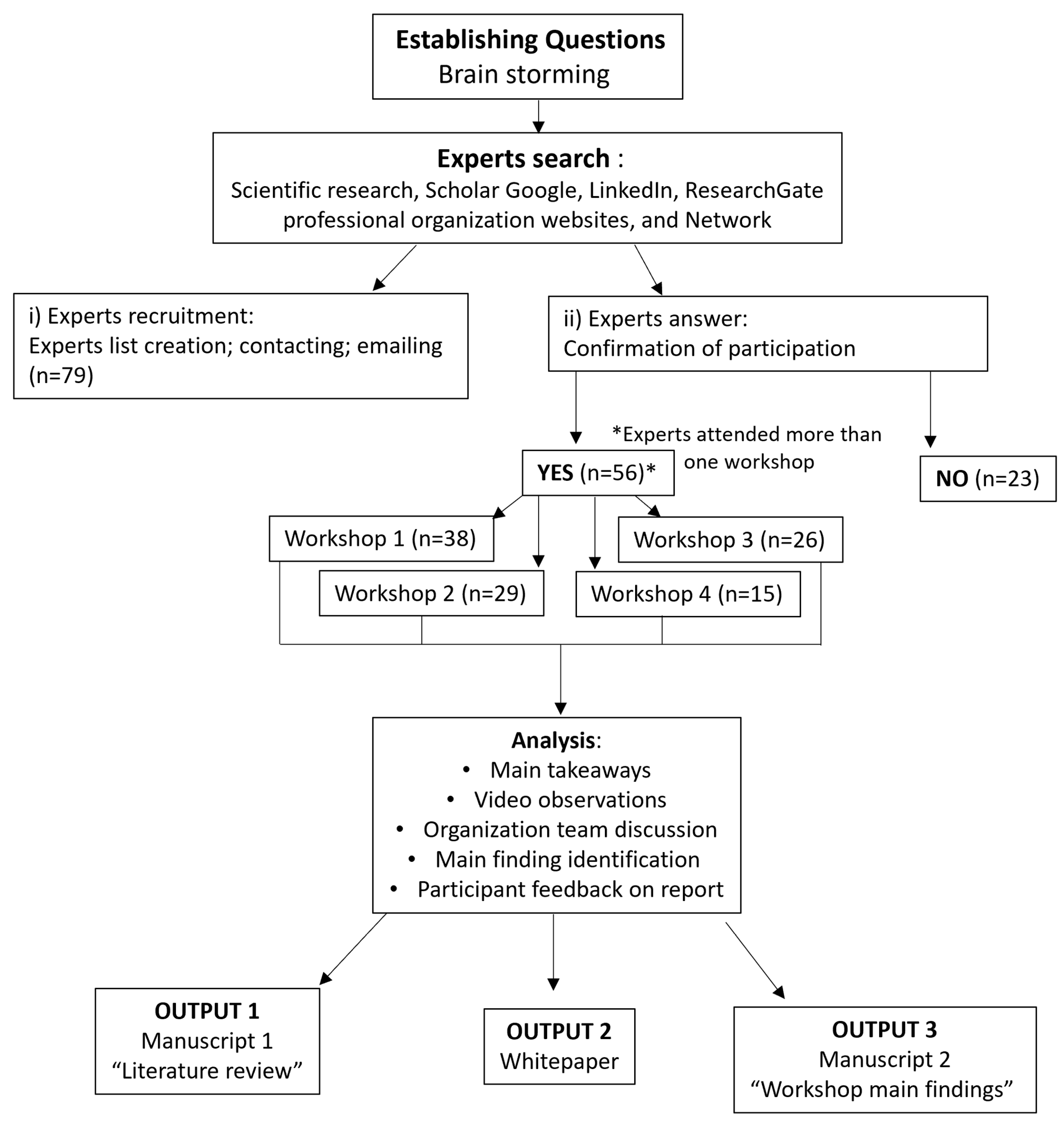
4.1. Participants
4.2. The Workshops
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. In Brief to The State of World Fisheries and Aquaculture 2022. Towards a Blue Transformation; Food and Agriculture Organization of the Nations United: Rome, Italy, 2022; ISBN 978-92-5-136367-6. [Google Scholar]
- Gephart, J.A.; Henriksson, P.J.G.; Parker, R.W.R.; Shepon, A.; Gorospe, K.D.; Bergman, K.; Eshel, G.; Golden, C.D.; Halpern, B.S.; Hornborg, S.; et al. Environmental performance of blue foods. Nature 2021, 597, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Tlusty, M.F. Animal health: The foundation for aquaculture sustainability. In Aquaculture Health Management. Design and Operation Approaches; Kibenge, F.S.B., Powell, M.D., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–15. [Google Scholar]
- Kautsky, N.; Rönnbäck, P.; Tedengren, M.; Troell, M. Ecosystem perspectives on management of disease in shrimp pond farming. Aquaculture 2000, 191, 145–161. [Google Scholar] [CrossRef]
- FAO. Impacts of Climate Change on Fisheries and Aquaculture, Synthesis of Current Knowledge, Adaptation and Mitigation Options; Food and Agriculture Organization of the Nations United: Rome, Italy, 2018; p. 654. [Google Scholar]
- Maulu, S.; Hasimuna, O.J.; Haambiya, L.H.; Monde, C.; Musuka, C.G.; Makorwa, T.H.; Munganga, B.P.; Phiri, K.J.; Nsekanabo, J.D. Climate Change Effects on Aquaculture Production: Sustainability Implications, Mitigation, and Adaptations. Front. Sustain. Food Syst. 2021, 5, 609097. [Google Scholar] [CrossRef]
- FAO. Monitoring and Survillance of Antimicrobial Resistance in Bacteria from Healthy Food Animals Intended for Consumption. Regional Antimicrobial Resistance Monitoring and Survillance Guidelines Bangkok; Food and Agriculture Organization of the Nations United: Rome, Italy, 2019; p. 84. [Google Scholar]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2019, 12, 640–663. [Google Scholar] [CrossRef]
- Coyne, L.; Arief, R.; Benigno, C.; Giang, V.N.; Huong, L.Q.; Jeamsripong, S.; Kalpravidh, W.; McGrane, J.; Padungtod, P.; Patrick, I.; et al. Characterizing antimicrobial use in the livestock sector in three South East Asian countries (Indonesia, Thailand, and Vietnam). Antibiotics 2019, 8, 33. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- Ríos, C.; Mutschke, E.; Montiel, A. Composición y Estructura de la Comunidad Macrobentónica en el Sistema Interior de Canales y Fiordos del Extremo Austral de Chile. An. Inst. Patagon. 2013, 41, 74–86. [Google Scholar] [CrossRef][Green Version]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef]
- St-Hilaire, S.; Chan, S.C.H.; Lim, K.Z.; MacKinnon, B.; Cheng, T.H.; Cheng, K.P.F.; Leung, A.C.F.; Lam, S.H.Y.; Bhardway, V.; Chan, O.S.K. Subsidized veterinary extension services may reduce antimicrobial resistance in aquaculture. Sci. Rep. 2023, 13, 10118. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Yuan, Y.; Xie, Y. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 2021, 798, 149205. [Google Scholar] [CrossRef] [PubMed]
