Returned Rate and Changed Patterns of Systemic Antibiotic Use in Ambulatory Care in Hungary after the Pandemic—A Longitudinal Ecological Study
Abstract
1. Introduction
2. Results
3. Discussion
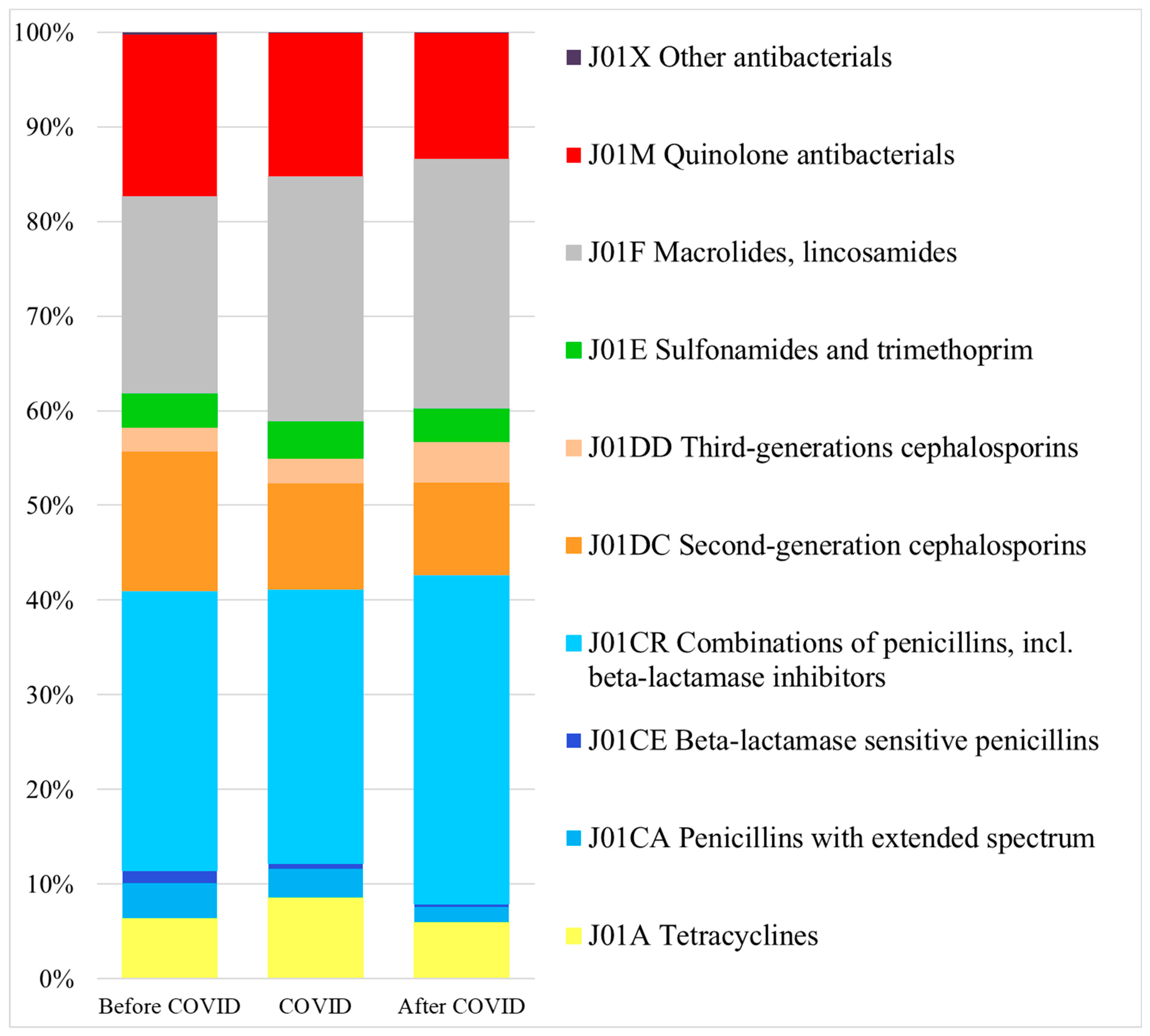
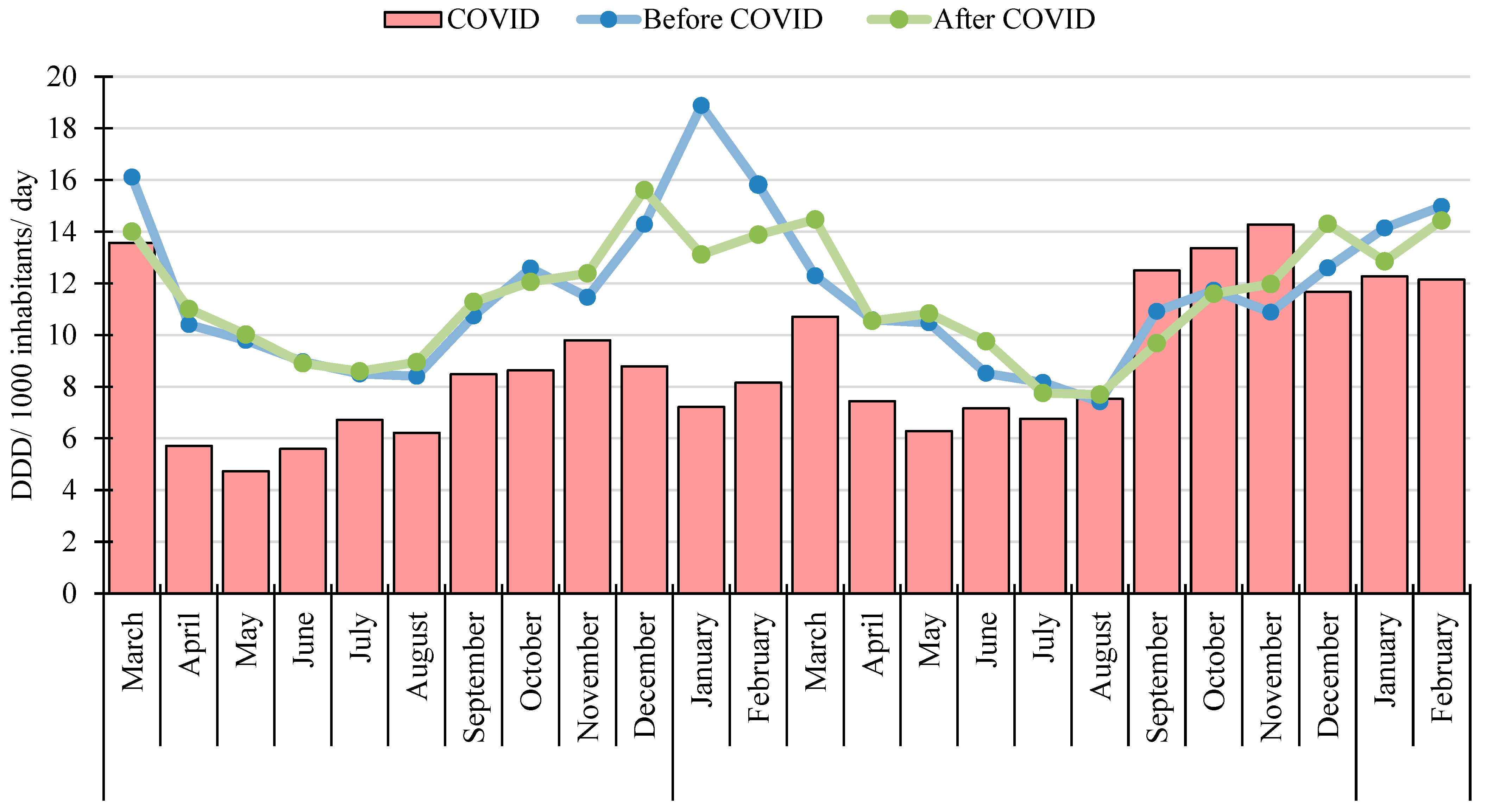
3.1. Scale of Systemic Antibacterial (J01) Use
3.2. Seasonal Variation
3.3. Pattern of Systemic Antibacterial (J01) Use
3.4. Strengths and Limitations
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.; Amann, M.; Ayeb-Karlsson, S.; Belesova, K.; Bouley, T.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; Chambers, J.; et al. The Lancet Countdown on Health and Climate Change: From 25 Years of Inaction to a Global Transformation for Public Health. Lancet 2018, 391, 581–630. [Google Scholar] [CrossRef] [PubMed]
- Matuz, M.; Soós, G.; Hajdú, E.; Papfalvi, E.; Visnyovszki, Á.; Viola, R.; Benkő, R. The characteristics and trends of Hungarian outpatient antibiotic use (2010–2019). Orv. Hetil. 2022, 163, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.R.; Bonomo, R.A. Overview: The Ongoing Threat of Antimicrobial Resistance. Infect. Dis. Clin. N. Am. 2020, 34, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Broughan, J.; Crowley, D.; O’Kelly, B.; Fawsitt, R.; Burke, M.C.; McCombe, G.; Lambert, J.S.; Cullen, W. COVID-19’s Impact on Primary Care and Related Mitigation Strategies: A Scoping Review. Eur. J. Gen. Pract. 2021, 27, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Elden, N.M.K.; Mandil, A.M.A.; Hegazy, A.A.; Nagy, N.; Mabry, R.M.; Khairy, W.A. Health Innovations in Response to the COVID-19 Pandemic: Perspectives from the Eastern Mediterranean Region. J. Public Health 2023, 45, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Walia, K.; Mendelson, M.; Kang, G.; Venkatasubramanian, R.; Sinha, R.; Vijay, S.; Veeraraghavan, B.; Basnyat, B.; Rodrigues, C.; Bansal, N.; et al. How Can Lessons from the COVID-19 Pandemic Enhance Antimicrobial Resistance Surveillance and Stewardship? Lancet Infect. Dis. 2023, 23, e301–e309. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, A.; Al Mutairi, N.; Baker, K.; M-Amen, K.; Darweesh, O.; Karwi, H.; Seaton, A.; Sneddon, J.; Godman, B. Impact of COVID-19 Pandemic on the Utilization and Quality of Antibiotic Use in the Primary Care Setting in England, March 2019–March 2023: A Segmented Interrupted Time Series Analysis of over 53 Million Individuals. Expert Rev. Anti-Infect. Ther. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Coenen, S.; Ferech, M.; Haaijer-Ruskamp, F.M.; Butler, C.C.; Vander Stichele, R.H.; Verheij, T.J.M.; Monnet, D.L.; Little, P.; Goossens, H. ESAC Project Group European Surveillance of Antimicrobial Consumption (ESAC): Quality Indicators for Outpatient Antibiotic Use in Europe. Qual. Saf. Health Care 2007, 16, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Gabarró, C.; Leung, V.H.; Vlahović-Palčevski, V.; Machowska, A.; Monnet, D.L.; Högberg, L.D.; Group, E.-N. study Rebound in Community Antibiotic Consumption after the Observed Decrease during the COVID-19 Pandemic, EU/EEA, 2022. Eurosurveillance 2023, 28, 2300604. [Google Scholar] [CrossRef]
- Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2022 (accessed on 4 July 2024).