- Bondad-Reantaso, M.G.; Fejzic, N.; MacKinnon, B.; Huchzermeyer, D.; Seric-Haracic, S.; Mardones, F.O.; Mohan, C.V.; Taylor, N.; Jansen, M.D.; Tavornpanich, S.; et al. A 12-point checklist for surveillance of diseases of aquatic organisms: A novel approach to assist multidisciplinary teams in developing countries. Rev. Aquac. 2021, 13, 1469–1487. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dolz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.E.M.; Schreier, H.J.; Lanska, L.; Hale, M.S. The rising tide of Antimicrobial Resistance in Aquaculture: Sources, sinks and solutions. Mar. Drugs 2017, 15, 158. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Y.; Zheng, Y.; Meng, F. Antibiotics in mariculture systems: A review of occurrence, environmental behavior, and ecological effects. Environ. Pollut. 2022, 293, 118541. [Google Scholar] [CrossRef]
- Rigos, G.; Troisi, G.M. Antibacterial Agents in Mediterranean Finfish Farming: A Synopsis of Drug Pharmacokinetics in Important Euryhaline Fish Species and Possible Environmental Implications. Rev. Fish Biol. Fish. 2005, 15, 53–73. [Google Scholar] [CrossRef]
- Rodgers, C.; Furones, M. Antimicrobial agents in aquaculture: Practices, needs and issues. In The Use of Veterinary Drugs and Vaccines in Mediterranean Aquaculture; Rogers, C., Basurco, B., Eds.; CIHEAM: Zaragoza, Spain, 2009; pp. 41–59. [Google Scholar]
- Hendriksen, R.S.; Munk, P.; Njage, P.; van Bunnik, B.; McNally, L.; Lukjancenko, O.; Roder, T.; Nieuwenhuijse, D.; Pedersen, S.K.; Kjeldgaard, J.; et al. Global monitoring of antimicrobial resistance based on metagenomics analyses of urban sewage. Nat. Commun. 2019, 10, 1124. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action; FAO: Rome, Italy, 2020; p. 224. ISBN 978-92-5-132692-3. [Google Scholar]
- World Bank. FISH TO 2030, Prospects for Fisheries and Aquaculture; World Bank: Washington, DC, USA, 2013; p. 102. [Google Scholar]
- Henriksson, P.J.G.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: A review from a systems perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef]
- Caputo, A.; Bondad-Reantaso, M.G.; Karunasagar, I.; Hao, B.; Gaunt, P.; Verner-Jeffreys, D.; Fridman, S.; Dorado-Garcia, A. Antimicrobial resistance in aquaculture: A global analysis of literature and national action plans. Rev. Aquac. 2022, 15, 568–578. [Google Scholar] [CrossRef]
- Avendaño-Herrera, R.; Mancilla, M.; Miranda, C. Use of antimicrobials in Chilean Salmon farming: Facts, myths and perspectives. Rev. Aquac. 2023, 15, 89–111. [Google Scholar] [CrossRef]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current Status of the Use of Antibiotics and the Antimicrobial Resistance in the Chilean Salmon Farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Mog, M.; Ngasotter, S.; Tesia, S.; Waikhom, D.; Priyadarshini Panda, S.; Sharma, S.; Varshney, S. Problems of antibiotic resistance associated with oxytetracycline use in aquaculture: A review. J. Entomol. Zool. Stud. 2020, 8, 1075–1083. [Google Scholar]
- Vincent, A.T.; Gauthier, J.; Derome, N.; Charette, S.J. The rise and fall of antibiotics in Aquaculture. In Microbial Communities in Aquaculture Ecosystems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–19. [Google Scholar]