- King, L.M.; Lovegrove, M.C.; Shehab, N.; Tsay, S.; Budnitz, D.S.; Geller, A.I.; Lind, J.N.; Roberts, R.M.; Hicks, L.A.; Kabbani, S. Trends in US Outpatient Antibiotic Prescriptions During the Coronavirus Disease 2019 Pandemic. Clin. Infect. Dis. 2021, 73, e652–e660. [Google Scholar] [CrossRef]
- Kitano, T.; Brown, K.A.; Daneman, N.; MacFadden, D.R.; Langford, B.J.; Leung, V.; So, M.; Leung, E.; Burrows, L.; Manuel, D.; et al. The Impact of COVID-19 on Outpatient Antibiotic Prescriptions in Ontario, Canada; An Interrupted Time Series Analysis. Open Forum Infect. Dis. 2021, 8, ofab533. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.M.; Estrela, M.; Gomes, E.R.; Piñeiro-Lamas, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The Impact of the COVID-19 Pandemic on Antibiotic Prescribing Trends in Outpatient Care: A Nationwide, Quasi-Experimental Approach. Antibiotics 2021, 10, 1040. [Google Scholar] [CrossRef]
- Colliers, A.; De Man, J.; Adriaenssens, N.; Verhoeven, V.; Anthierens, S.; De Loof, H.; Philips, H.; Coenen, S.; Morreel, S. Antibiotic Prescribing Trends in Belgian Out-of-Hours Primary Care during the COVID-19 Pandemic: Observational Study Using Routinely Collected Health Data. Antibiotics 2021, 10, 1488. [Google Scholar] [CrossRef]
- Högberg, L.D.; Vlahović-Palčevski, V.; Pereira, C.; Weist, K.; Monnet, D.L.; ESAC-Net study group. ESAC-Net study group participants Decrease in Community Antibiotic Consumption during the COVID-19 Pandemic, EU/EEA, 2020. Eurosurveillance 2021, 26, 2101020. [Google Scholar] [CrossRef]
- Antimicrobial Consumption—Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2019 (accessed on 4 July 2024).
- Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2020 (accessed on 4 July 2024).
- Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2021. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2021 (accessed on 4 July 2024).
- The WHO AWaRe (Access, Watch, Reserve) Antibiotic Book; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006238-2.
- AWaRe Classification of Antibiotics for Evaluation and Monitoring of Use, 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.04 (accessed on 4 July 2024).
- WHO Antibiotics Portal. Available online: https://aware.essentialmeds.org/groups (accessed on 4 July 2024).
- Adriaenssens, N.; Bruyndonckx, R.; Versporten, A.; Hens, N.; Monnet, D.L.; Molenberghs, G.; Goossens, H.; Weist, K.; Coenen, S. Quality Appraisal of Antibiotic Consumption in the Community, European Union/European Economic Area, 2009 and 2017. J. Antimicrob. Chemother. 2021, 76, ii60–ii67. [Google Scholar] [CrossRef] [PubMed]
- The Correct Use of Antimicorbal Agents [Hungarian]. Available online: https://efop180.antsz.hu/tajekoztatok/modszertani-anyagok/144-antimikrobas-szerek-helyes-hasznalata.html (accessed on 7 July 2024).
- Hambalek, H.; Matuz, M.; Ruzsa, R.; Engi, Z.; Visnyovszki, Á.; Papfalvi, E.; Hajdú, E.; Doró, P.; Viola, R.; Soós, G.; et al. Impact of the COVID-19 Pandemic on Ambulatory Care Antibiotic Use in Hungary: A Population-Based Observational Study. Antibiotics 2023, 12, 970. [Google Scholar] [CrossRef] [PubMed]
- Vitális, E. Management of Otitis in Outpatient Care; Version 1; Prepared by the Methodological Development Working Group I. of the EFOP 1.8.0-VEKOP-17-2017-00001 Project “Professional Methodological Development of the Healthcare System”. [hungarian] 2020. Available online: https://info.nevesforum.hu/wp-content/uploads/2021/03/otitis.pdf (accessed on 4 July 2024).
- Vitális, E. Management of Sinusitis in Outpatient Care; Version 1; Prepared by the Methodological Development Working Group I. of the EFOP 1.8.0-VEKOP-17-2017-00001 Project “Professional Methodological Development of the Healthcare System” [hungarian] 2020. Available online: https://info.nevesforum.hu/wp-content/uploads/2021/03/sinusitis.pdf (accessed on 4 July 2024).
- Hajdú, E.; Szabó, É. Home-Acquired Pneumonia Antimicrobial Treatment in Outpatient Care; Version 1; Prepared by the Methodological Development Working Group I of the EFOP 1.8.0-VEKOP-17-2017-00001 Project “Professional Methodological Development of the Healthcare System”. [Hungarian] 2020. Available online: https://info.nevesforum.hu/wp-content/uploads/2021/03/pneumonia.pdf (accessed on 4 July 2024).
- National Bacteriological Surveillance 2022 (NBS). Available online: https://www.nnk.gov.hu/index.php/mikrobiologiai-referencia-laboratoriumi-foosztaly/nemzeti-bakteriologiai-surveillance-nbs/category/347-nemzeti-bakteriologiai-surveillance-2022.html (accessed on 14 August 2024).
- WHO Model List of Essential Medicines—23rd List, 2023. Available online: https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2023.02 (accessed on 4 July 2024).
- Benkő, R. Guidelines for the Antibacterial Treatment of Acute Sore Throat (Tonsillopharyngitis) in Outpatient Care; Version 1; Prepared by the Methodological Development Working Group I. of the EFOP 1.8.0-VEKOP-17-2017-00001 Project “Professional Methodological Development of the Healthcare System” [hungarian] 2020. Available online: https://info.nevesforum.hu/wp-content/uploads/2021/03/torokfajas.pdf (accessed on 4 July 2024).
- Szabó, B.G. Antibiotic Treatment of Uncomplicated Acute Pyelonephritis in Outpatient Care; Version 1; Prepared by the Methodological Development Working Group I of the EFOP 1.8.0-VEKOP-17-2017-00001 Project “Professional Methodological Development of the Healthcare System”. [hungarian] 2020. Available online: https://info.nevesforum.hu/wp-content/uploads/2021/03/cystitis.pdf (accessed on 4 July 2024).
- Fluoroquinolone and Quinolone Antibiotics: PRAC Recommends New Restrictions on Use Following Review of Disabling and Potentially Long-Lasting Side Effects|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/fluoroquinolone-quinolone-antibiotics-prac-recommends-new-restrictions-use-following-review-disabling-potentially-long-lasting-side-effects (accessed on 7 July 2024).
- Pharmacovigilance Risk Assessment Committee (PRAC): 10–12 May 2023|European Medicines Agency. Available online: https://www.ema.europa.eu/en/events/pharmacovigilance-risk-assessment-committee-prac-10-12-may-2023 (accessed on 4 July 2024).
- Fluoroquinolone Antibiotics: Reminder of Measures to Reduce the Risk of Long-Lasting, Disabling and Potentially Irreversible Side Effects|European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/fluoroquinolone-antibiotics-reminder-measures-reduce-risk-long-lasting-disabling-potentially-irreversible-side-effects (accessed on 3 July 2024).
- Increase of Pertussis Cases in the EU/EEA. Available online: https://www.ecdc.europa.eu/en/publications-data/increase-pertussis-cases-eueea (accessed on 22 July 2024).
- Antibiotic Utilisation Data. Available online: https://www.neak.gov.hu/felso_menu/szakmai_oldalak/publikus_forgalmi_adatok/gyogyszer_forgalmi_adatok/gyogyszer_forgalmi_adatok (accessed on 3 July 2024).
- ATCDDD—ATC/DDD Index. Available online: https://atcddd.fhi.no/atc_ddd_index/?code=J01&showdescription=no (accessed on 4 July 2024).
- Hollingworth, S.; Kairuz, T. Measuring Medicine Use: Applying ATC/DDD Methodology to Real-World Data. Pharmacy 2021, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Hungarian Central Statistical Office. Information Data Base. Population. Population Movement. KSH. Main Indicators of Population and Vital Events (1941–2024). Available online: https://www.ksh.hu/stadat_eng?lang=en&theme=nep (accessed on 4 July 2024).