- Ve Van, L.; Quynh-Giao, T.; So-Ra, K.; Sang-Ah, L.; Hee-Mock, O.; HeeSik, K.; Chi-Yong, A. How do freshwater microalgae and cyanobacteria respond to antibiotics? Crit. Rev. Biotechnol. 2022, 43, 191–211. [Google Scholar] [CrossRef]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- Yin, Z. Distribution and ecological risk assessment of typical antibiotics in the surface waters of seven major rivers, China. Environ. Sci. Process. Impacts 2021, 23, 1088–1100. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzau, A.E.; Kiss, B.; Stefan, M.G.; Loghin, F. Antibiotics in the environment: Causes and consequences. Med. Pharm. Rep. 2020, 93, 231–240. [Google Scholar] [CrossRef]
- Ali, H.; Rahman, M.M.; Rico, A.; Jaman, A.; Basak, S.K.; Islam, M.M.; Khan, N.; Keus, H.J.; Mohan, C.V. An assessment of health management practices and occupational health hazards in tiger shrimp (Penaeus monodon) and freshwater prawn (Macrobrachium rosenbergii) aquaculture in Bangladesh. Vet. Anim. Sci. 2018, 5, 10–19. [Google Scholar] [CrossRef]
- Hedberg, N.; Stenson, I.; Pettersson, M.N.; Warshan, D.; Nguyen-Kim, H.; Tedengren, M.; Kautsky, N. Antibiotic use in Vietnamese fish and lobster sea cage farms; implications for coral reefs and human health. Aquaculture 2018, 495, 366–375. [Google Scholar] [CrossRef]
- WOAH. Report of the Meeting of the WOAH Working Group on Antimicrobial Resistance; Antimicrobial Resistance and Veterinary Products Department, World Organisation for Animal Health—WHOA: Paris, France, 2024; p. 26. [Google Scholar]
- Chowdhury, S.; Rheman, S.; Debnath, N.; Delamare-Deboutteville, J.; Akhtar, Z.; Ghosh, S.; Parveen, S.; Islam, K.; Islam, M.A.; Rashid, M.M.; et al. Antibiotics usage practices in aquaculture in Bangladesh and their associated factors. One Health 2022, 15, 100445. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, M.; Sanfilippo, G.; Fraix, A.; Sortino, G.; Barcellona, M.; Oliveri Conti, G.; Fragalà, M.E.; Ferrante, M.; Purrello, R.; D’Urso, A. Photodegradation of Antibiotics by Noncovalent Porphyrin-Functionalized TiO2 in Water for the Bacterial Antibiotic Resistance Risk Management. Int. J. Mol. Sci. 2020, 21, 3775. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.; Santos, E.B.H.; Esteves, V.I. Oxytetracycline in intensive aquaculture: Water quality during and after its administration, environmental fate, toxicity and bacterial resistance. Rev. Aquac. 2018, 11, 1176–1194. [Google Scholar] [CrossRef]
- Quinlan, E.L.; Nietch, C.T.; Blocksom, K.; Lazorchak, J.M.; Batt, A.L.; Griffiths, R.; Klemm, D.J. Temporal dynamics of periphyton exposed to tetracycline in stream mesocosms. Environ. Sci. Technol. 2011, 45, 10684–10690. [Google Scholar] [CrossRef]
- Samuelsen, O.L.; Torsvik, V.; Ervik, A. Long-range changes in oxytetracycline concentrations and bacterial resistance towards oxytetracycline in a fish farm sediment after medication. Sci. Total Environ. 1992, 114, 12. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Groumellec, M.L.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2022, 15, 1421–1451. [Google Scholar] [CrossRef]
- Jara, B.; Tucca, F.; Srain, B.M.; Mejanelle, L.; Aranda, M.; Fernandez, C.; Pantoja-Gutierrez, S. Antibiotics florfenicol and flumequine in the water column and sediments of Puyuhuapi Fjord, Chilean Patagonia. Chemosphere 2021, 275, 130029. [Google Scholar] [CrossRef]