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M. Outpatient Antibiotic Use in Europe and Association with Resistance: A Cross-National Database Study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Adriaenssens, N.; Coenen, S.; Versporten, A.; Muller, A.; Vankerckhoven, V.; Goossens, H.; ESAC Project Group. European Surveillance of Antimicrobial Consumption (ESAC): Quality Appraisal of Antibiotic Use in Europe. J. Antimicrob. Chemother. 2011, 66 (Suppl. 6), vi71–vi77. [Google Scholar] [CrossRef] [PubMed]
| Before COVID Period | COVID Period | After COVID Period | p * | ||||
|---|---|---|---|---|---|---|---|
| DID (Mean) ± SD | Min–Max | DID (Mean) ± SD | Min–Max | DID (Mean) ± SD | Min–Max | ||
| J01A Tetracyclines | 0.74 ± 0.15 | 0.45–1.05 | 0.77 ± 0.22 | 0.46–1.17 | 0.67 ± 0.11 | 0.49–0.92 | 0.157 |
| J01CA Penicillins with extended spectrum | 0.43 ± 0.10 | 0.27–0.68 | 0.27 ± 0.09 | 0.14–0.46 | 0.22 ± 0.17 | 0.01–0.43 | <0.001 |
| J01CE Beta-lactamase sensitive penicillins | 0.16 ± 0.04 | 0.08–0.24 | 0.05 ± 0.02 | 0.03–0.12 | 0.04 ± 0.03 | 0.04–0.03 | <0.001 |
| J01CR Combinations of penicillins, incl. beta-lactamase inhibitors | 3.44 ± 0.79 | 2.35–5.47 | 2.60 ± 0.79 | 1.44–4.21 | 3.87 ± 0.84 | 2.68–5.47 | 0.024 |
| J01C Beta-lactam antibacterials, penicillins | 4.03 ± 0.94 | 2.70–6.39 | 2.93 ± 0.90 | 1.60–4.78 | 4.12 ± 0.80 | 3.03–5.77 | 0.443 |
| J01DC Second-generation cephalosporins | 1.70 ± 0.48 | 0.93–2.88 | 1.01 ± 0.33 | 0.53–1.86 | 1.05 ± 0.20 | 0.70–1.51 | <0.001 |
| J01DD Third-generation cephalosporins | 0.29 ± 0.10 | 0.16–0.50 | 0.23 ± 0.11 | 0.09–0.43 | 0.45 ± 0.12 | 0.25–0.66 | <0.001 |
| J01D Cephalosporins | 2.00 ± 0.57 | 1.10–3.39 | 1.24 ± 0.41 | 0.63–2.20 | 1.50 ± 0.28 | 1.04–2.02 | 0.008 |
| J01E Sulfonamides and trimethoprim | 0.42 ± 0.06 | 0.33–0.56 | 0.36 ± 0.07 | 0.24–0.51 | 0.39 ± 0.06 | 0.32–0.51 | 0.364 |
| J01FA Macrolides | 1.91 ± 0.73 | 0.93–3.64 | 1.84 ± 1.13 | 0.45–4.22 | 2.41 ± 0.83 | 1.09–4.06 | 0.007 |
| J01FF Lincosamides | 0.49 ± 0.02 | 0.44–0.53 | 0.47 ± 0.03 | 0.41–0.55 | 0.47 ± 0.02 | 0.44–0.53 | 0.004 |
| J01F Macrolides, lincosamides | 2.40 ± 0.74 | 1.39–4.18 | 2.31 ± 1.15 | 0.87–4.71 | 2.89 ± 0.84 | 1.54–4.46 | 0.009 |
| J01M Quinolone antibacterials | 1.98 ± 0.52 | 1.28–3.30 | 1.36 ± 0.34 | 0.86–2.06 | 1.52 ± 0.33 | 1.01–2.12 | 0.001 |
| J01X Other antibacterials | 0.04 ± 0.04 | 0.00–0.11 | 0.01 ± 0.00 | 0.00–0.01 | 0.01 ± 0.00 | 0.01–0.02 | 0.006 |
| J01 Antibacterials | 11.61 ± 2.90 | 7.41–18.88 | 8.99 ± 2.87 | 4.73–14.28 | 11.11 ± 2.28 | 7.69–15.60 | 0.871 |
| J01 Access antibacterials | 5.68 ± 1.14 | 4.09–8.54 | 4.53 ± 1.13 | 2.77–6.72 | 5.78 ± 0.91 | 4.37–7.58 | 0.740 |
| J01 Watch antibacterials | 5.92 ± 1.78 | 3.32–10.33 | 4.45 ± 1.78 | 1.95–7.83 | 5.70 ± 1.40 | 3.31–8.01 | 0.628 |
| J01 Access % | 49.51 ± 2.79 | 43.58–55.20 | 51.63 ± 4.68 | 44.38–59.62 | 50.80 ± 2.93 | 46.11–57.13 | 0.124 |
| Before COVID Period | COVID Period | After COVID Period | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | ATC Code | Substance | DID 1 | % | Cum% 2 | ATC Code | Substance | DID 1 | % | Cum% 2 | ATC Code | Substance | DID | % | Cum% |
| 1. | J01CR02 | co-amoxiclav | 3.43 | 29.55 | 29.55 | J01CR02 | co-amoxiclav | 2.60 | 28.90 | 28.90 | J01CR02 | co-amoxiclav | 4.00 | 34.78 | 34.78 |
| 2. | J01DC02 | cefuroxime | 1.34 | 11.52 | 41.06 | J01FA10 | azithromycin | 1.40 | 15.55 | 44.45 | J01FA10 | azithromycin | 1.98 | 17.20 | 51.98 |
| 3. | J01FA10 | azithromycin | 1.22 | 10.47 | 51.54 | J01DC02 | cefuroxime | 0.70 | 8.84 | 53.29 | J01MA12 | levofloxacin | 0.73 | 6.40 | 58.38 |
| 4. | J01MA12 | levofloxacin | 1.00 | 8.59 | 60.13 | J01AA02 | doxycycline | 0.77 | 8.61 | 61.90 | J01AA02 | doxycycline | 0.68 | 5.95 | 64.33 |
| 5. | J01AA02 | doxycycline | 0.74 | 6.36 | 66.49 | J01MA12 | levofloxacin | 0.60 | 6.69 | 68.58 | J01DC02 | cefuroxime | 0.64 | 5.58 | 69.61 |
| 6. | J01FA09 | clarithromycin | 0.66 | 5.65 | 72.13 | J01FF01 | clindamycin | 0.47 | 5.27 | 73.85 | J01FA09 | clarithromycin | 0.57 | 4.99 | 74.91 |
| 7. | J01MA02 | ciprofloxacin | 0.58 | 4.97 | 77.11 | J01MA02 | ciprofloxacin | 0.46 | 5.15 | 79.01 | J01DD08 | cefixime | 0.49 | 4.30 | 79.20 |
| 8. | J01FF01 | clindamycin | 0.49 | 4.25 | 81.36 | J01FA09 | clarithromycin | 0.43 | 4.74 | 83.75 | J01MA02 | ciprofloxacin | 0.48 | 4.20 | 83.40 |
| 9. | J01CA04 | amoxicillin | 0.43 | 3.70 | 85.06 | J01EE01 | SMX-TMP 3 | 0.36 | 3.96 | 87.71 | J01FF01 | clindamycin | 0.47 | 4.13 | 87.53 |
| 10. | J01EE01 | SMX-TMP 3 | 0.42 | 3.62 | 88.68 | J01CA04 | amoxicillin | 0.27 | 2.99 | 90.70 | J01DC10 | cefprozil | 0.45 | 3.90 | 91.93 |
| Mean Monthly Number of Exposed Patients | |||||
|---|---|---|---|---|---|
| Before COVID Period | COVID Period | After COVID Period | |||
| J01CR02 | Amoxicillin and clavulanic acid (co-amoxiclav) | mean | 140,494 | 99,246 | 156,208 |
| min–max | 89,124–227,533 | 51,685–166,902 | 97,410–220,223 | ||
| J01FA10 | Azithromycin | mean | 66,869 | 66,538 | 97,367 |
| min–max | 30,329–129,604 | 13,831–160,368 | 37,441–160,572 | ||
| J01DC02 | Cefuroxim | mean | 41,890 | 23,251 | 18,391 |
| min–max | 22,458–69,290 | 12,828–44,588 | 10,824–29,549 | ||
| J01MA12 | Levofloxacin | mean | 39,450 | 22,909 | 28,240 |
| min–max | 17,716–82,602 | 9642–44,438 | 13,159–46,138 | ||