- Shiroma, L.S.; Soares, M.P.; Cardoso, I.L.; Ishikawa, M.M.; Jonsson, C.M.; Queiroz, S.C.N. Evaluation of health and environmental risks for juvenile tilapia (Oreochromis niloticus) exposed to florfenicol. Heliyon 2020, 6, e05716. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Variatza, E.; Balcazar, J.L. The role of aquatic ecosystems as reservoirs of antibiotic resistance. Trends Microbiol. 2014, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- FAO. Second Consultation Meeting on Regional AMR Monitoring and Survillance Guidelines—Volume 3 “Monitoring and Survillance of AMR in Aquaculture”; Food and Agriculture Organization of the Nations United: Rome, Italy, 2020; p. 8. [Google Scholar]
- FAO. Recommendation for Prudent and Responsible Use of Veterinary Medicines in Aquaculture; Food and Agriculture Organization of the United State: Rome, Italy, 2019. [Google Scholar]
- Ashbolt, N.J.; Amézquita, A.; Backhaus, T.; Borriello, P.; Brandt, K.K.; Collignon, P.; Coors, A.; Finley, R.; Gaze, W.H.; Heberer, T.; et al. Human Health Risk Assessment (HHRA) for Environmental Development and Transfer of Antibiotic Resistance. Environ. Health Perspect. 2013, 121, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- FAO. International Instruments on the Use of Antimicrobials across the Human, Animal and Plant Sectors; World Health Organization (WHO): Geneva, Switzerland; Food and Agriculture Organization (FAO): Rome, Italy; World Organisation for Animal Health (OIE): Paris, France, 2020; p. 60. [Google Scholar]
- FAO. The FAO Action Plan on Antimicrobial Resistance 2021–2025; Food and Agriculture Organization (FAO): Rome, Italy, 2021; p. 46. [Google Scholar]
- Gonzalez-Gaya, B.; Garcia-Bueno, N.; Buelow, E.; Marin, A.; Rico, A. Effects of aquaculture waste feeds and antibiotics on marine benthic ecosystems in the Mediterranean Sea. Sci. Total Environ. 2022, 806, 151190. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, P.; Chantziaras, I.; Vijay, D.; Bedi, J.S.; Makovska, I.; Biebaut, E.; Dewulf, J. Can Improved Farm Biosecurity Reduce the Need for Antimicrobials in Food Animals? A Scoping Review. Antibiotics 2023, 12, 893. [Google Scholar] [CrossRef]
- Lambraki, I.A.; Cousins, M.; Graells, T.; Léger, A.; Henriksson, P.; Harbarth, S.; Troell, M.; Wernli, D.; Jørgensen, P.S.; Desbois, A.P.; et al. Factors influencing antimicrobial resistance in the European food system and potential leverage points for intervention: A participatory, One Health study. PLoS ONE 2022, 17, e0263914. [Google Scholar] [CrossRef]
- WOAH. Aquatic Animal Health Code; World Organisation for Animal Health: Paris, France, 2019; p. 324. [Google Scholar]
- Stentiford, G.D.; Bateman, I.J.; Hinchliffe, S.J.; Bass, D.; Hartnell, R.; Santos, E.M.; Devlin, M.J.; Feist, S.W.; Taylor, N.G.H.; Verner-Jeffreys, D.W.; et al. Sustainable aquaculture through the One Health lens. Nat. Food 2020, 1, 468–474. [Google Scholar] [CrossRef]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Editorial: Assessing the antimicrobial susceptibility of bacteria obtained from animals. J. Antimicrob. Chemother. 2010, 65, 601–604. [Google Scholar] [CrossRef]
- Smith, P. Eight rules for improving the quality of papers on the antimicrobial susceptibility of bacteria isolated from aquatic animals. Dis. Aquat. Org. 2020, 139, 87–92. [Google Scholar] [CrossRef]
- Smith, P. Guideline 2.1 Laboratory Methodologies for Bacterial Antimicrobial Susceptibility Testing; WOAH: Paris, France, 2012; p. 11. [Google Scholar]
- Ibrahim, M.; Ahmad, F.; Yaqub, B.; Ramzan, A.; Imran, A.; Afzaal, M.; Mirza, S.A.; Mazhar, I.; Younus, M.; Akram, Q.; et al. Current trends of antimicrobials used in food animals and aquaculture. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–69. [Google Scholar]
- Weitzman, J.; Filgueira, R.; Grant, J. Development of best practices for more holistic assessments of carrying capacity of aquaculture. J Environ. Manag. 2021, 287, 112278. [Google Scholar] [CrossRef]
- Salgado-Caxito, M.; Zimin-Veselkoff, N.; Adell, A.D.; Olivares-Pacheco, J.; Mardones, F.O. Qualitative Risk Assessment for Antimicrobial Resistance among Humans from Salmon Fillet Consumption Due to the High Use of Antibiotics against Bacterial Infections in Farmed Salmon. Antibiotics 2022, 11, 662. [Google Scholar] [CrossRef] [PubMed]
| DIMENSION | DRIVER, GAPS | GENERAL RECOMMENDATIONS |
|---|---|---|
| ANTIBIOTIC USE: methodological and technical for assessing ecological impacts | ||
| Measuring impacts | Indirect impacts on the environment must be assessed for conducting environmental evaluations (e.g., ecological processes intervened by bacteria present in the environment or benthic organisms affected by ABs). | In the absence of clear information on the ecological effects caused by AMs, it is prudent to differentiate freshwater and marine environment impact assessments. Ecological assessment criteria for ABs should be incorporated into national policies. |
| Absence of models on cumulative impact (e.g., multiple sources or chronic discharge); validated models are required for evaluating impacts and delivering recommendations. | Implementing surveillance tools (e.g., benthic respiration, oxidative stress, oxygenation, primary production, environmental DNA, metabarcoding, metagenomics, and others) is required. | |
| Impact thresholds should be established. | The existing ERA used for drug approval is not enough for assessing environmental risks; therefore, implementing or improving ERA is necessary. | |
| Methodology | Lack of standardized methodology for measuring in situ ecological impacts; the methodology that is currently used is expensive and not standardized. | Conducting pilot assessment/field tests in key water bodies (e.g., fjords) is needed for obtaining valuable real data and determining impacts, as it is vital to have knowledge on measuring ecological impacts since an evaluation framework does already exist. |
| Monitoring | Missing information on AM passive monitoring in aquatic environments. | EQS (AMR and ecotoxicological) for emissions and the environment can serve as a valuable reference point in aquaculture. |
| ANTIBIOTIC USE AND ECOLOGICAL IMPACTS: regulatory aspects | ||
| Environmental Quality standards | Absence of international agreement and validation of EQS (i.e., AMR and ecotoxicological) for AM release. | |
| The current ERA used for drug approval is insufficient to assess environmental risk. | ||
| Risk assessment | Deficiency in standard risk assessment methodologies appropriate for developing countries, and barely applicable for aquaculture; risk assessment methodologies for pathogen risk analyses are well developed in aquatic food production systems. | |