| J01AA02 | Doxycyline | mean | 8292 | 8185 | 6987 |
| min–max | 4873–12,732 | 4600–13,208 | 4795–9914 | ||
| Seasonality Index + | |||
|---|---|---|---|
| Before COVID Period | COVID Period | After COVID Period | |
| J01A Tetracyclines | 38.92 | 60.29 | 22.48 |
| J01CA Penicillins with extended spectrum * | |||
| J01CE Beta-lactamase sensitive penicillins * | |||
| J01CR Combinations of penicillins, incl. beta-lactamase inhibitors | 44.52 | 37.97 | 38.53 |
| J01C Beta-lactam antibacterials, penicillins | 43.63 | 35.68 | 34.07 |
| J01DC Second-generation cephalosporins | 50.92 | 27.96 | 27.76 |
| J01DD Third-generation cephalosporins | 69.82 | 82.12 | 62.26 |
| J01D Cephalosporins | 53.39 | 36.26 | 37.32 |
| J01E Sulfomadies and trimethoprim | 28.11 | 30.17 | 22.54 |
| J01F Macrolides, lincosamides | 60.63 | 104.74 | 59.74 |
| J01M Quinolone antibacterials | 42.22 | 42.73 | 35.01 |
| J01X Other antibacterials * | |||
| J01 Antibacterials | 46.86 | 53.42 | 39.68 |
| Months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| Years | 2018 | ||||||||||||
| 2019 | |||||||||||||
| 2020 | |||||||||||||
| 2021 | |||||||||||||
| 2022 | |||||||||||||
| 2023 | |||||||||||||
| 2024 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hambalek, H.; Matuz, M.; Ruzsa, R.; Papfalvi, E.; Nacsa, R.; Engi, Z.; Csatordai, M.; Soós, G.; Hajdú, E.; Csupor, D.; et al. Returned Rate and Changed Patterns of Systemic Antibiotic Use in Ambulatory Care in Hungary after the Pandemic—A Longitudinal Ecological Study. Antibiotics 2024, 13, 848. https://doi.org/10.3390/antibiotics13090848
Hambalek H, Matuz M, Ruzsa R, Papfalvi E, Nacsa R, Engi Z, Csatordai M, Soós G, Hajdú E, Csupor D, et al. Returned Rate and Changed Patterns of Systemic Antibiotic Use in Ambulatory Care in Hungary after the Pandemic—A Longitudinal Ecological Study. Antibiotics. 2024; 13(9):848. https://doi.org/10.3390/antibiotics13090848
Chicago/Turabian StyleHambalek, Helga, Mária Matuz, Roxána Ruzsa, Erika Papfalvi, Róbert Nacsa, Zsófia Engi, Márta Csatordai, Gyöngyvér Soós, Edit Hajdú, Dezső Csupor, and et al. 2024. "Returned Rate and Changed Patterns of Systemic Antibiotic Use in Ambulatory Care in Hungary after the Pandemic—A Longitudinal Ecological Study" Antibiotics 13, no. 9: 848. https://doi.org/10.3390/antibiotics13090848
APA StyleHambalek, H., Matuz, M., Ruzsa, R., Papfalvi, E., Nacsa, R., Engi, Z., Csatordai, M., Soós, G., Hajdú, E., Csupor, D., & Benkő, R. (2024). Returned Rate and Changed Patterns of Systemic Antibiotic Use in Ambulatory Care in Hungary after the Pandemic—A Longitudinal Ecological Study. Antibiotics, 13(9), 848. https://doi.org/10.3390/antibiotics13090848