| Protection | Specific safety objectives for human health and ecosystems should be outlined. | |
| SOCIAL AND ECONOMIC | ||
| Economic incentives | Low-cost access in informal markets. | Identify informal sources of access to antibiotics and access to certifications for the proper use of antibiotics and design subsidies for small farmers that can demonstrate traceability in ABU. |
| Regulations | Inappropriate regulations to control the overuse of ABs and informal access. | Strict regulations should be established in each country regarding the sale, use, and monitoring of ABU in aquaculture. There should be more surveillance on the application of the regulation as there exist international documents that some countries do not apply. |
| Farmers’ attitudes and knowledge | Lack of risk perception and awareness of impacts of AB misuse. | Public communication programs for behavioral change. Research in stakeholders’ preferences and behaviors. |
| Lack of training in the sustainable use of ABs and disease management. | Improved small-scale disease management tools. Characterization of producers and identification of their knowledge gaps on the use of ABs and their impacts. Improve coordination between farms in the same zone. | |
| Biosecurity | Restricted access to freshwater and poor sanitation. | Training for farmers and field personnel is essential to improve biosecurity at a farm level. More trained personnel, ideally Veterinarians, to guide practices on AM and AB use. |
| Lack of professional veterinary services. | ||
| Scarcity of laboratory infrastructure in microbiological testing. | ||
| Inadequate access to AB. | Improve the technique of AB selection according to the type of disease. | |
| Lack of reporting on ABU and monitoring. | Need for traceability and tracking systems for the use of AB. | |
| THE ROLE OF AMR ON AQUACULTURE | ||
| Scientific research | While recent scientific research has emerged, substantial gaps in understanding AMR persist. | To collect more knowledge to answer relevant questions regarding AMR. More investigation is needed and the funding to conduct investigations. |
| Better knowledge is required regarding the relative magnitude of aquaculture contrasted with land-based animal farming systems/humans’ AM (over-)consumption. | ||
| Absence of detailed information about naturally occurring ARG to develop methodologies to establish impact. | ||
| Aquaculture systems boundaries | Deficient knowledge of how to determine aquaculture systems boundaries. | Strengthening knowledge of the role of AMR is fundamental. |
| Hazard | There is a need to categorize systems regarding hazards. | Enhancing knowledge of the theme. |
| Workshop 1 General AMU and Ecological Impacts | Workshop 2 Socioeconomics Perspective | Workshop 3 ABs in Aquaculture | Workshop 4 Methods for Determining Impacts | |
|---|---|---|---|---|
| Date | 27 May 2021 | 22 July 2021 | 23 September 2021 | 2 February 2022 |
| Objective | Evaluating the current state of knowledge on AMU and identifying existing gaps. Formulating strategies to identify ecologically relevant impact indicators and establish thresholds for assessment. | Identifying pivotal socioeconomic factors and effective governance mechanisms essential for implementing monitoring practices in aquaculture and extending them across sectors, and countries to enhance the sustainability of aquaculture. | Developing pathways to enhance our understanding of AB usage in aquaculture and AMR. | Exploring potential AB monitoring tools that can be universally adapted and implemented across regions and sectors. |
| Attendees | 38 attendees among experts (26), guests (4), and the AGAP team (8) from 22 institutions worldwide belonging to 11 countries; 71.1% men and 15.5% women | 29 attendees among experts (20), guests (5), and the AGAP team (4). The attendees were representatives of 10 countries and 15 institutions, including the FAO, WorldFish, and WOAH. 75.9% men and 9.2% women. | 26 attendees, among experts (17), the organizing team (3), and guests (6) from 14 institutions, including FAO, WorldFish, and WOAH, representing 13 countries, with 69.2% men and 11.6% women. | 15 attendees among the AGAP team (5) and experts (10) from 9 institutions belonging to 4 countries, with 60% mean and 10% women. |
| Field | Land-based animal growing systems, freshwater aquaculture, and marine-based aquaculture, as well as fields involving pathologists, animal health experts, ecologists, food services, and ecotoxicologists, were among other areas of expertise concerning prawn farms and fish farms. | Animal health experts, ecologists, experts in certification and food systems, livestock specialists, socioeconomists | Pathologists, epidemiologists, microbiologists, and data management and aquaculture system experts | Experts involved in pharmacology, food safety, and animal health, as well as environmental experts, researchers using analytic techniques, and ecotoxicologists |
| Duration | 3:30 h | 3:00 h | 3:00 h | 3:00 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farías, D.R.; Ibarra, R.; Estévez, R.A.; Tlusty, M.F.; Nyberg, O.; Troell, M.; Avendaño-Herrera, R.; Norden, W. Towards Sustainable Antibiotic Use in Aquaculture and Antimicrobial Resistance: Participatory Experts’ Overview and Recommendations. Antibiotics 2024, 13, 887. https://doi.org/10.3390/antibiotics13090887
Farías DR, Ibarra R, Estévez RA, Tlusty MF, Nyberg O, Troell M, Avendaño-Herrera R, Norden W. Towards Sustainable Antibiotic Use in Aquaculture and Antimicrobial Resistance: Participatory Experts’ Overview and Recommendations. Antibiotics. 2024; 13(9):887. https://doi.org/10.3390/antibiotics13090887
Chicago/Turabian StyleFarías, Daniela R., Rolando Ibarra, Rodrigo A. Estévez, Michael F. Tlusty, Oskar Nyberg, Max Troell, Ruben Avendaño-Herrera, and Wendy Norden. 2024. "Towards Sustainable Antibiotic Use in Aquaculture and Antimicrobial Resistance: Participatory Experts’ Overview and Recommendations" Antibiotics 13, no. 9: 887. https://doi.org/10.3390/antibiotics13090887
APA StyleFarías, D. R., Ibarra, R., Estévez, R. A., Tlusty, M. F., Nyberg, O., Troell, M., Avendaño-Herrera, R., & Norden, W. (2024). Towards Sustainable Antibiotic Use in Aquaculture and Antimicrobial Resistance: Participatory Experts’ Overview and Recommendations. Antibiotics, 13(9), 887. https://doi.org/10.3390/antibiotics13090887







